Severe Acquired Brain Injury: Prognostic Factors of Discharge Outcome in Older Adults
Abstract
:1. Introduction
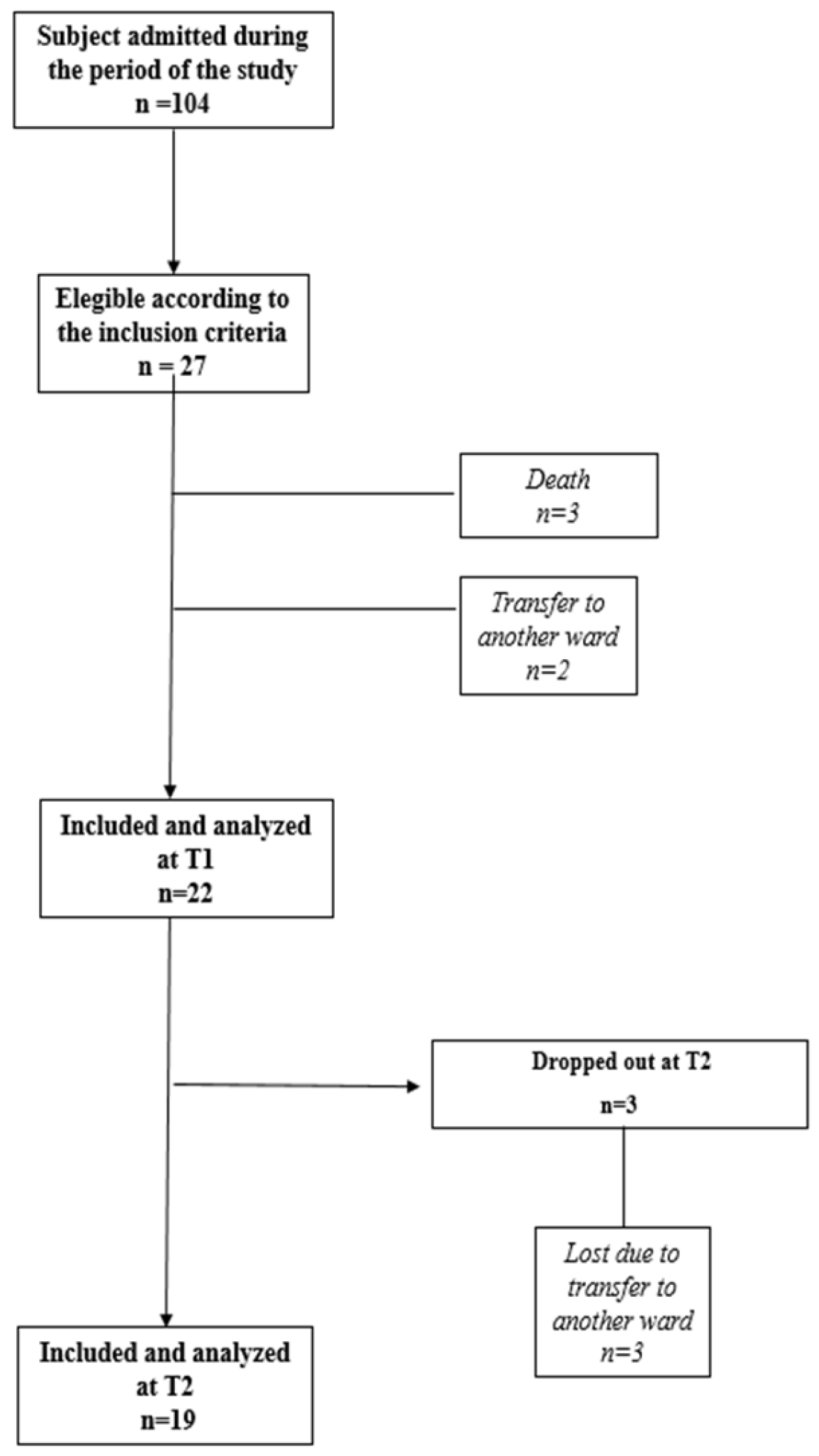
2. Materials and Methods
2.1. Study Design
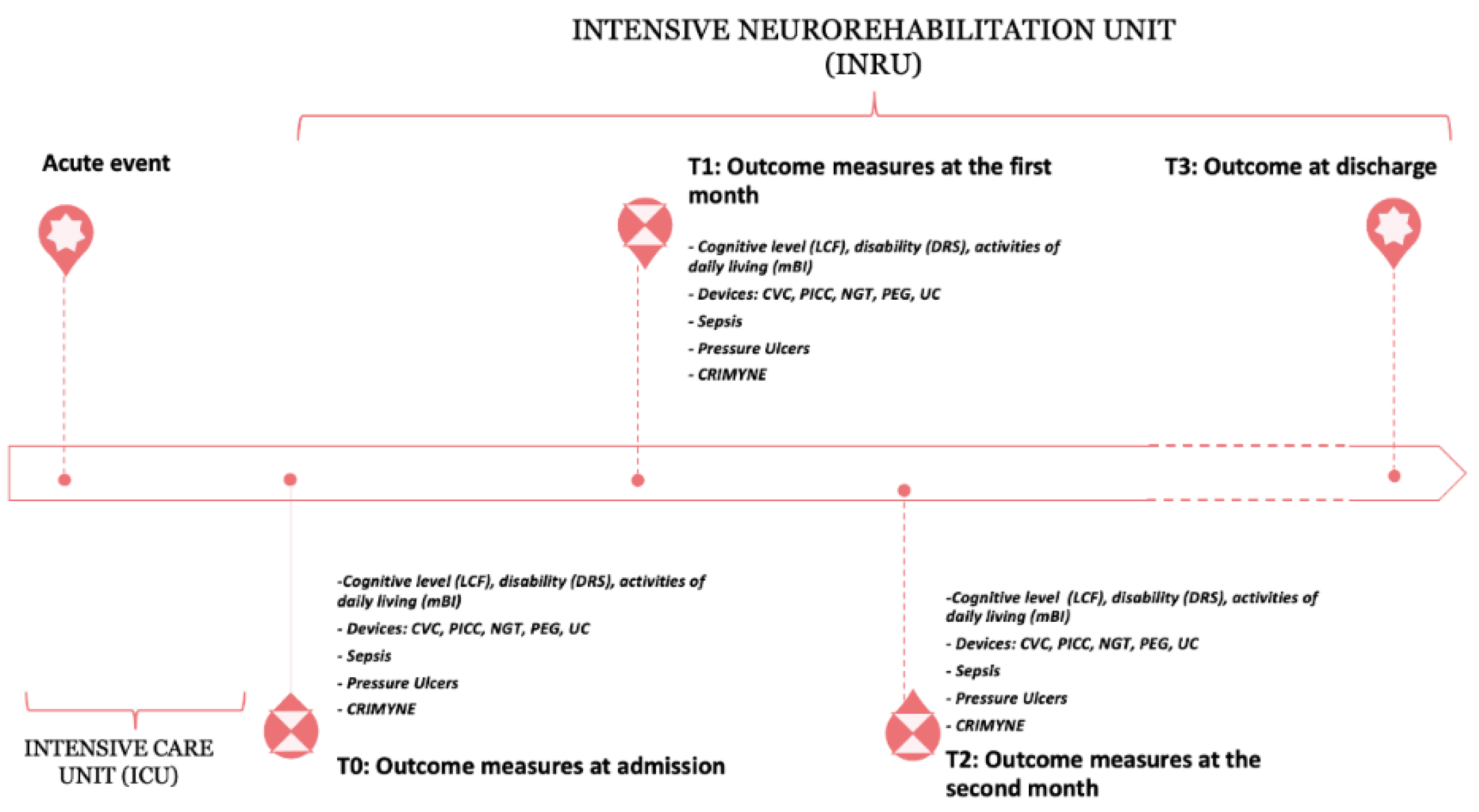
2.2. Timing of Assessment
2.3. Discharge Outcome
2.4. Assessment
2.5. Statistical Analysis
3. Results
3.1. Predictors of Good/Poor Outcomes at 1 Month
3.2. Predictors of Good/Poor Outcomes at 2 Months
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Creutzfeldt, C.J.; Longstreth, W.T.; Holloway, R.G. Predicting Decline and Survival in Severe Acute Brain Injury: The Fourth Trajectory. BMJ 2015, 351, h3904. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, A.F.; Lauzier, F.; Simard, J.F.; Scales, D.C.; Burns, K.E.A.; Moore, L.; Zygun, D.A.; Bernard, F.; Meade, M.O.; Dung, T.C.; et al. Mortality Associated with Withdrawal of Life-Sustaining Therapy for Patients with Severe Traumatic Brain Injury: A Canadian Multicentre Cohort Study. CMAJ 2011, 183, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, V.; Hough, C.L.; Vavilala, M.S.; Komisarow, J.; Chaikittisilpa, N.; Lele, A.V.; Raghunathan, K.; Creutzfeldt, C.J. Tracheostomy After Severe Acute Brain Injury: Trends and Variability in the USA. Neurocrit. Care 2019, 30, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.D.; Corrigan, J.D.; Dijkers, M.P. Traumatic Brain Injury Rehabilitation Comparative Effectiveness Research: Introduction to the Traumatic Brain Injury-Practice Based Evidence Archives Supplement. Arch. Phys. Med. Rehabil. 2015, 96, S173–S177. [Google Scholar] [CrossRef]
- Ràbago, C.A.; Wilken, J.M. Application of a Mild Traumatic Brain Injury Rehabilitation Program in a Virtual Realty Environment: A Case Study. J. Neurol. Phys. Ther. 2011, 35, 185–193. [Google Scholar] [CrossRef]
- Giovannetti, A.M.; Covelli, V.; Sattin, D.; Leonardi, M. Caregivers of Patients with Disorder of Consciousness: Burden, Quality of Life and Social Support. Acta Neurol. Scand. 2015, 132, 259–269. [Google Scholar] [CrossRef]
- Fischer, D.; Edlow, B.L.; Giacino, J.T.; Greer, D.M. Neuroprognostication: A Conceptual Framework. Nat. Rev. Neurol. 2022, 18, 419–427. [Google Scholar] [CrossRef]
- Estraneo, A.; Loreto, V.; Masotta, O.; Pascarella, A.; Trojano, L. Do Medical Complications Impact Long-Term Outcomes in Prolonged Disorders of Consciousness? Arch. Phys. Med. Rehabil. 2018, 99, 2523–2531.e3. [Google Scholar] [CrossRef]
- Estraneo, A.; Masotta, O.; Bartolo, M.; Pistoia, F.; Perin, C.; Marino, S.; Lucca, L.; Pingue, V.; Casanova, E.; Romoli, A.; et al. Multi-Center Study on Overall Clinical Complexity of Patients with Prolonged Disorders of Consciousness of Different Etiologies. Brain Inj. 2021, 35, 1–7. [Google Scholar] [CrossRef]
- Whyte, J.; Gosseries, O.; Chervoneva, I.; DiPasquale, M.C.; Giacino, J.; Kalmar, K.; Katz, D.I.; Novak, P.; Long, D.; Childs, N.; et al. Predictors of Short-Term Outcome in Brain-Injured Patients with Disorders of Consciousness. Prog. Brain Res. 2009, 177, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Kowalski, R.G.; Hammond, F.M.; Weintraub, A.H.; Nakase-Richardson, R.; Zafonte, R.D.; Whyte, J.; Giacino, J.T. Recovery of Consciousness and Functional Outcome in Moderate and Severe Traumatic Brain Injury. JAMA Neurol. 2021, 78, 548–557. [Google Scholar] [CrossRef]
- Vetrano, D.L.; Collamati, A.; Magnavita, N.; Sowa, A.; Topinkova, E.; Finne-Soveri, H.; van der Roest, H.G.; Tobiasz-Adamczyk, B.; Giovannini, S.; Ricciardi, W.; et al. Health determinants and survival in nursing home residents in Europe: Results from the SHELTER study. Maturitas 2018, 107, 19–25. [Google Scholar] [CrossRef]
- Giovannini, S.; Onder, G.; Lattanzio, F.; Bustacchini, S.; di Stefano, G.; Moresi, R.; Russo, A.; Bernabei, R.; Landi, F. Selenium Concentrations and Mortality Among Community-Dwelling Older Adults: Results from IlSIRENTE Study. J. Nutr. Health Aging 2018, 22, 608–612. [Google Scholar] [CrossRef]
- Giovannini, S.; Onder, G.; Leeuwenburgh, C.; Carter, C.; Marzetti, E.; Russo, A.; Capoluongo, E.; Pahor, M.; Bernabei, R.; Landi, F. Myeloperoxidase Levels and Mortality in Frail Community-Living Elderly Individuals. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010, 65, 369–376. [Google Scholar] [CrossRef]
- Laureys, S.; Celesia, G.G.; Cohadon, F.; Lavrijsen, J.; León-Carrión, J.; Sannita, W.G.; Sazbon, L.; Schmutzhard, E.; von Wild, K.R.; Zeman, A.; et al. Unresponsive Wakefulness Syndrome: A New Name for the Vegetative State or Apallic Syndrome. BMC Med. 2010, 8, 68. [Google Scholar] [CrossRef]
- Giacino, J.T.; Ashwal, S.; Childs, N.; Cranford, R.; Jennett, B.; Katz, D.I.; Kelly, J.P.; Rosenberg, J.H.; Whyte, J.; Zafonte, R.D.; et al. The Minimally Conscious State: Definition and Diagnostic Criteria. Neurology 2002, 58, 349–353. [Google Scholar] [CrossRef]
- Prigatano, G.P. Personality Disturbances Associated With Traumatic Brain Injury. J. Consult. Clin. Psychol. 1992, 60, 360–368. [Google Scholar] [CrossRef]
- Gouvier, W.D.; Blanton, P.D.; LaPorte, K.K.; Nepomuceno, C. Reliability and Validity of the Disability Rating Scale and the Levels of Cognitive Functioning Scale in Monitoring Recovery from Severe Head Injury. Arch. Phys. Med. Rehabil. 1987, 68, 94–97. [Google Scholar] [CrossRef]
- Rappaport, M.; Hall, K.M.; Hopkins, K.; Belleza, T.; Cope, D.N. Disability Rating Scale for Severe Head Trauma: Coma to Community. Arch. Phys. Med. Rehabil. 1982, 63, 118–123. [Google Scholar]
- Hall, K.M.; Bushnik, T.; Lakisic-Kazazic, B.; Wright, J.; Cantagallo, A. Assessing Traumatic Brain Injury Outcome Measures for Long-Term Follow-Up of Community-Based Individuals. Arch. Phys. Med. Rehabil. 2001, 82, 367–374. [Google Scholar] [CrossRef]
- Hammond, F.M.; Grattan, K.D.; Sasser, H.; Corrigan, J.D.; Rosenthal, M.; Bushnik, T.; Shull, W. Five Years after Traumatic Brain Injury: A Study of Individual Outcomes and Predictors of Change in Function. NeuroRehabilitation 2004, 19, 25–35. [Google Scholar] [CrossRef]
- Shah, S.; Vanclay, F.; Cooper, B. Improving the Sensitivity of the Barthel Index for Stroke Rehabilitation. J. Clin. Epidemiol. 1989, 42, 703–709. [Google Scholar] [CrossRef]
- Wade, D.T.; Collin, C. The Barthel ADL Index: A Standard Measure of Physical Disability? Int. Disabil. Stud. 1988, 10, 64–67. [Google Scholar] [CrossRef]
- Kantor, J.; Margolis, D.J. A multicentre study of percentage change in venous leg ulcer area as a prognostic index of healing at 24 weeks. Br. J. Dermatol. 2000, 142, 960–964. [Google Scholar] [CrossRef]
- Sweeney, N.; O’Sullivan, C.; Kelly, G. Multifidus muscle size and percentage thickness changes among patients with unilateral chronic low back pain (CLBP) and healthy controls in prone and standing. Man. Ther. 2014, 19, 433–439. [Google Scholar] [CrossRef]
- Austevoll, I.M.; Gjestad, R.; Grotle, M.; Solberg, T.; Brox, J.I.; Hermansen, E.; Rekeland, F.; Indrekvam, K.; Storheim, K.; Hellum, C. Follow-up score, change score or percentage change score for determining clinical important outcome following surgery? An observational study from the Norwegian registry for Spine surgery evaluating patient reported outcome measures in lumbar spinal stenosis and lumbar degenerative spondylolisthesis. BMC Musculoskelet. Disord. 2019, 20, 31. [Google Scholar] [CrossRef]
- de Goumoëns, V.; Rio, L.M.; Jaques, C.; Ramelet, A.S. Family-Oriented Interventions for Adults with Acquired Brain Injury and Their Families: A Scoping Review. JBI Database Syst. Rev. Implement. Rep. 2018, 16, 2330–2367. [Google Scholar] [CrossRef]
- Mabire, C.; Dwyer, A.; Garnier, A.; Pellet, J. Effectiveness of Nursing Discharge Planning Interventions on Health-Related Outcomes in Discharged Elderly Inpatients: A Systematic Review. JBI Database Syst. Rev. Implement. Rep. 2016, 14, 217–260. [Google Scholar] [CrossRef]
- Onder, G.; Giovannini, S.; Sganga, F.; Manes-Gravina, E.; Topinkova, E.; Finne-Soveri, H.; Garms-Homolová, V.; Declercq, A.; van der Roest, H.G.; Jónsson, P.V.; et al. Interactions between Drugs and Geriatric Syndromes in Nursing Home and Home Care: Results from Shelter and IBenC Projects. Aging Clin. Exp. Res. 2018, 30, 1015–1021. [Google Scholar] [CrossRef]
- Dijkland, S.A.; Foks, K.A.; Polinder, S.; Dippel, D.W.J.; Maas, A.I.R.; Lingsma, H.F.; Steyerberg, E.W. Prognosis in Moderate and Severe Traumatic Brain Injury: A Systematic Review of Contemporary Models and Validation Studies. J. Neurotrauma 2020, 37, 1–13. [Google Scholar] [CrossRef]
- Watson, P.A.; Gignac, G.E.; Weinborn, M.; Green, S.; Pestell, C. A Meta-Analysis of Neuropsychological Predictors of Outcome Following Stroke and Other Non-Traumatic Acquired Brain Injuries in Adults. Neuropsychol. Rev. 2020, 30, 194–223. [Google Scholar] [CrossRef] [PubMed]
- Godbolt, A.K.; DeBoussard, C.N.; Stenberg, M.; Lindgren, M.; Ulfarsson, T.; Borg, J. Disorders of Consciousness after Severe Traumatic Brain Injury: A Swedish-Icelandic Study of Incidence, Outcomes and Implications for Optimizing Care Pathways. J. Rehabil. Med. 2013, 45, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Vujasinovic, M.; Ingre, C.; Baldaque Silva, F.; Frederiksen, F.; Yu, J.; Elbe, P. Complications and Outcome of Percutaneous Endoscopic Gastrostomy in a High-Volume Centre. Scand. J. Gastroenterol. 2019, 54, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Anis, M.K.; Abid, S.; Jafri, W.; Abbas, Z.; Shah, H.A.; Hamid, S.; Wasaya, R. Acceptability and Outcomes of the Percutaneous Endoscopic Gastrostomy (PEG) Tube Placement—Patients’ and Care Givers’ Perspectives. BMC Gastroenterol. 2006, 6, 37. [Google Scholar] [CrossRef]
- Chaudhry, R.; Kukreja, N.; Tse, A.; Pednekar, G.; Mouchli, A.; Young, L.; Didyuk, O.; Wegner, R.C.; Grewal, N.; Williams, G.W. Trends and Outcomes of Early Versus Late Percutaneous Endoscopic Gastrostomy Placement in Patients With Traumatic Brain Injury: Nationwide Population-Based Study. J. Neurosurg. Anesthesiol. 2018, 30, 251–257. [Google Scholar] [CrossRef]
- Borgaonkar, M.R.; Irvine, E.J. Quality of Life Measurement in Gastrointestinal and Liver Disorders. Gut 2000, 47, 444–454. [Google Scholar] [CrossRef]
- Schnakers, C.; Vanhaudenhuyse, A.; Giacino, J.; Ventura, M.; Boly, M.; Majerus, S.; Moonen, G.; Laureys, S. Diagnostic Accuracy of the Vegetative and Minimally Conscious State: Clinical Consensus versus Standardized Neurobehavioral Assessment. BMC Neurol. 2009, 9, 35. [Google Scholar] [CrossRef]
- Whyte, J.; Nakase-Richardson, R.; Hammond, F.M.; McNamee, S.; Giacino, J.T.; Kalmar, K.; Greenwald, B.D.; Yablon, S.A.; Horn, L.J. Functional Outcomes in Traumatic Disorders of Consciousness: 5-Year Outcomes from the National Institute on Disability and Rehabilitation Research Traumatic Brain Injury Model Systems. Arch. Phys. Med. Rehabil. 2013, 94, 1855–1860. [Google Scholar] [CrossRef]
- Katz, D.I.; Polyak, M.; Coughlan, D.; Nichols, M.; Roche, A. Natural History of Recovery from Brain Injury after Prolonged Disorders of Consciousness: Outcome of Patients Admitted to Inpatient Rehabilitation with 1-4 Year Follow-Up. Prog. Brain Res. 2009, 177, 73–88. [Google Scholar] [CrossRef]
- Padua, L.; Cuccagna, C.; Pazzaglia, C. Novel sensory paradigms for neuromodulation in disorders of consciousness in traumatic brain injury. Curr. Opin. Neurol. 2019, 32, 844–849. [Google Scholar] [CrossRef]
- Hussain, I.; Park, S.J. HealthSOS: Real-Time Health Monitoring System for Stroke Prognostics. IEEE Access 2020, 8, 213574–213586. [Google Scholar] [CrossRef]
- Hussain, I.; Hossain, M.A.; Jany, R.; Bari, M.A.; Uddin, M.; Kamal, A.R.M.; Ku, Y.; Kim, J.S. Quantitative Evaluation of EEG-Biomarkers for Prediction of Sleep Stages. Sensors 2022, 22, 3079. [Google Scholar] [CrossRef]
- Hussain, I.; Young, S.; Kim, C.H.; Benjamin, H.C.M.; Park, S.J. Quantifying Physiological Biomarkers of a Microwave Brain Stimulation Device. Sensors 2021, 21, 1896. [Google Scholar] [CrossRef]
- Biscetti, F.; Giovannini, S.; Straface, G.; Bertucci, F.; Angelini, F.; Porreca, C.; Landolfi, R.; Flex, A. RANK/RANKL/OPG pathway: Genetic association with history of ischemic stroke in Italian population. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4574–4580. [Google Scholar]
- Wang, R.; Wang, L.; Zhang, J.; He, M.; Xu, J. XGBoost Machine Learning Algorism Performed Better Than Regression Models in Predicting Mortality of Moderate-to-Severe Traumatic Brain Injury. World Neurosurg. 2022, 163, e617–e622. [Google Scholar] [CrossRef]
- Gravesteijn, B.Y.; Nieboer, D.; Ercole, A.; Lingsma, H.F.; Nelson, D.; van Calster, B.; Steyerberg, E.W.; CENTER-TBI collaborators. Machine learning algorithms performed no better than regression models for prognostication in traumatic brain injury. J. Clin. Epidemiol. 2020, 122, 95–107. [Google Scholar] [CrossRef]
- Churpek, M.M.; Yuen, T.C.; Winslow, C.; Meltzer, D.O.; Kattan, M.W.; Edelson, D.P. Multicenter Comparison of Machine Learning Methods and Conventional Regression for Predicting Clinical Deterioration on the Wards. Crit. Care Med. 2016, 44, 368–374. [Google Scholar] [CrossRef]
- Christodoulou, E.; Ma, J.; Collins, G.S.; Steyerberg, E.W.; Verbakel, J.Y.; Van Calster, B. A systematic review shows no performance benefit of machine learning over logistic regression for clinical prediction models. J. Clin. Epidemiol. 2019, 110, 12–22. [Google Scholar] [CrossRef]
- Rapp, P.E.; Keyser, D.O.; Albano, A.; Hernandez, R.; Gibson, D.B.; Zambon, R.A.; Hairston, W.D.; Hughes, J.D.; Krystal, A.; Nichols, A.S. Traumatic brain injury detection using electrophysiological methods. Front. Hum. Neurosci. 2015, 9, 11. [Google Scholar] [CrossRef]
- Vishwanath, M.; Jafarlou, S.; Shin, I.; Lim, M.M.; Dutt, N.; Rahmani, A.M.; Cao, H. Investigation of Machine Learning Approaches for Traumatic Brain Injury Classification via EEG Assessment in Mice. Sensors 2020, 20, 2027. [Google Scholar] [CrossRef] [Green Version]
| Age at Injury, Median (Range), y | 77.3 (Range 70.1–86.2) |
|---|---|
| Cause of sABI, n (%) | |
| Traumatic | 3 (11.1) |
| Stroke (ischemic/hemorrhagic) | 12 (44.4) |
| Hypoxic (heart arrest) | 5 (18.5) |
| Infections/Tumor | 5 (18.5) |
| Post COVID-19 | 2 (7.4) |
| Elapsed time from event to INRU (days), median (range) | 39 (8–404) |
| Length of stay (days), median (range) | 82 (11–357) |
| DOC, n (%) | 10 (37.0) |
| CRIMYNE, n (%) | 5 (18.5) |
| Sepsis, n (%) | 15 (55.6) |
| Pressure ulcers, n (%) | 15 (55.6) |
| Tracheostomy, n (%) | 19 (70.4) |
| Days, median (range) | 50 (10–205) |
| NGT, n (%) | 15 (55.6) |
| Days, median (range) | 43 (7–113) |
| PEG, n (%) | 9 (33.3) |
| Days, median (range) | 114 (12–235) |
| CVC/PICC, n (%) | 15 (55.6) |
| Days, median (range) | 37 (2–89) |
| UC, n (%) | 26 (96.3) |
| Days, median (range) | 72 (11–357) |
| Comorbidity, n (%) | 26 (96.3) |
| Numbers of comorbidity, median (range) | 3 (1–5) |
| All N = 22 | Good Outcome N = 15 | Poor Outcome N = 7 | p-Value * | |
|---|---|---|---|---|
| Male, n (%) | 12 (54.6) | 9 (60.0) | 3 (42.9) | 0.65 |
| Age (years), median (range) | 77.5 (70.1–86.2) | 77.3 (72.4–85.6) | 78.5 (70.1–86.2) | 1.0 |
| Elapsed time from event to ICRU (days), median (range) | 39 (8–153) | 39 (8–93) | 69 (25–153) | 0.22 |
| Length of stay (days), median (range) | 98 (19–357) | 93 (41–357) | 103 (19–205) | 0.99 |
| Body Mass Index (BMI), median (range) | 25.2 (20.1–29.3) | 24.8 (20.1–28.6) | 25.9 (24.2–29.3) | 0.048 |
| Cause of injury, n (%) | - | |||
| Trauma | 3 (13.6) | 2 (13.3) | 1 (14.3) | - |
| Stroke | 9 (40.9) | 4 (26.7) | 5 (71.4) | - |
| Hypoxic (heart arrest) | 4 (18.2) | 4 (26.7) | 0 (0.0) | - |
| Infection/Tumor | 4 (18.2) | 3 (20.0) | 1 (14.3) | - |
| Post COVID-19 | 2 (9.1) | 2 (13.3) | 0 (0.0) | - |
| DOC, n (%) | 8 (36.4) | 3 (20.0) | 5 (71.4) | 0.052 |
| CRIMYNE, n (%) | 5 (22.7) | 4 (26.7) | 1 (14.3) | 1.0 |
| Rehabilitation Gradient: (T1 − T0/T0) × 100 | ||||
| DRS, median (range) | −3.9 (−75.0–38.1) | −5.9 (−75.0–0.0) | 0.0 (−5.3–38.1) | 0.025 |
| LCF, median (range) | 0.0 (−33.3–166.7) | 33.3 (−33.3–166.7) | 0.0 (−33.3–0.0) | 0.011 |
| mBI, median (range) | 0.0 (0.0–550.0) | 0.0 (0.0–550.0) | 0.0 (0.0–0.0) | 0.057 |
| Sepsis T0-T1, n (%) | 7 (31.8) | 2 (13.3) | 5 (71.4) | 0.014 |
| Pressure Ulcers#, n (%) | 12 (54.6) | 6 (40.0) | 6 (85.7) | 0.074 |
| Devices (T0-T1), n (%) | ||||
| Tracheostomy | 10 (45.5) | 6 (40.0) | 4 (57.1) | 0.65 |
| NGT | 9 (40.9) | 7 (46.7) | 2 (28.6) | 0.65 |
| PEG | 7 (31.8) | 3 (20.0) | 4 (57.1) | 0.15 |
| CVC/PICC | 7 (31.8) | 3 (20.0) | 4 (57.1) | 0.15 |
| UC | 18 (81.8) | 13 (86.7) | 5 (71.4) | 0.57 |
| Comorbidity, n (%) | 21 (95.5) | 15 (100) | 6 (85.7) | 0.32 |
| Numbers of Comorbidity, median (range) | 2 (1–5) | 2 (1–4) | 3 (1–5) | 0.81 |
| All N = 19 | Good Outcome N = 14 | Poor Outcome N = 5 | p-Value * | |
|---|---|---|---|---|
| Male, n (%) | 11 (57.9) | 8 (57.1) | 3 (60.0) | 1.0 |
| Age (years), median (range) | 76.0 (70.1–85.6) | 76.7 (72.4–85.6) | 73.9 (70.1–80.7) | 0.26 |
| Elapsed time from event to ICRU (days), median (range) | 39 (8–153) | 37 (8–93) | 69 (27–153) | 0.16 |
| Length of stay (days), median (range) | 105 (50–357) | 99 (52–357) | 114 (50–205) | 0.67 |
| Body Mass Index (BMI), median (range) | 25.0 (20.1–29.3) | 24.9 (20.1–28.6) | 25.9 (24.2–29.3) | 0.12 |
| Cause of injury, n (%) | - | |||
| Trauma | 2 (10.5) | 1 (7.1) | 1 (20.0) | - |
| Stroke | 8 (42.2) | 4 (28.5) | 4 (80.0) | - |
| Hypoxic (heart arrest) | 4 (21.1) | 4 (28.6) | 0 (0.0) | - |
| Infection/tumor | 3 (15.8) | 3 (21.4) | 0 (0.0) | - |
| Post COVID-19 | 2 (10.5) | 2 (14.3) | 0 (0.0) | - |
| DOC, n (%) | 7 (36.8) | 3 (21.4) | 4 (80.0) | 0.038 |
| CRIMYNE, n (%) | 5 (26.3) | 4 (28.6) | 1 (20.0) | 1.0 |
| Rehabilitation gradient: (T2 − T0/T0) × 100 | ||||
| DRS, median (range) | −16.7 (−58.3–14.3) | −17.5 (−58.3–−4.8) | 0.0 (−17.2–14.3) | 0.024 |
| LCF, median (range) | 50.0 (−16.7–166.7) | 58.3 (−16.7–166.7) | 0.0 (0.0–50.0) | 0.049 |
| mBI, median (range) | 0.0 (−33.3–1000.0) | 10.0 (−33.3–1000.0) | 0.0 (0.0–0.0) | 0.16 |
| Rehabilitation gradient: (T2 − T1/T1) × 100 | ||||
| DRS, median (range) | −12.5 (−58.3–0.0) | −12.5 (−58.3–0.0) | −11.5 (−17.2–0.0) | 0.42 |
| LCF, median (range) | 0.0 (0.0–100.0) | 0.0 (0.0–100.0) | 0.0 (0.0–50.0) | 1.0 |
| mBI, median (range) | 20.0 (−50.0–1100.0) | 55.0 (−50.0–1100.0) | 0.0 (0.0–0.0) | 0.031 |
| Sepsis (T0-T2), n (%) | 8 (42.1) | 4 (28.6) | 4 (80.0) | 0.11 |
| Pressure Ulcers #, n (%) | 10 (52.6) | 6 (42.9) | 4 (80.0) | 0.30 |
| Devices (T0-T2), n (%) | ||||
| Tracheostomy | 13 (68.4) | 8 (57.1) | 5 (100) | 0.13 |
| NGT | 10 (52.6) | 8 (57.1) | 2 (40.0) | 0.63 |
| PEG | 7 (36.8) | 3 (21.4) | 4 (80.0) | 0.038 |
| CVC/PICC | 10 (52.6) | 6 (42.9) | 4 (80.0) | 0.30 |
| UC | 19 (100) | 14 (100) | 5 (100) | - |
| Devices (T1-T2), n (%) | ||||
| Tracheostomy | 8 (42.1) | 5 (35.7) | 3 (60.0) | 0.60 |
| NGT | 3 (15.8) | 3 (21.4) | 0 (0.0) | 0.53 |
| PEG | 7 (36.8) | 3 (21.4) | 4 (80.0) | 0.038 |
| CVC/PICC | 3 (15.8) | 1 (7.1) | 2 (40.0) | 0.16 |
| UC | 14 (73.7) | 10 (71.4) | 4 (80.0) | 1.0 |
| Comorbidity, n (%) | 18 (94.7) | 14 (100) | 4 (80.0) | 0.26 |
| Numbers of Comorbidity, median (range) | 3 (1–5) | 3 (1–4) | 2 (1–5) | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fusco, A.; Galluccio, C.; Castelli, L.; Pazzaglia, C.; Pastorino, R.; Pires Marafon, D.; Bernabei, R.; Giovannini, S.; Padua, L. Severe Acquired Brain Injury: Prognostic Factors of Discharge Outcome in Older Adults. Brain Sci. 2022, 12, 1232. https://doi.org/10.3390/brainsci12091232
Fusco A, Galluccio C, Castelli L, Pazzaglia C, Pastorino R, Pires Marafon D, Bernabei R, Giovannini S, Padua L. Severe Acquired Brain Injury: Prognostic Factors of Discharge Outcome in Older Adults. Brain Sciences. 2022; 12(9):1232. https://doi.org/10.3390/brainsci12091232
Chicago/Turabian StyleFusco, Augusto, Caterina Galluccio, Letizia Castelli, Costanza Pazzaglia, Roberta Pastorino, Denise Pires Marafon, Roberto Bernabei, Silvia Giovannini, and Luca Padua. 2022. "Severe Acquired Brain Injury: Prognostic Factors of Discharge Outcome in Older Adults" Brain Sciences 12, no. 9: 1232. https://doi.org/10.3390/brainsci12091232






