Neurofeedback for the Education of Children with ADHD and Specific Learning Disorders: A Review
Abstract
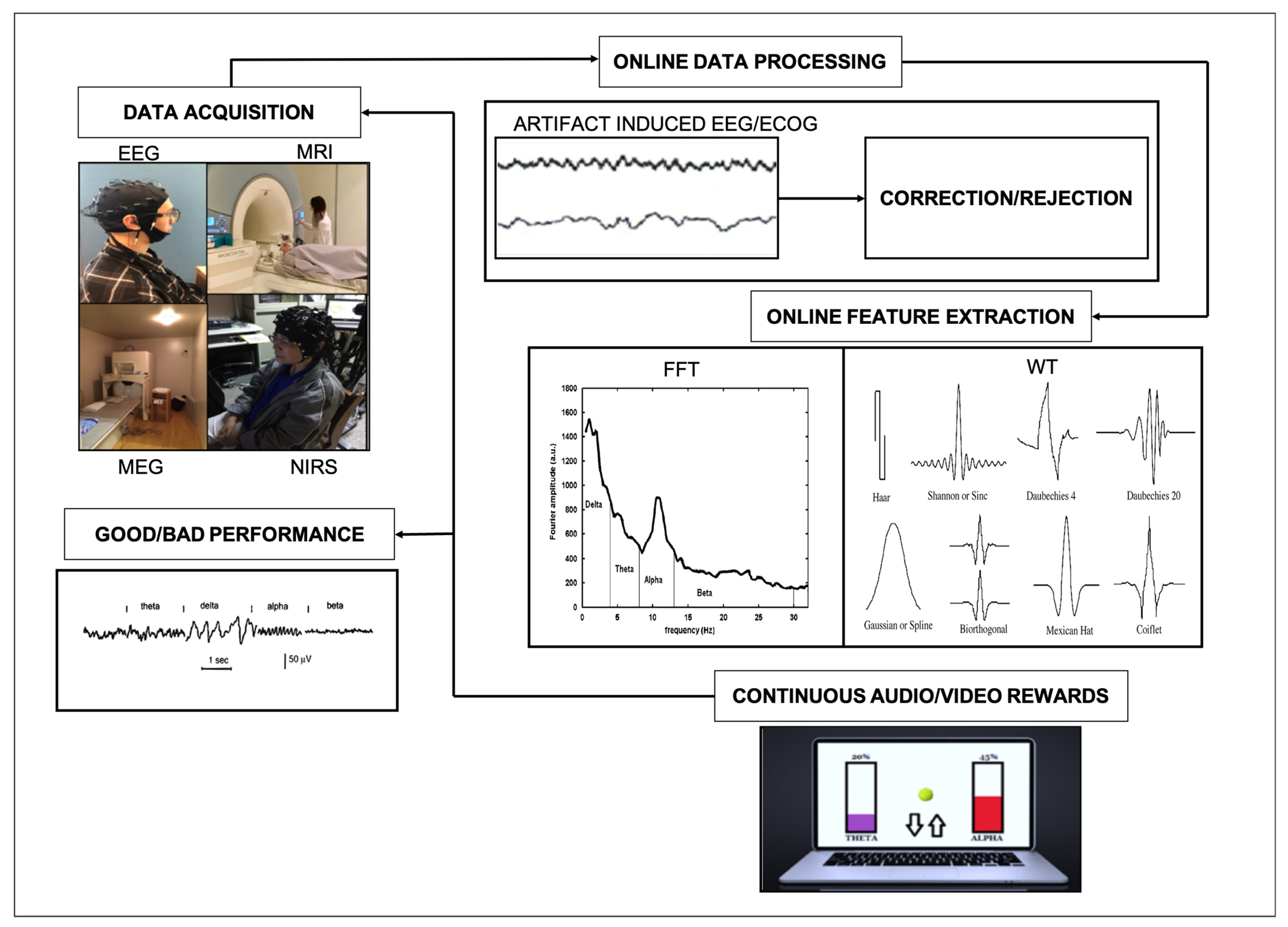
:1. Introduction
2. Methods
3. Results
3.1. EEG–Neurofeedback for Assisting Children with Dyslexia
| Author(s) | Characteristics of the Sample | Groups | NF Details | Post-NF | ||
|---|---|---|---|---|---|---|
| Brain Activities | Behavior (Short Term) | Behavior (Long Term) | ||||
| Breteler et. al. [45] | n = 19 (11 male) with dyslexia were randomized into 2 groups; age range = 8–16 years, mean age = 10.33 years. | Exp. = 10, control = 9. | 1. Increased activity in delta with Z >1.5 times normal at T6. 2. Increased coherence in alpha/beta band at F7–FC3 or F7–C3 with Z > 1.5. 3. Increased coherence at T3–T4 with Z > 1.5. | Increased coherence in delta and alpha bands, and decreased coherence in the beta band. | Improvement in spelling but no clear improvement in reading abilities. | There was a significant improvement in spelling due to NF training. Attentional modulation can be assumed to be involved in this improvement. |
| Nazari et. al. [60] | n = 6 (all male) with dyslexia; age range = 8–10 years, mean age = 9 years. | Exp. = 6, no control group. | EEG was converted to specific frequency bands using FFT (delta: 1–4 Hz, theta: 4–8 Hz, alpha: 8–12 Hz; beta: 12–25 Hz); z-scores of coherence, absolute power, and relative power were calculated for all the above bands; the study examined individual differences in subjects. | Increased coherence in beta, theta, and delta band. No change in power for all bands. | Reduction in reading errors and reading time following NF sessions. | Sensory–motor integration and cerebral maturity in children with dyslexia. |
3.2. EEG–Neurofeedback for Assisting Children with ADHD Symptoms
| Author(s) | Characteristics of the Sample | Groups | NF Details | Post-NF | ||
|---|---|---|---|---|---|---|
| Brain Activities | Behavior (Short Term) | Behavior (Long Term) | ||||
| Kropotov et al., 2005 [34] | n = 86 (9 female) with ADHD; age range = 9–14 years, mean age = 11.4 years. | Exp. = 86, no control group. | 1. Beta training on C3-Fz 2. SMR training on C4-Pz; 15–22 sessions. | At least a 25% increase in within sessional beta or SMR power in the 1st session. | Improvement in go/no-go response time and go/no-go SD. | Children showed improvements in symptoms of ADHD. |
| Doren et al., 2017 [76] | n = 22 (1 female) with ADHD; age range = 10–15 years, mean age = 13.4 years. | Exp. = 22, no control group. | Theta/beta power training was provided based on the signal at Cz, NF session—2 NF phases: 1. Puzzle task and 2. Attention task, one acquaintance session, and two theta/beta NF sessions with pre- and post-NF behavioral and EEG assessments | Reduction in the theta/beta ratio and theta power during reading. | Improved reading ability. | No long-term measures. |
| Escolano et al., 2014 [78] | n = 20 with ADHD (1 female); age range = 9–13 years, mean age = 11.8 years. | Exp. = 20, no control group. | Increase upper alpha power averaged over six feedback electrodes (frontocentral) at IAF; 18 sessions, 5 trails per session. | Enhanced upper alpha. Average increase of 13% in upper alpha power in task-related activity. | Improvement in working memory, concentration, and impulsivity. | A significant positive learning and improved cognitive performance over sessions. |
| Rajabi et al., 2019 [79] | n = 32 (32 male) with ADHD; randomized double-blind trial with two groups; age range = 6–11 years, mean age = 10.25 years. | Exp. group = 16, control = 16 | 1. Beta training on FCz electrode (15 min). 2. SMR training on C1–C5 (15 min). | Increased activity of beta at Cz and SMR at Cz and FCz and decreased theta/beta at Cz. | NF with computer training resulted in significant improvements in control of motor behavior and inhibition of attention to disturbing stimuli. | Children demonstrated improvements in symptoms of ADHD |
| Christiansen et al., 2014 [80] | n = 58 (10 female) with ADHD; age range = 7–11 years, mean age = 8.42 years. | Exp. = 32 * randomly divided into two groups; exp. group was given SCP NF training only; the control group was given medication only | SCP NF protocol; 30 sessions of NF training with 40 trials of 8 min each. | Positive SCPs indicated a relaxed state and negative SCPs indicated an attentive state | Psychopathology ratings increased in children who did a follow-up, improved ADHD symptoms. | Psychopathology ratings showed no differences from a period post-treatment to 6 months after treatment. |
| Okumura et al., 2019 [81] | n = 22 with ADHD; age range = 7.83–16.25 years, mean age = 12.11 years | Participants were divided into two groups, learners and non-learners, based on their pretraining indices and SCP regulation in training using decision tree analysis. | SCP NF protocol; 10 sessions (2 sessions/day), 60 trials/session; SCP recorded at Cz. | Enhancement of positive SCP in learners only. | No significant changes in the symptoms of ADHD. | SCP might not be an effective protocol for all ADHD children. |
| Janssen et al., 2017 [77] | n = 38 (9 female) with ADHD; age range = 7–13 years, mean age = 9.87 years. | Exp. = 38, no control group. | Theta/beta NF protocol, 29 sessions (3 sessions/week), each session = 45 min, 10 trials/session; inhibit theta and reinforce beta at Cz. | A linear decrease in theta/beta index over sessions. | No behavioral changes. | Learning improved with an increase in beta power over sessions. |
| Strehl et al., 2006 [82] | n = 23 (9 female) with ADHD; age range = 8–13 years, mean age = not provided. | Exp. = 23; no control group. | SCP NF protocol; 30 sessions (3 blocks of 10) Each session = 45 min, 39 trials/run, 3–5 runs/session; SCP recorded at Cz. Follow-up after 6 months. | The good performance is indicated by differences in the mean amplitude of SCPs. | Improvement in behavior, attention, and IQ following NF sessions. | Clinical improvement is seen only in good learners. |
| Drechsler et al., 2007 [83] | n = 30 (7 female) with ADHD; age range = 9–13 years, mean age = 10.85 years. | Randomization of group assignment was incomplete; two groups: NFT group = 17, control group = 13. | SCP NF protocol; 30 sessions; 2 sessions = 45 min, 40 trials/run; SCP recorded at Cz. | The good performance is indicated by differences in the mean amplitude of SCPs. | Improvement in positive behavioral effects following NF sessions. | Clinical improvement in ADHD symptoms is seen only in good learners. |
| Leins et al., 2007 [84] | n = 38 (6 female) with ADHD and were blind to group assignment; age range = 8–13 years, mean age = 9.16 years. | Two groups: theta/beta NF group = 19; SCP NF group = 19. | 1. Theta/beta group: theta/beta NF protocol; 30 sessions, each session = 1 hr; theta/beta recorded at C3f, C4f. 2. SCP group: SCP NF protocol; 30 sessions; each session = 1 hr; SCP recorded at Cz. | Both groups demonstrated EEG-based learning by improvements in respective cortical activity. | Both groups demonstrated improvements in attention and IQ. | Clinical effects for both groups remained stable six months after treatment. |
| Bakhshayesh et al., 2011 [75] | n = 35 (9 female) with ADHD and were blind to group assignment; age range = 6–14 years, mean age = 9.34 years. | Two groups: NF group = 18, BF group = 17. | Theta/beta NF protocol, 30 sessions, each session = 30 min, 2–3 sessions/week, theta/beta recorded at Cz. | Reduced theta/beta ratios in the NF group. | Improved reaction times and attention following NF sessions. | Improvement in ADHD symptoms was seen by parents only in the NF group. |
| DeBeus et al., 2011 [85] | n = 42 (29 female) with ADHD and were double blind to randomization; age range = 7–11 years, mean age = 8.8 years. | Two groups: NF group = 18, placebo group = 17. | NF group: suppress theta + alpha, enhance beta including SMR at Fz; 20 sessions. | Engagement index (beta/(theta + alpha) increase) improved following NF sessions in 74% of the children. | Behavioral symptoms rated by teachers; improvements in the continuous performance test. | Improvements rated by teachers correlated with the engagement index. |
| Gevensleben et al., 2014 [86] | n = 10 with ADHD; randomized controlled trials; age range = 10–13 years, mean age = 11.4 years. | Exp. = 10; no control group. | SCP NF protocol; 13 double sessions, each session = 105 min, 36–38 trials/session; SCP recorded at Cz. | Mean amplitude increase in SCP negativity in all trials. | Decrease in inattention symptoms, and an association between mean amplitude and SCP negativity. | No long-term changes. |
| Takahashi et al., 2014 [87] | n = 10 (3 female) with ADHD; age range = 8.4–16.6 years, mean age = 12.5 years. | Exp. = 10; no control group. | SCP NF protocol; 16 sessions; 2 sessions/week, each session = 12 min, 60 trials/session; SCP recorded at Cz. | Positive shift increase in amplitude in sessions 9 and 13, and negative shift increase in amplitude in sessions 11 and 12. | No behavioral changes following NF sessions. | No long-term changes |
3.3. EEG–Neurofeedback for Assisting Children with Other Specific Learning Disorders
| Author(s) | Details of the Sample | Conditions | NF Details | Post-NF |
|---|---|---|---|---|
| Becerra et al. (2006) [46] | n = 10 (2 female) children with learning disability; age range = 7–11 years, mean age = 11.65 years. | Children diagnosed with learning disorders were divided into two groups: experimental group (n = 5, age = 11.2 ± 1.4, 1 female) and control group (n = 4 *, age = 12.1 ± 1.6, 1 female). | Each child received 20 NF sessions in the experimental group, with each session lasting 30 min, and 2 sessions per week over a period of 10–12 weeks. | This follow-up study lasted for 2 years with two groups: the experimental group receiving NF sessions and the control group receiving placebo treatment; verbal scores decreased after NF sessions; EEG maturational lag in control group children increased, reaching abnormally high theta values. By contrast, children in the experimental group exhibited positive behavioral changes. |
| Jacobs (2006) Case I [104] | 15-year-old boy. | Diagnosed with ADHD, learning disabilities in writing, reading, and spelling, and bipolar and developmental disorders. | Received 40 NF sessions including right and intra-hemispheric training. | Improvements in some learning deficits. Symptoms of anxiety, depression, phobias, interpersonal sensitivity, etc. improved significantly; improved focus. |
| Jacobs (2006) Case II [104] | 10-year-old boy. | Serious deficits in social interactions, attention, and anxiety affect his home and school functions. | Received 39 NF sessions including right-hemispheric training. | Improvement in inhibition and executive functions. Improved attention, social acceptability, social interaction, and control over anger. |
| Thornton and Carmody (2005), Case II [105] | 9-year-old girl. | History of learning problems. No academic records or neuropsychological testing was completed to verify the severity of the learning disability. Did not exhibit a high theta/low beta pattern as with other children with a learning disability. | 40 sessions including alpha coherence (input stage) and alpha and beta coherence (recall period). | Post-NF sessions showed improvement in auditory and reading memory. |
| Thornton and Carmody (2005), Case III [105] | Boy (age not mentioned). | History of reading problems. He exhibited abnormalities in connectivity and coherence. | 25 NF training sessions at occipital positions indicating issues in the posterior regions. | Increased auditory memory functioning following the 25 sessions. In addition demonstrated improved reading scores. |
| Thornton and Carmody (2005), Case IV [105] | 17-year-old. | Issues relating to reading disability. | 20 NF sessions were provided to the participant. | Following the 20 NF sessions, the comprehension scores improved from 45% to 90% (8th grade level) and 20% to 70% (10th grade level); the story recall performance score also increased. |
| Fernández et al. [47,103] | n = 16 with LD randomly assigned to two groups: experimental group (n = 11; 6 females; age range = 7–11 years; mean age = 8.94 years): received NF training. Control group (n = 5; age range = 7–11 years; mean age = 9.7 years): received placebo treatment. | Children with learning disability. | Experimental group: Before NF training, 2–3 EEG recordings were taken for every child. Every child received 20 NF sessions (30 mins/session) for 10–12 weeks (2 sessions/week); theta/alpha ratio was calculated at the beginning and end of every session. Control group: in similar conditions, only the tone onset and its duration were randomly assigned. | All children learned to decrease the theta/alpha ratio during the NF sessions. Post NF sessions, the control group did not find significant reductions in the EEG power bands. In contrast, the experimental group reduced delta and theta power levels and increased the alpha and beta power levels. |
| Fernández et al. [47,103] | n = 20 with LD randomly assigned to two groups: auditory group (n = 10; mean age = 9.10 years): NF training using an auditory stimulus. Visual group (n = 10; mean age = 9.08 years): NF training using a visual stimulus. | Children with learning disability. | Before NF training, 2–3 EEG recordings were taken for every child. Every child received 20 NF sessions (30 mins/session) for 10–12 weeks (2 sessions/week). | After the NF training, both groups significantly reduced the z-score of theta/alpha quotient; However, more children with normalized z-score theta/alpha quotient were found in the NF enforced auditory group. |
4. Discussion
4.1. Potential for Using EEG-NF in Education
4.2. Challenges in the Adoption of EEG-NF in Education
4.3. Future Directions for EEG-NF Applications in Education
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nestoriuc, Y.; Martin, A.; Rief, W.; Andrasik, F. Biofeedback Treatment for Headache Disorders: A Comprehensive Efficacy Review. Appl. Psychophysiol. Biofeedback 2008, 33, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Arns, M.; de Ridder, S.; Strehl, U.; Breteler, M.; Coenen, A. Efficacy of Neurofeedback Treatment in ADHD: The Effects on Inattention, Impulsivity and Hyperactivity: A Meta-Analysis. Clin. EEG Neurosci. 2009, 40, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.K.; Sneha, C.S. EEG Neurofeedback Brain Training for Epilepsy to Reduce Seizures: EEG Neurofeedback Training for Epilepsy. Int. J. Child Dev. Ment. Health 2019, 7, 28–33. [Google Scholar]
- Wu, Y.-L.; Fang, S.-C.; Chen, S.-C.; Tai, C.-J.; Tsai, P.-S. Effects of Neurofeedback on Fibromyalgia: A Randomized Controlled Trial. Pain Manag. Nurs. 2021, 22, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Elvira, R.; Oltra-Cucarella, J.; Carrobles, J.; Teodoru, M.; Bacila, C.; Neamtu, B. Individual Alpha Peak Frequency, an Important Biomarker for Live Z-Score Training Neurofeedback in Adolescents with Learning Disabilities. Brain Sci. 2021, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Gruzelier, J.H. EEG-neurofeedback for optimising performance. I: A review of cognitive and affective outcome in healthy participants. Neurosci. Biobehav. Rev. 2014, 44, 124–141. [Google Scholar] [CrossRef]
- Huster, R.J.; Debener, S.; Eichele, T.; Herrmann, C.S. Methods for Simultaneous EEG-fMRI: An Introductory Review. J. Neurosci. 2012, 32, 6053–6060. [Google Scholar] [CrossRef]
- Amano, K.; Shibata, K.; Kawato, M.; Sasaki, Y.; Watanabe, T. Learning to Associate Orientation with Color in Early Visual Areas by Associative Decoded fMRI Neurofeedback. Curr. Biol. 2016, 26, 1861–1866. [Google Scholar] [CrossRef]
- Cortese, A.; Amano, K.; Koizumi, A.; Lau, H.; Kawato, M. Decoded fMRI neurofeedback can induce bidirectional confidence changes within single participants. NeuroImage 2017, 149, 323–337. [Google Scholar] [CrossRef]
- Megumi, F.; Yamashita, A.; Kawato, M.; Imamizu, H. Functional MRI neurofeedback training on connectivity between two regions induces long-lasting changes in intrinsic functional network. Front. Hum. Neurosci. 2015, 9, 160. [Google Scholar] [CrossRef]
- Yamashita, A.; Hayasaka, S.; Kawato, M.; Imamizu, H. Connectivity Neurofeedback Training Can Differentially Change Functional Connectivity and Cognitive Performance. Cereb. Cortex 2017, 27, 4960–4970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musall, S.; Von Pföstl, V.; Rauch, A.; Logothetis, N.K.; Whittingstall, K. Effects of Neural Synchrony on Surface EEG. Cereb. Cortex 2014, 24, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.H.; Memon, A.A.; Memon, R.A.; Joop, A.; Pilkington, J.; Catiul, C.; Gerstenecker, A.; Triebel, K.; Cutter, G.; Bamman, M.M.; et al. Slow Wave Sleep and EEG Delta Spectral Power are Associated with Cognitive Function in Parkinson’s Disease. J. Park. Dis. 2021, 11, 703–714. [Google Scholar] [CrossRef]
- Harmony, T. The functional significance of delta oscillations in cognitive processing. Front. Integr. Neurosci. 2013, 7, 83. [Google Scholar] [CrossRef]
- Gevins, A.; Smith, M.E.; Leong, H.; McEvoy, L.; Whitfield, S.; Du, R.; Rush, G. Monitoring Working Memory Load during Computer-Based Tasks with EEG Pattern Recognition Methods. Hum. Factors 1998, 40, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Lo, Y.-H.; Pan, C.-Y.; Chen, F.-C.; Liang, W.-K.; Tsai, C.-L. Frontal midline theta as a neurophysiological correlate for deficits of attentional orienting in children with developmental coordination disorder. Psychophysiology 2015, 52, 801–812. [Google Scholar] [CrossRef]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. EEG alpha oscillations: The inhibition–timing hypothesis. Brain Res. Rev. 2007, 53, 63–88. [Google Scholar] [CrossRef] [PubMed]
- Bonnefond, M.; Jensen, O. The role of gamma and alpha oscillations for blocking out distraction. Commun. Integr. Biol. 2013, 6, e22702. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Li, P.; Martens, S.; Luo, Y. Beta-gamma oscillation reveals learning from unexpected reward in learners versus non-learners. Neuropsychologia 2019, 131, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Rodríguez, O.; Zavala-Calderón, E.; Magaña-García, N.; González-Campos, R.; López-Loeza, E.; Rangel-Argueta, A.R.; López-Vázquez, M.; Olvera-Cortés, M.E. Diabetic patients are deficient in intentional visuospatial learning and show different learning-related patterns of theta and gamma EEG activity. J. Clin. Exp. Neuropsychol. 2021, 43, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Modolo, J.; Hassan, M.; Wendling, F.; Benquet, P. Decoding the circuitry of consciousness: From local microcircuits to brain-scale networks. Netw. Neurosci. 2020, 4, 315–337. [Google Scholar] [CrossRef]
- Dempster, T.J.T. An Investigation into the Optimum Training Paradigm for Alpha Electroencephalographic Biofeedback; Canterbury Christ Church University: Canterbury, UK, 2012. [Google Scholar]
- Congedo, M.; Gouy-Pailler, C.; Jutten, C. On the blind source separation of human electroencephalogram by approximate joint diagonalization of second order statistics. Clin. Neurophysiol. 2008, 119, 2677–2686. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Sejnowski, T.; Makeig, S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. NeuroImage 2006, 34, 1443–1449. [Google Scholar] [CrossRef]
- Delorme, A.; Miyakoshi, M.; Jung, T.-P.; Makeig, S. Grand average ERP-image plotting and statistics: A method for comparing variability in event-related single-trial EEG activities across subjects and conditions. J. Neurosci. Methods 2015, 250, 3–6. [Google Scholar] [CrossRef]
- Romero, S.; Mañanas, M.A.; Barbanoj, M.J. A comparative study of automatic techniques for ocular artifact reduction in spontaneous EEG signals based on clinical target variables: A simulation case. Comput. Biol. Med. 2008, 38, 348–360. [Google Scholar] [CrossRef]
- Onton, J.; Westerfield, M.; Townsend, J.; Makeig, S. Imaging human EEG dynamics using independent component analysis. Neurosci. Biobehav. Rev. 2006, 30, 808–822. [Google Scholar] [CrossRef] [PubMed]
- Sulzer, J.; Haller, S.; Scharnowski, F.; Weiskopf, N.; Birbaumer, N.; Blefari, M.; Bruehl, A.; Cohen, L.; Decharms, R.; Gassert, R.; et al. Real-time fMRI neurofeedback: Progress and challenges. NeuroImage 2013, 76, 386–399. [Google Scholar] [CrossRef]
- Watanabe, T.; Sasaki, Y.; Shibata, K.; Kawato, M. Advances in fMRI Real-Time Neurofeedback. Trends Cogn. Sci. 2017, 21, 997–1010. [Google Scholar] [CrossRef]
- Weiskopf, N.; Scharnowski, F.; Veit, R.; Goebel, R.; Birbaumer, N.; Mathiak, K. Self-regulation of local brain activity using real-time functional magnetic resonance imaging (fMRI). J. Physiol. 2004, 98, 357–373. [Google Scholar] [CrossRef]
- Zich, C.; Debener, S.; Kranczioch, C.; Bleichner, M.G.; Gutberlet, I.; De Vos, M. Real-time EEG feedback during simultaneous EEG–fMRI identifies the cortical signature of motor imagery. NeuroImage 2015, 114, 438–447. [Google Scholar] [CrossRef]
- Eros, T.; Baars, B.; Lanius, R.A.; Vuilleumier, P. Tuning pathological brain oscillations with neurofeedback: A systems neuroscience framework. Front. Hum. Neurosci. 2014, 8, 1008. [Google Scholar] [CrossRef]
- Kropotov, J.D.; Grin-Yatsenko, V.A.; Ponomarev, V.A.; Chutko, L.S.; Yakovenko, E.A.; Nikishena, I.S. ERPs correlates of EEG relative beta training in ADHD children. Int. J. Psychophysiol. 2005, 55, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Sterman, M.B.; Macdonald, L.R.; Stone, R.K. Biofeedback Training of the Sensorimotor Electroencephalogram Rhythm in Man: Effects on Epilepsy. Epilepsia 1974, 15, 395–416. [Google Scholar] [CrossRef]
- Budzynski, T.H.; Budzynski, H.K.; Evans, J.R.; Abarbanel, A. Introduction to Quantitative EEG and Neuro-Feedback: Advanced Theory and Applications, 2nd ed.; Academic Press: Cambridge, MA, USA, 2009; ISBN 9780123745347. [Google Scholar]
- Horrell, T.; El-Baz, A.; Baruth, J.; Tasman, A.; Sokhadze, G.; Stewart, C.; Sokhadze, E. Neurofeedback Effects on Evoked and Induced EEG Gamma Band Reactivity to Drug-Related Cues in Cocaine Addiction. J. Neurother. 2010, 14, 195–216. [Google Scholar] [CrossRef]
- Nan, W.; Chang, L.; Rodrigues, J.P.; Wan, F.; Mak, P.U.; Mak, P.I.; Vai, M.I.; Rosa, A. Neurofeedback for the Treatment of Schizophrenia: Case Study. In Proceedings of the Proceedings of IEEE International Conference on Virtual Environments, Human-Computer Interfaces, and Measurement Systems, VECIMS, Tianjin, China, 2–4 July 2012; Volume 139, pp. 78–81. [Google Scholar]
- McCarthy-Jones, S. Taking Back the Brain: Could Neurofeedback Training Be Effective for Relieving Distressing Auditory Verbal Hallucinations in Patients with Schizophrenia? Schizophr. Bull. 2012, 38, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Surmeli, T.; Ertem, A.; Eralp, E.; Kos, I.H. Schizophrenia and the Efficacy of qEEG-Guided Neurofeedback Treatment: A Clinical Case Series. Clin. EEG Neurosci. 2012, 43, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Hammer, B.U.; Colbert, A.P.; Brown, K.A.; Ilioi, E.C. Neurofeedback for Insomnia: A Pilot Study of Z-Score SMR and Individualized Protocols. Appl. Psychophysiol. Biofeedback 2011, 36, 251–264. [Google Scholar] [CrossRef]
- Gevensleben, H.; Rothenberger, A.; Moll, G.H.; Heinrich, H. Neurofeedback in children with ADHD: Validation and challenges. Expert Rev. Neurother. 2012, 12, 447–460. [Google Scholar] [CrossRef]
- Azizi, A.; Drikvand, F.M.; Sepahvani, M.A. Comparison of the Effect of Cognitive Rehabilitation and Neurofeedback on Sustained Attention Among Elementary School Students With Specific Learning Disorder: A Preliminary Randomized Controlled Clinical Trial. Basic Clin. Neurosci. J. 2020, 11, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Haghshenas, S.; Rostami, R. Neurofeedback and autism spectrum: A case study. Procedia-Soc. Behav. Sci. 2011, 30, 1472–1475. [Google Scholar] [CrossRef] [Green Version]
- Breteler, M.H.M.; Arns, M.; Peters, S.; Giepmans, I.; Verhoeven, L. Improvements in Spelling after QEEG-Based Neurofeedback in Dyslexia: A Randomized Controlled Treatment Study. Appl. Psychophysiol. Biofeedback 2010, 35, 5–11. [Google Scholar] [CrossRef]
- Becerra, J.; Fernández, T.; Harmony, T.; Caballero, M.; Garcia, F.; Fernández-Bouzas, A.; Santiago-Rodriguez, E.; Prado-Alcalá, R. Follow-up study of learning-disabled children treated with neurofeedback or placebo. Clin. EEG Neurosci. 2006, 37, 198–203. [Google Scholar] [CrossRef]
- Fernández, T.; Harmony, T.; Fernández-Bouzas, A.; Díaz-Comas, L.; Prado-Alcalá, R.A.; Valdés-Sosa, P.; Otero, G.; Bosch, J.; Galán, L.; Santiago-Rodriguez, E.; et al. Changes in EEG Current Sources Induced by Neurofeedback in Learning Disabled Children. An Exploratory Study. Appl. Psychophysiol. Biofeedback 2007, 32, 169–183. [Google Scholar] [CrossRef]
- Haufler, A.J.; Spalding, T.W.; Maria, D.S.; Hatfield, B.D. Neuro-cognitive activity during a self-paced visuospatial task: Comparative EEG profiles in marksmen and novice shooters. Biol. Psychol. 2000, 53, 131–160. [Google Scholar] [CrossRef]
- Markovska-Simoska, S.; Pop-Jordanova, N.; Georgiev, D. Simultaneous EEG and EMG biofeedback for peak performance in musicians. Prilozi 2008, 29, 239–252. [Google Scholar]
- Moradi, A.; Pouladi, F.; Pishva, N.; Rezaei, B.; Torshabi, M.; Alam Mehrjerdi, Z. Treatment of Anxiety Disorder with Neurofeedback: Case Study. Procedia-Soc. Behav. Sci. 2011, 30, 103–107. [Google Scholar] [CrossRef]
- Plotkin, W.B.; Rice, K.M. Biofeedback as a placebo: Anxiety reduction facilitated by training in either suppression or enhancement of alpha brainwaves. J. Consult. Clin. Psychol. 1981, 49, 590–596. [Google Scholar] [CrossRef]
- Thibault, R.T.; Lifshitz, M.; Raz, A. The self-regulating brain and neurofeedback: Experimental science and clinical promise. Cortex 2016, 74, 247–261. [Google Scholar] [CrossRef]
- Thibault, R.T.; Lifshitz, M.; Birbaumer, N.; Raz, A. Neurofeedback, Self-Regulation, and Brain Imaging: Clinical Science and Fad in the Service of Mental Disorders. Psychother. Psychosom. 2015, 84, 193–207. [Google Scholar] [CrossRef]
- Thibault, R.T.; Raz, A. The psychology of neurofeedback: Clinical intervention even if applied placebo. Am. Psychol. 2017, 72, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.E.; Norman, C.A. The Neurophysiology of Dyslexia: A Selective Review with Implications for Neurofeedback Remediation and Results of Treatment in Twelve Consecutive Patients. J. Neurother. 2008, 10, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Pugh, K.R.; Sandak, R.; Frost, S.J.; Moore, D.; Mencl, W.E. Examining Reading Development and Reading Disability in English Language Learners: Potential Contributions from Functional Neuroimaging. Learn. Disabil. Res. Pract. 2005, 20, 24–30. [Google Scholar] [CrossRef]
- Barquero, L.A.; Davis, N.; Cutting, L.E. Neuroimaging of Reading Intervention: A Systematic Review and Activation Likelihood Estimate Meta-Analysis. PLoS ONE 2014, 9, e83668. [Google Scholar] [CrossRef]
- Klingberg, T.; Hedehus, M.; Temple, E.; Salz, T.; Gabrieli, J.D.; Moseley, M.E.; Poldrack, R.A. Microstructure of Temporo-Parietal White Matter as a Basis for Reading Ability: Evidence from Diffusion Tensor Magnetic Resonance Imaging. Neuron 2000, 25, 493–500. [Google Scholar] [CrossRef]
- Papagiannopoulou, E.A.; Lagopoulos, J. Resting State EEG Hemispheric Power Asymmetry in Children with Dyslexia. Front. Pediatr. 2016, 4, 11. [Google Scholar] [CrossRef]
- Nazari, M.A.; Mosanezhad, E.; Hashemi, T.; Jahan, A. The Effectiveness of Neurofeedback Training on EEG Coherence and Neuropsychological Functions in Children With Reading Disability. Clin. EEG Neurosci. 2012, 43, 315–322. [Google Scholar] [CrossRef]
- Rippon, G.; Brunswick, N. Trait and state EEG indices of information processing in developmental dyslexia. Int. J. Psychophysiol. 2000, 36, 251–265. [Google Scholar] [CrossRef]
- Palsson, O.; Pope, A. Morphing beyond Recognition: The Future of Biofeedback Technologies. Biofeedback 2002, 30, 14–18. [Google Scholar]
- López-Villalobos, J.A.; Andrés-De Llano, J.; López-Sánchez, M.V.; Rodríguez-Molinero, L.; Garrido-Redondo, M.; Sacristán-Martín, A.M.; Martínez-Rivera, M.T.; Alberola-López, S. Criterion validity and clinical usefulness of Attention Deficit Hyperactivity Disorder Rating Scale IV in attention deficit hyperactivity disorder (ADHD) as a function of method and age. Psicothema 2017, 29, 103–110. [Google Scholar] [CrossRef]
- Rubia, K. Functional brain imaging across development. Eur. Child Adolesc. Psychiatry 2013, 22, 719–731. [Google Scholar] [CrossRef]
- Thomas, R.; Sanders, S.; Doust, J.; Beller, E.; Glasziou, P. Prevalence of Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-analysis. Pediatrics 2015, 135, e994–e1001. [Google Scholar] [CrossRef]
- Norman, L.J.; Carlisi, C.; Lukito, S.; Hart, H.; Mataix-Cols, D.; Radua, J.; Rubia, K. Structural and Functional Brain Abnormalities in Attention-Deficit/Hyperactivity Disorder and Obsessive-Compulsive Disorder: A Comparative Meta-Analysis. JAMA Psychiatry 2016, 73, 815–825. [Google Scholar] [CrossRef]
- Shaw, P.; Malek, M.; Watson, B.; Sharp, W.; Evans, A.; Greenstein, D. Development of Cortical Surface Area and Gyrification in Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry 2012, 72, 191–197. [Google Scholar] [CrossRef]
- Lubar, J.F. Neurofeedback for the Management of Attention Deficit Disorder. In Biofeedback: A Practitioner’s Guide; The Guilford Press: New York, NY, USA, 2003; pp. 409–437. [Google Scholar]
- Nooner, K.B.; Leaberry, K.D.; Keith, J.R.; Ogle, R.L. Clinic Outcome Assessment of a Brief Course Neurofeedback for Childhood ADHD Symptoms. J. Behav. Health Adm. 2017, 44, 506–514. [Google Scholar] [CrossRef]
- Sterman, M.B. Physiological origins and functional correlates of EEG rhythmic activities: Implications for self-regulation. Biofeedback Self-Regul. 1996, 21, 3–33. [Google Scholar] [CrossRef]
- Lubar, J.F. Discourse on the development of EEG diagnostics and biofeedback for attention-deficit/hyperactivity disorders. Biofeedback Self-Regul. 1991, 16, 201–225. [Google Scholar] [CrossRef]
- Monastra, V.J.; Lubar, J.F.; Linden, M.; VanDeusen, P.; Green, G.; Wing, W.; Phillips, A.; Fenger, T.N. Assessing attention deficit hyperactivity disorder via quantitative electroencephalography: An initial validation study. Neuropsychology 1999, 13, 424–433. [Google Scholar] [CrossRef]
- El-Sayed, E.; Larsson, J.-O.; Persson, H.E.; Rydelius, P.-A. Altered Cortical Activity in Children With Attention-Deficit/Hyperactivity Disorder During Attentional Load Task. J. Am. Acad. Child Adolesc. Psychiatry 2002, 41, 811–819. [Google Scholar] [CrossRef]
- Holtmann, M.; Sonuga-Barke, E.; Cortese, S.; Brandeis, D. Neurofeedback for ADHD: A Review of Current Evidence. Child Adolesc. Psychiatr. Clin. N. Am. 2014, 23, 789–806. [Google Scholar] [CrossRef]
- Bakhshayesh, A.R.; Hänsch, S.; Wyschkon, A.; Rezai, M.J.; Esser, G. Neurofeedback in ADHD: A single-blind randomized controlled trial. Eur. Child Adolesc. Psychiatry 2011, 20, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Van Doren, J.; Heinrich, H.; Bezold, M.; Reuter, N.; Kratz, O.; Horndasch, S.; Berking, M.; Ros, T.; Gevensleben, H.; Moll, G.H.; et al. Theta/beta neurofeedback in children with ADHD: Feasibility of a short-term setting and plasticity effects. Int. J. Psychophysiol. 2017, 112, 80–88. [Google Scholar] [CrossRef]
- Janssen, T.W.P.; Bink, M.; Weeda, W.D.; Geladé, K.; Van Mourik, R.; Maras, A.; Oosterlaan, J. Learning curves of theta/beta neurofeedback in children with ADHD. Eur. Child Adolesc. Psychiatry 2016, 26, 573–582. [Google Scholar] [CrossRef] [Green Version]
- Escolano, C.; Navarro-Gil, M.; Garcia-Campayo, J.; Congedo, M.; Minguez, J. The Effects of Individual Upper Alpha Neurofeedback in ADHD: An Open-Label Pilot Study. Appl. Psychophysiol. Biofeedback 2014, 39, 193–202. [Google Scholar] [CrossRef]
- Rajabi, S.; Pakize, A.; Moradi, N. Effect of combined neurofeedback and game-based cognitive training on the treatment of ADHD: A randomized controlled study. Appl. Neuropsychol. Child 2019, 9, 193–205. [Google Scholar] [CrossRef]
- Christiansen, H.; Reh, V.; Schmidt, M.H.; Rief, W. Slow cortical potential neurofeedback and self-management training in outpatient care for children with ADHD: Study protocol and first preliminary results of a randomized controlled trial. Front. Hum. Neurosci. 2014, 8, 943. [Google Scholar] [CrossRef]
- Okumura, Y.; Kita, Y.; Omori, M.; Suzuki, K.; Yasumura, A.; Fukuda, A.; Inagaki, M. Predictive factors of success in neurofeedback training for children with ADHD. Dev. Neurorehabilit. 2019, 22, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Strehl, U.; Leins, U.; Goth, G.; Klinger, C.; Hinterberger, T.; Birbaumer, N. Self-regulation of Slow Cortical Potentials: A New Treatment for Children With Attention-Deficit/Hyperactivity Disorder. Pediatrics 2006, 118, e1530–e1540. [Google Scholar] [CrossRef]
- Drechsler, R.; Straub, M.; Doehnert, M.; Heinrich, H.; Steinhausen, H.-C.; Brandeis, D. 1Controlled evaluation of a neurofeedback training of slow cortical potentials in children with Attention Deficit/Hyperactivity Disorder (ADHD). Behav. Brain Funct. 2007, 3, 35. [Google Scholar] [CrossRef]
- Leins, U.; Goth, G.; Hinterberger, T.; Klinger, C.; Rumpf, N.; Strehl, U. Neurofeedback for Children with ADHD: A Comparison of SCP and Theta/Beta Protocols. Appl. Psychophysiol. Biofeedback 2007, 32, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Debeus, R.J.; Kaiser, D.A. Neurofeedback with Children with Attention Deficit Hyperactivity Disorder. A Randomized Double-Blind Placebo-Controlled Study; Elsevier Inc.: Amsterdam, The Netherlands, 2011; ISBN 9780123822352. [Google Scholar] [CrossRef]
- Gevensleben, H.; Moll, G.H.; Rothenberger, A.; Heinrich, H. Neurofeedback in attention-deficit/hyperactivity disorder—Different models, different ways of application. Front. Hum. Neurosci. 2014, 8, 846. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.; Yasumura, A.; Nakagawa, E.; Inagaki, M. Changes in Negative and Positive EEG Shifts during Slow Cortical Potential Training in Children with Attention-Deficit/Hyperactivity Disorder: A Preliminary Investigation. Neuroreport 2014, 25, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Monastra, V. Electroencephalographic biofeedback (neurotherapy) as a treatment for attention deficit hyperactivity disorder: Rationale and empirical foundation. Child Adolesc. Psychiatr. Clin. N. Am. 2005, 14, 55–82. [Google Scholar] [CrossRef]
- Albrecht, B.; Brandeis, D.; Uebel, H.; Valko, L.; Heinrich, H.; Drechsler, R.; Heise, A.; Müller, U.C.; Steinhausen, H.-C.; Rothenberger, A.; et al. Familiality of neural preparation and response control in childhood attention deficit-hyperactivity disorder. Psychol. Med. 2012, 43, 1997–2011. [Google Scholar] [CrossRef]
- Heinrich, H.; Gevensleben, H.; Strehl, U. Annotation: Neurofeedback-train your brain to train behaviour. J. Child Psychol. Psychiatry 2007, 48, 3–16. [Google Scholar] [CrossRef]
- Arns, M.; Heinrich, H.; Strehl, U. Evaluation of neurofeedback in ADHD: The long and winding road. Biol. Psychol. 2014, 95, 108–115. [Google Scholar] [CrossRef]
- Cortese, S.; Ferrin, M.; Brandeis, D.; Holtmann, M.; Aggensteiner, P.; Daley, D.; Santosh, P.; Simonoff, E.; Stevenson, J.; Stringaris, A.; et al. Neurofeedback for Attention-Deficit/Hyperactivity Disorder: Meta-Analysis of Clinical and Neuropsychological Outcomes From Randomized Controlled Trials. J. Am. Acad. Child Adolesc. Psychiatry 2016, 55, 444–455. [Google Scholar] [CrossRef]
- Micoulaud-Franchi, J.-A.; Salvo, F.; Bioulac, S.; Fovet, T. Neurofeedback in Attention-Deficit/Hyperactivity Disorder: Efficacy. J. Am. Acad. Child Adolesc. Psychiatry 2016, 55, 1091–1092. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; (DSM-5); American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Shalev, R.S.; Manor, O.; Gross-Tsur, V. Developmental dyscalculia: A prospective six-year follow-up. Dev. Med. Child Neurol. 2005, 47, 121–125. [Google Scholar] [CrossRef]
- Shalev, R.S.; Auerbach, J.; Manor, O.; Gross-Tsur, V. Developmental dyscalculia: Prevalence and prognosis. Eur. Child Adolesc. Psychiatry 2000, 9, S58–S64. [Google Scholar] [CrossRef]
- Menon, V.; Rivera, S.; White, C.; Glover, G.; Reiss, A. Dissociating Prefrontal and Parietal Cortex Activation during Arithmetic Processing. NeuroImage 2000, 12, 357–365. [Google Scholar] [CrossRef]
- Schmithorst, V.J.; Brown, R. Empirical validation of the triple-code model of numerical processing for complex math operations using functional MRI and group Independent Component Analysis of the mental addition and subtraction of fractions. NeuroImage 2004, 22, 1414–1420. [Google Scholar] [CrossRef]
- Levy, L.M.; Reis, I.L.; Grafman, J. Metabolic abnormalities detected by 1H-MRS in dyscalculia and dysgraphia. Neurology 1999, 53, 639. [Google Scholar] [CrossRef]
- Dehaene, S.; Piazza, M.; Pinel, P.; Cohen, L. Three parietal circuits for number processing. Cogn. Neuropsychol. 2003, 20, 487–506. [Google Scholar] [CrossRef]
- Van Hartingsveldt, M.J.; De Groot, I.J.M.; Aarts, P.B.M.; Der Sanden, M.W.G.N.-V. Standardized tests of handwriting readiness: A systematic review of the literature. Dev. Med. Child Neurol. 2011, 53, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Richards, T.L.; Berninger, V.W.; Stock, P.; Altemeier, L.; Trivedi, P.; Maravilla, K. Functional magnetic resonance imaging sequential-finger movement activation differentiating good and poor writers. J. Clin. Exp. Neuropsychol. 2009, 31, 967–983. [Google Scholar] [CrossRef]
- Fernández, T.; Bosch-Bayard, J.; Harmony, T.; Caballero, M.I.; Díaz-Comas, L.; Galán, L.; Ricardo-Garcell, J.; Aubert, E.; Otero-Ojeda, G. Neurofeedback in Learning Disabled Children: Visual versus Auditory Reinforcement. Appl. Psychophysiol. Biofeedback 2015, 41, 27–37. [Google Scholar] [CrossRef]
- Jacobs, E.H. Neurofeedback Treatment of Two Children with Learning, Attention, Mood, Social, and Developmental Deficits. J. Neurother. 2006, 9, 55–70. [Google Scholar] [CrossRef]
- Thornton, K.E.; Carmody, D.P. Electroencephalogram biofeedback for reading disability and traumatic brain injury. Child Adolesc. Psychiatr. Clin. N. Am. 2005, 14, 137–162. [Google Scholar] [CrossRef]
- Sterman, M.B.; Howe, R.C.; Macdonald, L.R. Facilitation of Spindle-Burst Sleep by Conditioning of Electroencephalographic Activity While Awake. Science 1970, 167, 1146–1148. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Stancák, A.; Neuper, C. Event-related synchronization (ERS) in the alpha band—An electrophysiological correlate of cortical idling: A review. Int. J. Psychophysiol. 1996, 24, 39–46. [Google Scholar] [CrossRef]
- Huang, H.W.; King, J.T.; Lee, C.L. The New Science of Learning: Using the Power and Potential of the Brain to Inform Digital Learning. Educ. Technol. Soc. 2020, 23, 1–13. [Google Scholar]
- Gaume, A.; Vialatte, A.; Mora-Sánchez, A.; Ramdani, C.; Vialatte, F. A psychoengineering paradigm for the neurocognitive mechanisms of biofeedback and neurofeedback. Neurosci. Biobehav. Rev. 2016, 68, 891–910. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.L. Application of Motor Learning Principles in Occupational Therapy. Am. J. Occup. Ther. 1991, 45, 531–537. [Google Scholar] [CrossRef]
- Lansbergen, M.M.; Arns, M.; van Dongen-Boomsma, M.; Spronk, D.; Buitelaar, J.K. The increase in theta/beta ratio on resting-state EEG in boys with attention-deficit/hyperactivity disorder is mediated by slow alpha peak frequency. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 47–52. [Google Scholar] [CrossRef]
- Dong, M.Y.; Sandberg, K.; Bibby, B.M.; Pedersen, M.N.; Overgaard, M. The development of a sense of control scale. Front. Psychol. 2015, 6, 1733. [Google Scholar] [CrossRef]
- Reichert, J.L.; Kober, S.E.; Neuper, C.; Wood, G. Resting-state sensorimotor rhythm (SMR) power predicts the ability to up-regulate SMR in an EEG-instrumental conditioning paradigm. Clin. Neurophysiol. 2015, 126, 2068–2077. [Google Scholar] [CrossRef]
- Sonuga-Barke, E.J.; Brandeis, D.; Cortese, S.; Daley, D.; Ferrin, M.; Holtmann, M.; Stevenson, J.; Danckaerts, M.; Van Der Oord, S.; Döpfner, M.; et al. Nonpharmacological Interventions for ADHD: Systematic Review and Meta-Analyses of Randomized Controlled Trials of Dietary and Psychological Treatments. Am. J. Psychiatry 2013, 170, 275–289. [Google Scholar] [CrossRef]
- Van Doren, J.; Arns, M.; Heinrich, H.; Vollebregt, M.A.; Strehl, U.; Loo, S.K. Sustained effects of neurofeedback in ADHD: A systematic review and meta-analysis. Eur. Child Adolesc. Psychiatry 2018, 28, 293–305. [Google Scholar] [CrossRef]
- Razoki, B. Neurofeedback versus psychostimulants in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: A systematic review. Neuropsychiatr. Dis. Treat. 2018, 14, 2905–2913. [Google Scholar] [CrossRef]
- Duric, N.S.; Assmus, J.; Gundersen, D.; Golos, A.D.; Elgen, I.B. Multimodal treatment in children and adolescents with attention-deficit/hyperactivity disorder: A 6-month follow-up. Nord. J. Psychiatry 2017, 71, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Monastra, V.J.; Monastra, D.M.; George, S. The Effects of Stimulant Therapy, EEG Biofeedback, and Parenting Style on the Primary Symptoms of Attention-Deficit/Hyperactivity Disorder. Appl. Psychophysiol. Biofeedback 2002, 27, 231–249. [Google Scholar] [CrossRef] [PubMed]
- Geladé, K.; Janssen, T.W.P.; Bink, M.; Van Mourik, R.; Maras, A.; Oosterlaan, J. Behavioral Effects of Neurofeedback Compared to Stimulants and Physical Activity in Attention-Deficit/Hyperactivity Disorder: A Randomized Controlled Trial. J. Clin. Psychiatry 2016, 77, e1270–e1277. [Google Scholar] [CrossRef]
- Krepel, N.; Egtberts, T.; Sack, A.T.; Heinrich, H.; Ryan, M.; Arns, M. A multicenter effectiveness trial of QEEG-informed neurofeedback in ADHD: Replication and treatment prediction. NeuroImage Clin. 2020, 28, 102399. [Google Scholar] [CrossRef]
- Steiner, N.J.; Frenette, E.C.; Rene, K.M.; Brennan, R.T.; Perrin, E.C. In-School Neurofeedback Training for ADHD: Sustained Improvements From a Randomized Control Trial. Pediatrics 2014, 133, 483–492. [Google Scholar] [CrossRef]
- Berger, A.M.; Davelaar, E.J. Frontal Alpha Oscillations and Attentional Control: A Virtual Reality Neurofeedback Study. Neuroscience 2018, 378, 189–197. [Google Scholar] [CrossRef]
- Putman, J. The Effects of Brief, Eyes-Open Alpha Brain Wave Training with Audio and Video Relaxation Induction on the EEG of 77 Army Reservists. J. Neurother. 2000, 4, 17–28. [Google Scholar] [CrossRef]
- Angelakis, E.; Stathopoulou, S.; Frymiare, J.L.; Green, D.L.; Lubar, J.F.; Kounios, J. EEG Neurofeedback: A Brief Overview and an Example of Peak Alpha Frequency Training for Cognitive Enhancement in the Elderly. Clin. Neuropsychol. 2007, 21, 110–129. [Google Scholar] [CrossRef]
- Zoefel, B.; Huster, R.J.; Herrmann, C.S. Neurofeedback training of the upper alpha frequency band in EEG improves cognitive performance. NeuroImage 2011, 54, 1427–1431. [Google Scholar] [CrossRef]
- Subramaniam, K.; Vinogradov, S. Improving the neural mechanisms of cognition through the pursuit of happiness. Front. Hum. Neurosci. 2013, 7, 452. [Google Scholar] [CrossRef]
- Kleih, S.C.; Kübler, A. Empathy, motivation, and P300 BCI performance. Front. Hum. Neurosci. 2013, 7, 642. [Google Scholar] [CrossRef] [PubMed]
- Schabus, M.; Griessenberger, H.; Gnjezda, M.-T.; Heib, D.P.J.; Wislowska, M.; Hoedlmoser, K. Better than sham? A double-blind placebo-controlled neurofeedback study in primary insomnia. Brain 2017, 140, 1041–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| EEg Waves | Frequency Range | Band Characteristics | ||
|---|---|---|---|---|
| Delta | 0.5–4 Hz | Unconsciousness, deep sleep, complex problem solving | ||
| Theta | 4–8 Hz | Anxiety, creativity, depression | ||
| Alpha | Lower | 8–10 Hz | Recall | Relaxation, alertness |
| Upper | 10–12 Hz | Cognition tasks | Peacefulness/calmness | |
| Sensorimotor rhythm (SMR) | 12–16 Hz | Relaxation, alertness | ||
| Beta | Lower | 16–20 Hz | Focus, coherent thinking, attention | |
| Upper | 20–30 Hz | Anxiety, attention (focused) | ||
| Gamma | 30–100 Hz | Learning, task solving | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, A.U.; Madathil, D.; Fan, Y.-T.; Tzeng, O.J.L.; Huang, C.-M.; Huang, H.-W. Neurofeedback for the Education of Children with ADHD and Specific Learning Disorders: A Review. Brain Sci. 2022, 12, 1238. https://doi.org/10.3390/brainsci12091238
Patil AU, Madathil D, Fan Y-T, Tzeng OJL, Huang C-M, Huang H-W. Neurofeedback for the Education of Children with ADHD and Specific Learning Disorders: A Review. Brain Sciences. 2022; 12(9):1238. https://doi.org/10.3390/brainsci12091238
Chicago/Turabian StylePatil, Abhishek Uday, Deepa Madathil, Yang-Tang Fan, Ovid J. L. Tzeng, Chih-Mao Huang, and Hsu-Wen Huang. 2022. "Neurofeedback for the Education of Children with ADHD and Specific Learning Disorders: A Review" Brain Sciences 12, no. 9: 1238. https://doi.org/10.3390/brainsci12091238







