Preliminary Study of Efficacy and Safety of Self-Administered Virtual Exposure Therapy for Social Anxiety Disorder vs. Cognitive-Behavioral Therapy
Abstract
:1. Introduction
2. Design of the Study
- 1
- A self-administered VR group (experimental group), where the patients were gradually exposed to social situations in virtual reality. The patients had several exposures at their disposal in VR, which they selected themselves, taking into account the severity of the anxiety. The patients underwent the therapy without any kind of help or intervention from a therapist.
- 2
- A CBT + VR group (experimental group), where virtual reality was used in the CBT protocol. Within this arm, a therapy analogous to that which took place in the CBT group was carried out, while the exposure in virtual reality replaced the exposure in the patient’s imagination.
- 3
- A CBT group (active control group) in which work was based on a cognitive-behavioral therapy protocol.
2.1. Participants
2.2. Measures
2.3. Treatment
2.4. Procedure
2.5. Statistical Analysis
3. Results
3.1. T0—The Severity of SAD Symptoms
3.2. Primary Endpoints
3.2.1. Efficacy
3.2.2. Safety Assessment
3.3. Secondary Endpoint—Efficacy
3.4. Other Analyses
3.4.1. Patient Dropouts
3.4.2. VR System
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rabe-Jabłonska, J. Fobia społeczna. Rozpowszechnienie, kryteria rozpoznawania, podtypy, przebieg, wspołchorobowość leczenie. Psychiatr. W Prakt. Ogolnolekarskiej 2002, 2, 161–166. [Google Scholar]
- Bruce, S.E.; Yonkers, K.A.; Otto, M.W.; Eisen, J.L.; Weisberg, R.; Pagano, M.E.; Shea, M.T.; Keller, M.B. Influence of psychiatric comorbidity on recovery and recurrence in generalised anxiety disorder, social phobia, and panic disorder: A 12-year prospective study. Am. J. Psychiatry 2005, 162, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Otto, M.W.; Pollack, M.H.; Maki, K.M.; Gould, R.A.; Worthington, J.J., 3rd; Smoller, J.W.; Rosenbaum, J.F. Childhood history of anxiety disorders among adults with social phobia: Rates, correlates, and comparisons with patients with panic disorder. Depress. Anxiety 2001, 14, 209–213. [Google Scholar] [CrossRef]
- Lijster, J.M.; Dierckx, B.; Utens, E.M.W.J.; Verhulst, F.C.; Zieldorff, C.; Dieleman, G.C.; Legerstee, J.S. The age of onset of anxiety disorders: A meta-analysis. Can. J. Psychiatry 2017, 62, 237–246. [Google Scholar] [CrossRef] [PubMed]
- First, M.B.; Allen, F.; Harold, A.P. DSM-IV-TR Guideb; American Psychiatric Publishing, Inc.: Wahington, DC, USA, 2004. [Google Scholar]
- Furmark, T. Social phobia: Overview of community surveys. Acta Psychiatr. Scand. 2002, 105, 84–93. [Google Scholar] [CrossRef]
- Rush, A.J.; Zimmerman, M.; Wisniewski, S.R.; Fava, M.; Hollon, S.D.; Warden, D.; Biggs, M.M.; Shores-Wilson, K.; Shelton, R.C.; Luther, J.F.; et al. Comorbid psychiatric disorders in depressed outpatients: Demographic and clinical features. J. Affect. Disord. 2005, 87, 43–55. [Google Scholar] [CrossRef]
- Carrigan, M.H.; Randall, C.L. Self-medication in social phobia: A review of the alcohol literature. Addict. Behav. 2003, 28, 269–284. [Google Scholar] [CrossRef]
- Saarni, S.I.; Suvisaari, J.; Sintonen, H.; Pirkola, S.; Koskinen, S.; Aromaa, A.; Lönnqvist, J. Impact of psychiatric disorders on health-related quality of life: General population survey. Br. J. Psychiatry 2007, 190, 326–332. [Google Scholar] [CrossRef]
- Clark, D.M.; Ehlers, A.; McManus, F.; Hackmann, A.; Fennell, M.; Campbell, H.; Flower, T.; Davenport, C.; Louis, B. Cognitive therapy vs. fluoxetine in generalised social phobia: A randomised placebo-controlled trial. J. Consult. Clin. Psychol. 2003, 71, 1058–1067. [Google Scholar] [CrossRef]
- Clark, D.M.; Ehlers, A.; Hackmann, A.; McManus, F.; Fennell, M.; Grey, N.; Waddington, L.; Wild, J. Cognitive therapy versus exposure and applied relaxation in social phobia: A randomised controlled trial. J. Consult. Clin. Psychol. 2006, 74, 568–578. [Google Scholar] [CrossRef]
- Mayo-Wilson, E.; Dias, S.; Mavranezouli, I.; Kew, K.; Clark, D.M.; E Ades, A.; Pilling, S. Psychological and pharmacological interventions for social anxiety disorder in adults: A systematic review and network meta-analysis. Lancet Psychiatry 2014, 1, 368–376. [Google Scholar] [CrossRef] [Green Version]
- Clark, D.M.; Wells, A. A cognitive model of social phobia. In Social Phobia: Diagnosis, Assessment and Treatment; Heimberg, R.G., Liebowitz, M.R., Hope, D.A., Schneier, F.R., Eds.; Guilford Press: New York, NY, USA, 1995; pp. 69–93. [Google Scholar]
- Rapee, R.M.; Heimberg, R.G. A cognitive-behavioral model of anxiety in social phobia. Behav. Res. Ther. 1997, 35, 741–756. [Google Scholar] [CrossRef]
- Heimberg, R.G.; Magee, L. Social Anxiety Disorder. Clinical Handbook of Psychological Disorders, 5th ed.; Guilford Publications: New York, NY, USA, 2014. [Google Scholar]
- Kaczkurkin, A.N.; Foa, E.B. Cognitive-behavioral therapy for anxiety disorders: An update on the empirical evidence. Dialogues Clin. Neurosci. 2015, 17, 337–346. [Google Scholar] [CrossRef]
- Scaini, S.; Belotti, R.; Ogliari, A.; Battaglia, M.A. Comprehensive meta-analysis of cognitive-behavioral interventions for social anxiety disorder in children and adolescents. J. Anxiety Disord. 2016, 42, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.G. Cognitive mediation of treatment change in social phobia. J. Consult. Clin. Psychol. 2004, 72, 392–399. [Google Scholar] [CrossRef]
- Mersch, P.P. The treatment of social phobia: The differential effectiveness of exposure in vivo and and integration of exposure in vivo, rational emotive therapy and social skills training. Behav. Res. Ther. 1995, 33, 259–269. [Google Scholar] [CrossRef]
- Emmelkamp, P.M.G.; Meyerbroker, K.; Morina, N. Virtual reality therapy in social anxiety disorder. Curr. Psychiatry Rep. 2020, 22, 1–9. [Google Scholar] [CrossRef]
- Rachman, S. The conditioning theory of fear-acquisition: A critical examination. Behav. Res. Ther. 1977, 15, 375–387. [Google Scholar] [CrossRef]
- Feske, U.; Chambless, D.L. Cognitive behavioral versus exposure only treatment for social phobia: A meta-analysis. Behav. Ther. 1995, 26, 695–720. [Google Scholar] [CrossRef]
- Acarturk, C.; Cuijpers, P.; van Straten, A.; de Graaf, R. Psychological treatment of social anxiety disorder: A meta-analysis. Psychol. Med. 2009, 39, 241–254. [Google Scholar] [CrossRef]
- Powers, M.B.; Sigmarsson, S.R.; Emmelkamp, P.M.G. A meta-analytic review of psychological treatments for social anxiety disorder. Int. J. Cogn. Ther. 2008, 1, 94–113. [Google Scholar] [CrossRef]
- Salehi, E.; Mehrabi, M.; Fatehi, F.; Salehi, A. Virtual reality therapy for social phobia: A scoping review. Stud. Health Technol. Inform. 2020, 16, 713–717. [Google Scholar] [CrossRef]
- Park, M.J.; Kim, D.J.; Lee, U.; Na, E.J.; Jeon, H.J. Literature overview of virtuavlreality in treatment of psychiatric disorders: Recent advances and limitations. Front. Psychiatry 2019, 10, 505. [Google Scholar] [CrossRef] [PubMed]
- Carl, E.; Stein, A.T.; Levihn-Coon, A.; Pogue, J.R.; Rothbaum, B.; Emmelkamp, P.; Asmundson, G.J.; Carlbring, P.; Powers, M.B. Virtual reality exposure therapy for anxiety and related disorders: A meta-analysis of randomised controlled trials. J. Anxiety Disord. 2019, 61, 27–36. [Google Scholar] [CrossRef]
- Powers, M.B.; Emmelkamp, P.M. Virtual reality exposure therapy for anxiety disorders: A meta-analysis. J. Anxiety Disord. 2008, 22, 561–569. [Google Scholar] [CrossRef]
- Powers, M.B.; Briceno, N.F.; Gresham, R.; Jouriles, E.N.; Emmelkamp, P.M.; Smits, J. Do conversations with virtual avatars increase feelings of social anxiety? J. Anxiety Disord. 2013, 27, 398–403. [Google Scholar] [CrossRef]
- Botella, C.; Fernández-Álvarez, J.; Guillén, V.; García-Palacios, A.; Baños, R. Recent Progress in Virtual Reality Exposure Therapy for Phobias: A Systematic Review. Curr. Psychiatry Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Kampmann, I.L.; Emmelkamp, P.M.; Hartanto, D.; Brinkman, W.P.; Zijlstra, B.J.; Morina, N. Exposure to virtual social interactions in the treatment of social anxiety disorder: A randomized controlled trial. Behav. Res. Ther. 2016, 77, 147–156. [Google Scholar] [CrossRef]
- Chesham, R.K.; Malouff, J.M.; Schutte, N.S. Meta-analysis of the efficacy of virtual reality exposure therapy for social anxiety. Behav. Change 2018, 35, 152–166. [Google Scholar] [CrossRef]
- Wechsler, T.; Kumpers, F.; Muhlberger, A. Inferiority or even superiority of virtual reality exposure¨ therapy in phobias? a systematic review and quantitative meta-analysis on randomized controlled trials specifically comparing the efficacy of virtual reality exposure to gold standard in vivo exposure in agoraphobia, specific phobia, and social phobia. Front. Psychol. 2019, 10, 1758. [Google Scholar] [CrossRef]
- Bouchard, S.; Dumoulin, S.; Robillard, G.; Guitard, T.; Klinger, E.; Forget, H.; Loranger, C.; Roucaut, F.X. Virtual reality compared with in vivo exposure in the treatment of social anxiety disorder: A three-arm randomised controlled trial. Br. J. Psychiatry 2017, 210, 276–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parrish, D.E.; Oxhandler, H.K.; Duron, J.F.; Swank, P.; Bordnick, P. Feasibility of virtual reality environments for adolescent social anxiety disorder. Res. Soc. Work Pract. 2016, 26, 825–835. [Google Scholar] [CrossRef]
- Hartanto, D.; Kampmann, I.; Morina, N.; Emmelkamp, P.; Neerincx, M.; Brinkman, W. Controlling social stress in virtual reality environments. PLoS ONE 2014, 9, e92804. [Google Scholar] [CrossRef] [PubMed]
- Hartanto, D.; Brinkman, W.; Kampmann, I.; Morina, N.; Emmelkamp, P.; Neerincx, M. Home-based virtual reality exposure therapy with virtual health agent. In Pervasive Computing Paradigms for Mental Health. Communications in Computer and Information Science; Serino, S., Matic, A., Giakoumis, D., Lopez, G., Cipresso, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 85–98. [Google Scholar]
- Yoshinaga, N.; Matsuki, S.; Niitsu, T.; Sato, Y.; Tanaka, M.; Ibuki, H.; Takanashi, R.; Ohshiro, K.; Ohshima, F.; Asano, K.; et al. Cognitive behavioral therapy for patients with social anxiety disorder who remain symptomatic following antidepressant treatment: A randomised, assessor-blinded, controlled trial. Psychother. Psychosom. 2016, 85, 208–217. [Google Scholar] [CrossRef]
- Liebowitz, M.R. Social phobia. Mod. Probl. Pharm. 1987, 22, 147–173. [Google Scholar]
- Michael, B.F.; Williams, J.B.W.; Benjamin, L.S.B.; Spitzer, R.L. Structured Clinical Interview for DSM-IV Axis II Personality Disorders SCID-II; American Psychiatric Press: Washington, DC, USA, 2014. [Google Scholar]
- Guy, W. ECDEU Assessment Manual for Psychopharmacology; US Department of Health, Education, and Welfare: Rockville, MD, USA, 1976.
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef]
- Kennedy, R.; Lane, N.; Berbaum, K.; Lilienthal, M. Simulator sickness questionnaire: An enhanced method of quantifying simulator sickness. Int. J. Aviat. Psychol. 1993, 3, 203–220. [Google Scholar] [CrossRef]
- Balk, S.A.; Bertola, M.A.; Inman, V.W. Simulator sickness questionnaire: Twenty years later. In Proceedings of the Seventh International Driving Symposium on Human Factors in Driver Assessment, Owa City, IA, USA, 17–20 June 2013; pp. 257–263. [Google Scholar]
- Galluci, M. Gamlj: General Analyses for Linear Models. [Dataset]. 2019. Available online: https://gamlj.github.io/ (accessed on 12 August 2022).
- Horigome, T.; Kurokawa, S.; Sawada, K.; Kudo, S.; Shiga, K.; Mimura, M.; Kishimoto, T. Virtual reality exposure therapy for social anxiety disorder: A systematic review and meta-analysis. Psychol. Med. 2020, 50, 2487–2497. [Google Scholar] [CrossRef]
- Zainal, N.H.; Chan, W.W.; Saxena, A.P.; Taylor, C.B.; Newman, M.G. Pilot randomized trial of self-guided virtual reality exposure therapy for social anxiety disorder. Behav. Res. Ther. 2021, 147, 103984. [Google Scholar] [CrossRef]
- Pelissolo, A.; Abou Kassm, S.; Delhay, L. Therapeutic strategies for social anxiety disorder: Where are we now? Expert Rev. Neurother. 2019, 1179–1189. [Google Scholar] [CrossRef]
- Boeldt, D.; McMahon, E.; McFaul, M.; Greenleaf, W. Using virtual reality exposure therapy to enhance treatment of anxiety disorders: Identifying areas of clinical adoption and potential obstacles. Front. Psychiatry 2019, 10, 773. [Google Scholar] [CrossRef] [PubMed]
- Deacon, B.J.; Farrell, N.R.; Kemp, J.J.; Dixon, L.J.; Sy, J.T.; Zhang, A.R.; McGrath, P.B. Assessing therapist reservations about exposure therapy for anxiety disorders: The therapist beliefs about exposure scale. J. Anxiety Disord. 2013, 27, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Pittig, A.; Kotter, R.; Hoyer, J. The struggle of behavioral therapists with exposure: Self-reported practicability, negative beliefs, and therapist distress about exposure-based interventions. Behav. Ther. 2019, 50, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, B.K.; Wiederhold, M.D. The effect of presence on virtual reality treatment. In Virtual Reality Therapy for Anxiety disorders: Advances in Evaluation and Treatment; Wiederhold, M.D., Ed.; American Psychological Association: Washington, DC, USA, 2005; pp. 77–86. [Google Scholar]
- Duzmanska, N.; Strojny, P.; Strojny, A. Can simulator sickness be avoided? a review on temporal aspects of simulator sickness. Front. Psycholology 2018, 9, 2132. [Google Scholar] [CrossRef]
- Saredakis, D.; Szpak, A.; Birckhead, B.; Keage, H.A.; Rizzo, A.; Loetscher, T. Factors associated with virtual reality sickness in head-mounted displays: A systematic review and meta-analysis. Front. Hum. Neurosci. 2020, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- Stanney, K.M.; Kennedy, R.S. The psychometrics of cybersickness. Commun. ACM 1997, 40, 66–68. [Google Scholar] [CrossRef]


| Characteristics | CBT | CBT + VR | Self-Administered VR -Exposure |
|---|---|---|---|
| N | 30 | 31 | 30 |
| Age, M(SD) | 31.44 (7.68) | 31.98 (7.06) | 31.39 (7.73) |
| Gender, n female (%) | 17 (56) | 18 (58) | 17 (56) |
| Past treatment, n (%) none | 9 (30) | 13 (42) | 8(26) |
| Pharmacotherapy | 14 (46) | 11 (35) | 18 (60) |
| Psychotherapy | 20 (66) | 22 (70) | 24 (80) |
| Both | 12 (43) | 8 (25) | 18 (60) |
| Current pharmacotherapy, n (%) | 8 (26) | 8 (25) | 11 (36) |
| Session completed, n 1 | 30 | 31 | 30 |
| 2 | 30 | 31 | 30 |
| 3 | 30 | 30 | 26 |
| 4 | 28 | 30 | 22 |
| 5 | 28 | 30 | 21 |
| 6 | 28 | 30 | 20 |
| 7 | 28 | 30 | 19 |
| 8 | 28 | 30 | 19 |
| 9 | 26 | 29 | 19 |
| 10 | 26 | 29 | 19 |
| 11 | 25 | 29 | 19 |
| 12 | 25 | 29 | 19 |
| 13 | 25 | 29 | 19 |
| 14 | 25 | 29 | 19 |
| Dropouts (%) | 5 (16) | 2 (6) | 11 (36) |
| Scales | |||
|---|---|---|---|
| Structured Clinical Interview for Axis I Disorders DSM-IV-TR (SCID-I) | Clinician | Confirming social anxiety disorder | T0 |
| Liebowitz Social Anxiety Scale (LSAS) | Patient | Primary outcome | T0, T1,T2 |
| Clinical Global Impression Scale (CGI) | Clinician | Severity of SAD symptoms | T0 |
| Patient Global Impression Scale (PGI) | Patient | Severity of SAD symptoms | T0 |
| Beck Depression Inventory (BDI) | Patient | Secondary outcome | T0, T2 |
| Clinician Global Impressions of Improvement (CGI-I) | Clinician | Secondary outcome | T0,T2 |
| Patient Global Impression of Change Scale (PGI-CS) | Patient | Secondary outcome | T0, T2 |
| Simulator Sickness Questionnaire (SSQ)-pre | Patient | Before each exposure session | |
| Simulator Sickness Questionnaire (SSQ)-post | Patient | After each exposure session |
| Session (Duration: 45 Min. Each Arm) | Therapy Protocol: CBT | Therapy Protocol: CBT + VR | Therapy Protocol: Self-Administered VR Exposure |
|---|---|---|---|
| 2 | Presentation of general information about the therapy Identification of therapy goals and problems. Signing of a therapy contract. Homework | Presentation of general information about the therapy Identification of therapy goals and problems. Signing of a therapy contract. Homework | Turning on the application. Selection of a scenario. Reading the instructions for the scenario VR exposure. Self-reflection |
| 3 | Familiarization of the patient with the cognitive model of anxiety Psychoeducation on social anxiety Homework | Familiarization of the patient with the cognitive model of anxiety Psychoeducation on social anxiety Homework | Turning on the application. Analysis of the summary of results from the previous sessions. Selection of a scenario. Reading the instructions for the scenario. VR exposure. Self-reflection |
| 4 | Introduction of the cognitive model of social anxiety Homework | Introduction of the cognitive model of social anxiety Homework | See above |
| 5 | Psychoeducation on cognitive distortions. Introduction of cognitive restructuring as part of discussion with negative automatic thoughts. Homework | Psychoeducation on cognitive distortions. Introduction of cognitive restructuring as part of discussion with negative automatic thoughts. Homework | See above |
| 6 | Introduction to behavioral techniques. Homework | Introduction to behavioral techniques. Homework | See above |
| 7–12 | Imagination exposure. Discussion of exposure, discussion of a list of safety behaviors, and discussion of beliefs and perceptions about oneself in social situations. Cognitive reformulation. Homework | Virtual exposure. Discussion of exposure, discussion of a list of safety behaviors, and discussion of beliefs and perceptions about oneself in social situations Cognitive reformulation. Homework | See above |
| 13 | Therapy summary | Therapy summary | See above |
| CBT | CBT + VR | VR | F | p | ||
|---|---|---|---|---|---|---|
| CGI | 4.36 (0.85) | 4.22 (0.76) | 4.36 (0.96) | 0.27 a,b | 0.00 | 0.764 |
| PGI | 3.2 (0.55) | 3.26 (0.58) | 3.33 (0.66) | 0.37 a,c | 0.00 | 0.692 |
| CBT | CBT + VR | VR | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T0 | T1 | T2 | T0 | T1 | T2 | |
| LSAS-anxiety | 44.37 (14.13) | 40.08 (13.89) | 29.16 (13.79) | 43.61 (13.71) | 39.57 (11.78) | 32 (14.46) | 46.47 (10.89) | 44.05 (10.23) | 41.53 (10.96) |
| LSAS-avoidance | 39.47 (16.48) | 35.36 (13.71) | 26.09 (13.18) | 38.94 (13.36) | 34.18 (13.35) | 24.83 (14.8) | 41.43 (11.9) | 37.11 (8.19) | 34.58 (14.96) |
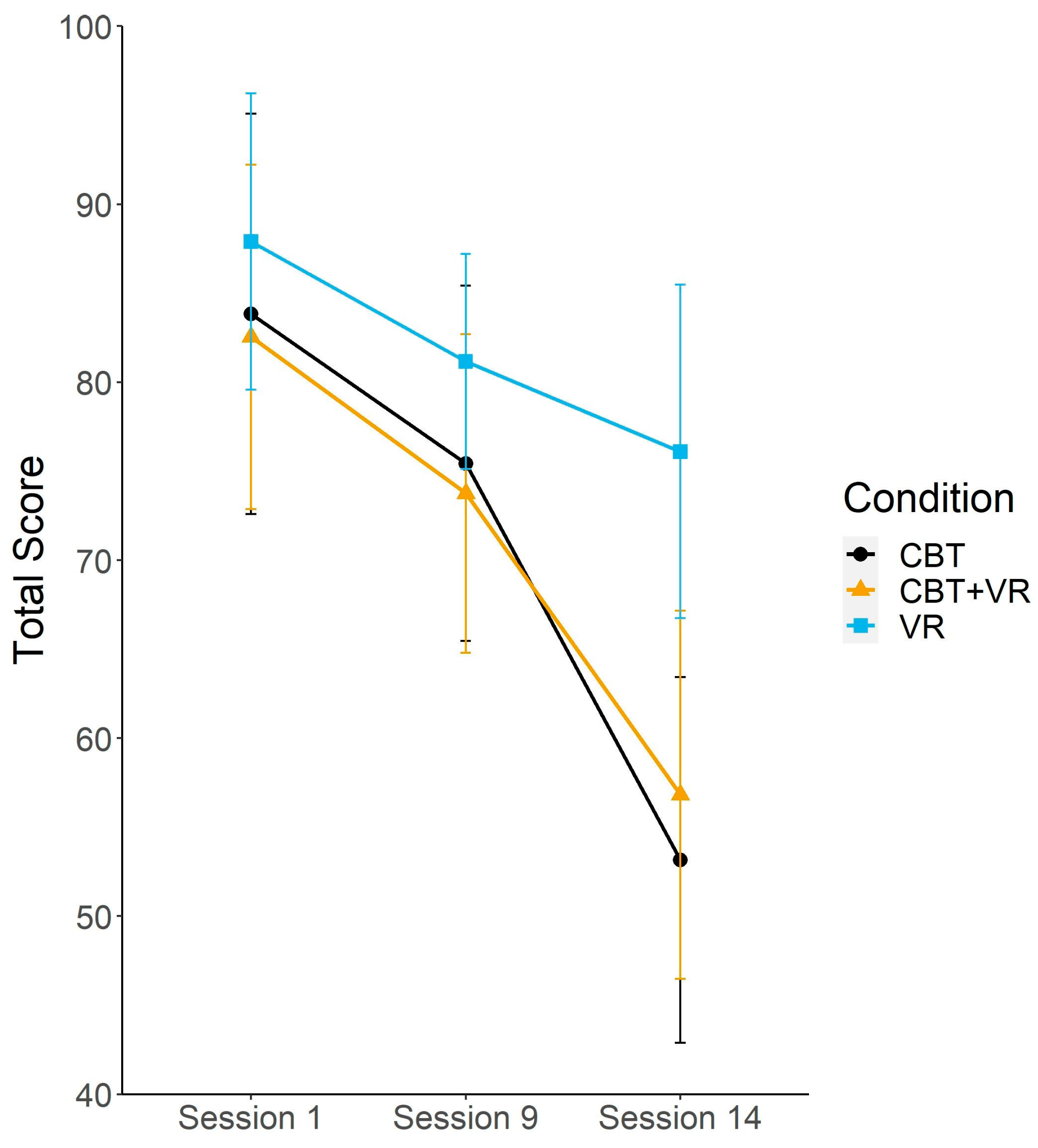
| LSAS-total score | 83.83 (30.12) | 75.44 (26.74) | 53.16 (27.49) | 82.55 (26.36) | 73.75 (24.42) | 56.83 (28.19) | 87.9 (22.29) | 81.16 (16.19) | 76.11 (25.1) |
| BDI | 17.62 (9.96) | - | 12.23 (9.6) | 14.39 (9.51) | - | 8.96 (9.21) | 19.83 (10.5) | - | 16.17 (12.05) |
| CGII | - | - | 2.56 (0.71) | - | - | 2.55 (0.69) | - | - | 3.21 (0.63) |
| PGICS | - | - | 5 (1.12) | - | - | 5.1 (1.18) | - | - | 3.74 (1.37) |
| Random Intercept + Time | Random Intercept + Time + Condition | Random Intercept + Time + Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B | CI | p | B | CI | p | B | CI | p | |||
| Total Score | Intercept | 74.1 | 69.2 to 79 | <0.001 | 74.23 | 69.34 to 79.12 | <0.001 | 74.45 | 69.57 to 79.33 | <0.001 | |
| Session | 9–1 | −8.18 | −12.7 to −3.65 | <0.001 | −8.02 | −12.56 to −3.5 | <0.001 | −8.13 | −12.62 to −3.36 | <0.001 | |
| 14–1 | −23.73 | −28.23 to −19.23 | <0.001 | −23.57 | −28.08 to −19.06 | <0.001 | −22.78 | −27.26 to −18.31 | <0.001 | ||
| Condition | CBT + VR-CBT | −0.18 | −11.98 to −11.61 | 0.975 | 0.04 | −11.72 to 11.81 | 0.995 | ||||
| Session * Condition | VR-CBT | −7.62 | −4.45 to 19.71 | 0.219 | 8.8 | −3.34 to 20.92 | 0.159 | ||||
| 9–1 * CBT + VR-CBT | −0.83 | −11.26 to 9.6 | 0.876 | ||||||||
| 14–1 * CBT + VR-CBT | 4.81 | −5.56 to 15.17 | 0.365 | ||||||||
| 9–1 * VR-CBT | −1.52 | −12.95 to 9.9 | 0.793 | ||||||||
| 14–1 * VR-CBT | 15.7 | 4.27 to 27.12 | 0.008 | ||||||||
| Random parts | AIC | 2100.42 | 2102.28 | 2099.57 | |||||||
| σ2 | 475.43 | 472.57 | 471.41 | ||||||||
| τ00, Participant | 198.43 | 198.9 | 191.04 | ||||||||
| N Participant | 91 | 91 | 91 | ||||||||
| ICC Participant | 0.7 | 0.7 | 0.71 | ||||||||
| Observations | 236 | 236 | 236 | ||||||||
| R2m/R2c | 0.127/743 | 0.145/0.747 | 0.157/0.757 | ||||||||
| Anxiety subscale | Intercept | 39.89 | 37.44 to 42.34 | <0.001 | 39.96 | 37.53 to 42.4 | <0.001 | 40.11 | 37.68 to 42.54 | <0.001 | |
| Session | 9–1 | −3.7 | −5.93 to −1.47 | 0.001 | −3.6 | −5.83 to −1.38 | 0.002 | −3.57 | −5.77 to −1.38 | 0.002 | |
| 14–1 | −11.05 | −13.26 to −8.83 | <0.001 | −10.95 | −13.17 to −8.74 | <0.001 | −10.53 | −12.72 to −8.35 | <0.001 | ||
| Condition | CBT + VR-CBT | 0.13 | −4.74 to 6 | 0.966 | 0.29 | −5.55 to 6.14 | 0.922 | ||||
| VR-CBT | 4.58 | −1.43 to 10.59 | 0.138 | 5.39 | −0.64 to 11.42 | 0.083 | |||||
| Session * Condition | 9–1 * CBT + VR-CBT | −0.17 | −5.26 to 4.92 | 0.947 | |||||||
| 14–1 * CBT + VR-CBT | 3.31 | −1.75 to 8.38 | 0.202 | ||||||||
| 9–1 * VR-CBT | 0.73 | −4.83 to 6.32 | 0.769 | ||||||||
| 14–1 * VR-CBT | 9.13 | 3.66 to 14.71 | 0.002 | ||||||||
| Random parts | AIC | 1768.02 | 1769.08 | 1764.53 | |||||||
| σ2 | 119.3 | 117.5 | 117.2 | ||||||||
| τ00, Participant | 47.9 | 48 | 45.6 | ||||||||
| N Participant | 91 | 91 | 91 | ||||||||
| ICC Participant | 0.713 | 0.71 | 72 | ||||||||
| Observations | 236 | 236 | 236 | ||||||||
| R2m / R2c | 0.113/0.746 | 0.137/0.75 | 0.152/0.763 | ||||||||
| Intercept | 34.31 | 31.74 to 36.89 | <0.001 | 34.37 | 31.79 to 36.96 | <0.001 | 34.43 | 31.83 to 37.02 | <0.001 | ||
| Session | 9–1 | −4.49 | −7.01 to −1.98 | <0.001 | −4.42 | −6.94 to −1.9 | <0.001 | −4.55 | −7.07 to −2.03 | <0.001 | |
| 14–1 | −12.37 | −14.9 to −9.84 | <0.001 | −12.3 | −14.84 to −9.77 | <0.001 | −11.98 | −14.5 to −9.46 | <0.001 | ||
| Condition | CBT + VR-CBT | −0.6 | −6.82 to 5.63 | 0.951 | −0.5 | −6.74 to 5.73 | 0.874 | ||||
| VR-CBT | 2.83 | −3.55 to 9.22 | 0.387 | 3.2 | −3.24 to 9.65 | 0.333 | |||||
| Session * Condition | 9–1 * CBT + VR-CBT | −0.66 | −6.5 to 5.2 | 0.825 | |||||||
| Avioidance subscale | 14–1 * CBT + VR-CBT | 0.74 | −5.18 to 6.66 | 0.805 | |||||||
| 9–1 * VR-CBT | −2.19 | −8.6 to 4.22 | 0.504 | ||||||||
| 14–1 * VR-CBT | 5.9 | −0.6 to 12.4 | 0.077 | ||||||||
| Random parts | AIC | 1798.95 | 1801.63 | 1802.6 | |||||||
| σ2 | 128.2 | 128.9 | 129.31 | ||||||||
| τ00, Participant | 61.6 | 61.7 | 60.46 | ||||||||
| N Participant | 91 | 91 | 91 | ||||||||
| ICC Participant | 0.67 | 0.67 | 0.68 | ||||||||
| Observations | 234 | 234 | 234 | ||||||||
| R2m/R2c | 0.122/0.715 | 0.132/0.719 | 0.14/0.726 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefaniak, I.; Hanusz, K.; Mierzejewski, P.; Bieńkowski, P.; Parnowski, T.; Murawiec, S. Preliminary Study of Efficacy and Safety of Self-Administered Virtual Exposure Therapy for Social Anxiety Disorder vs. Cognitive-Behavioral Therapy. Brain Sci. 2022, 12, 1236. https://doi.org/10.3390/brainsci12091236
Stefaniak I, Hanusz K, Mierzejewski P, Bieńkowski P, Parnowski T, Murawiec S. Preliminary Study of Efficacy and Safety of Self-Administered Virtual Exposure Therapy for Social Anxiety Disorder vs. Cognitive-Behavioral Therapy. Brain Sciences. 2022; 12(9):1236. https://doi.org/10.3390/brainsci12091236
Chicago/Turabian StyleStefaniak, Izabela, Krzysztof Hanusz, Paweł Mierzejewski, Przemysław Bieńkowski, Tadeusz Parnowski, and Sławomir Murawiec. 2022. "Preliminary Study of Efficacy and Safety of Self-Administered Virtual Exposure Therapy for Social Anxiety Disorder vs. Cognitive-Behavioral Therapy" Brain Sciences 12, no. 9: 1236. https://doi.org/10.3390/brainsci12091236
APA StyleStefaniak, I., Hanusz, K., Mierzejewski, P., Bieńkowski, P., Parnowski, T., & Murawiec, S. (2022). Preliminary Study of Efficacy and Safety of Self-Administered Virtual Exposure Therapy for Social Anxiety Disorder vs. Cognitive-Behavioral Therapy. Brain Sciences, 12(9), 1236. https://doi.org/10.3390/brainsci12091236






