Recognition and Processing of Visual Information after Neuronavigated Transcranial Magnetic Stimulation Session
Abstract
:1. Introduction
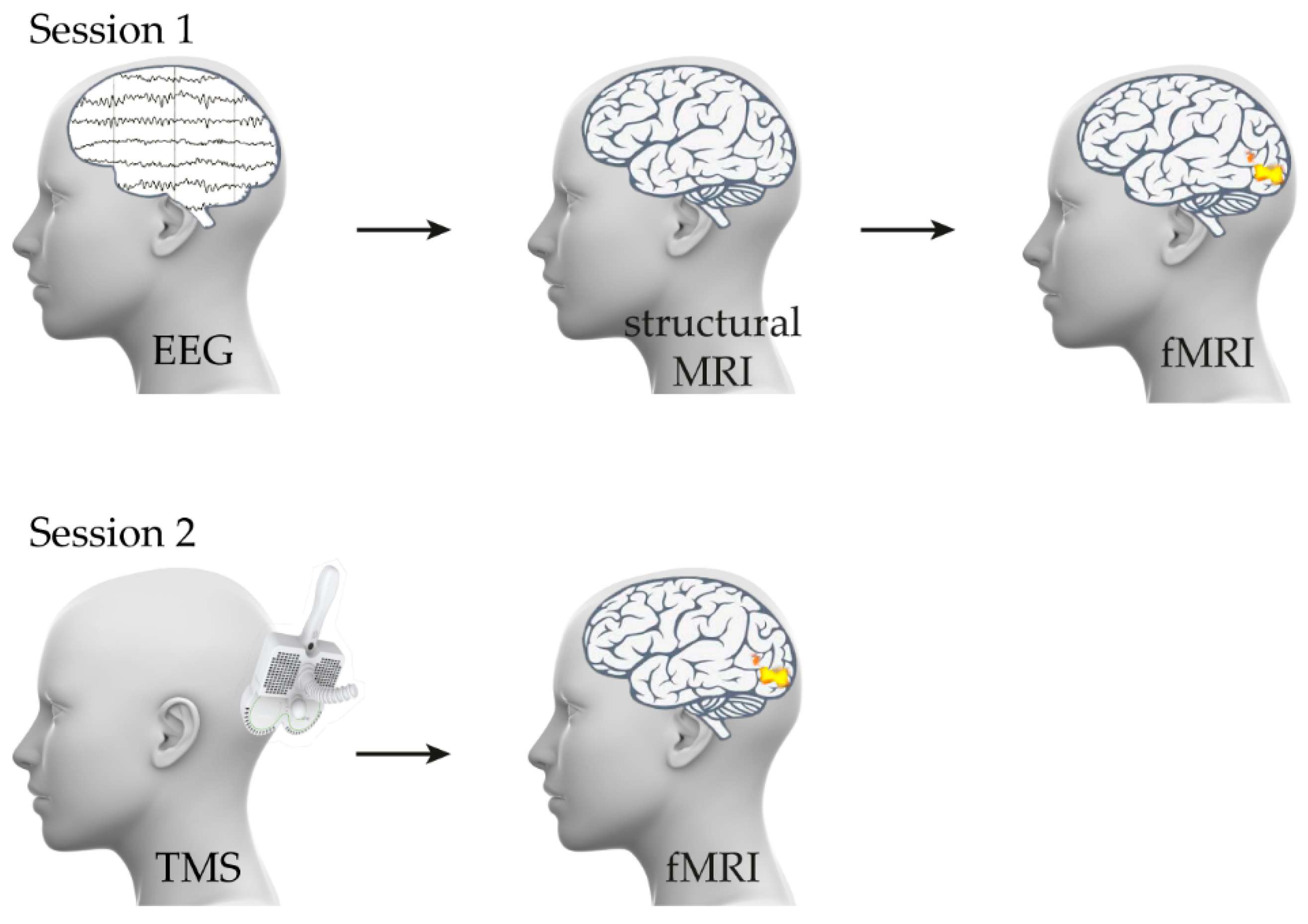
2. Materials and Methods
2.1. Participants and Safety of Research
2.2. fMRI: Task and Stimuli
2.3. TMS Stimulation
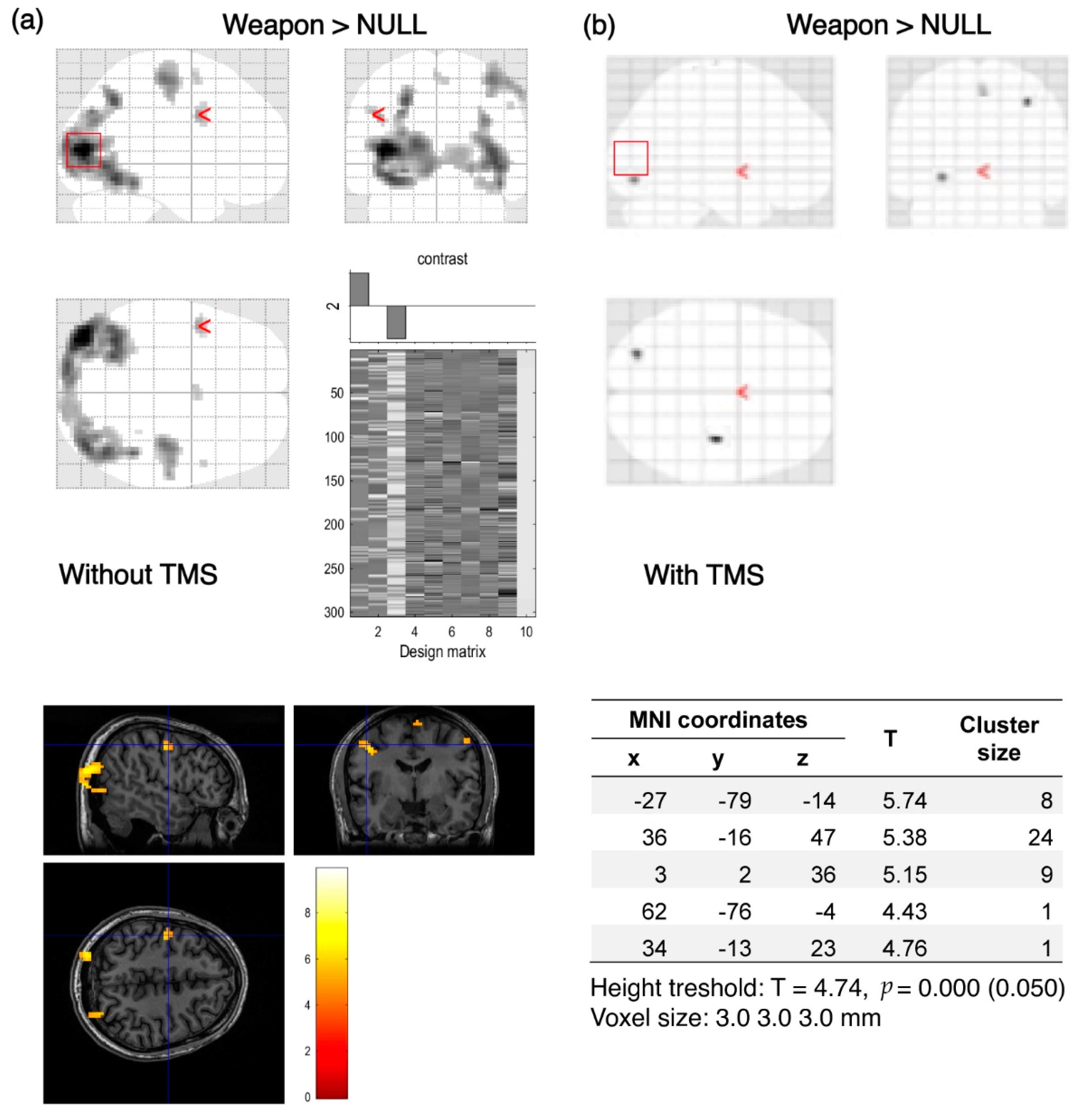
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hallett, M.; di Iorio, R.; Rossini, P.M.; Park, J.E.; Chen, R.; Celnik, P.; Strafella, A.P.; Matsumoto, H.; Ugawa, Y. Contribution of Transcranial Magnetic Stimulation to Assessment of Brain Connectivity and Networks. Clin. Neurophysiol. 2017, 128, 2125–2139. [Google Scholar] [CrossRef] [PubMed]
- Kozel, F.A.; Motes, M.A.; Didehbani, N.; DeLaRosa, B.; Bass, C.; Schraufnagel, C.D.; Jones, P.; Morgan, C.R.; Spence, J.S.; Kraut, M.A.; et al. Repetitive TMS to Augment Cognitive Processing Therapy in Combat Veterans of Recent Conflicts with PTSD: A Randomized Clinical Trial. J Affect. Disord. 2018, 229, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Vucic, S.; Ziemann, U.; Eisen, A.; Hallett, M.; Kiernan, M.C. Transcranial Magnetic Stimulation and Amyotrophic Lateral Sclerosis: Pathophysiological Insights. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Annavarapu, R.N.; Kathi, S.; Vadla, V.K. Non-Invasive Imaging Modalities to Study Neurodegenerative Diseases of Aging Brain. J. Chem. Neuroanat. 2019, 95, 54–69. [Google Scholar] [CrossRef]
- Rossini, P.M.; Barker, A.T.; Berardelli, A.; Caramia, M.D.; Caruso, G.; Cracco, R.Q.; Dimitrijević, M.R.; Hallett, M.; Katayama, Y.; Lücking, C.H.; et al. Non-Invasive Electrical and Magnetic Stimulation of the Brain, Spinal Cord and Roots: Basic Principles and Procedures for Routine Clinical Application. Report of an IFCN Committee. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 79–92. [Google Scholar] [CrossRef]
- O’Reardon, J.P.; Solvason, H.B.; Janicak, P.G.; Sampson, S.; Isenberg, K.E.; Nahas, Z.; McDonald, W.M.; Avery, D.; Fitzgerald, P.B.; Loo, C.; et al. Efficacy and Safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: A Multisite Randomized Controlled Trial. Biol. Psychiatry 2007, 62, 1208–1216. [Google Scholar] [CrossRef]
- Becker, J.E.; Shultz, E.K.B.; Maley, C.T. Transcranial Magnetic Stimulation in Conditions Other than Major Depressive Disorder. Child Adolesc. Psychiatr. Clin. N. Am. 2019, 28, 45–52. [Google Scholar] [CrossRef]
- Barker, A.T.; Shields, K. Transcranial Magnetic Stimulation: Basic Principles and Clinical Applications in Migraine. Headache J. Head Face Pain 2017, 57, 517–524. [Google Scholar] [CrossRef]
- Burke, M.J.; Fried, P.J.; Pascual-Leone, A. Transcranial Magnetic Stimulation: Neurophysiological and Clinical Applications. Handb. Clin. Neurol. 2019, 163, 73–92. [Google Scholar] [CrossRef]
- Iriarte, I.G.; George, M.S. Transcranial Magnetic Stimulation (TMS) in the Elderly. Curr. Psychiatry Rep. 2018, 20, 6. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-Based Guidelines on the Therapeutic Use of Repetitive Transcranial Magnetic Stimulation (RTMS): An Update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.C.; Stinear, C.M. Transcranial Magnetic Stimulation (TMS) in Stroke: Ready for Clinical Practice? J. Clin. Neurosci. 2016, 31, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Zyss, T.; Rachel, W.; Datka, W.; Dudek, D.; Zięba, A.; Gorczyca, P.; Hese, R.T.; Szwajca, K.; Piekoszewski, W. Transcranial Magnetic Stimulation in Psychiatric Therapy. Przegl. Lek. 2015, 72, 371–375. [Google Scholar] [PubMed]
- Fierro, B.; Brighina, F.; Vitello, G.; Piazza, A.; Scalia, S.; Giglia, G.; Daniele, O.; Pascual-Leone, A. Modulatory Effects of Low- and High-Frequency Repetitive Transcranial Magnetic Stimulation on Visual Cortex of Healthy Subjects Undergoing Light Deprivation. J. Physiol. 2005, 565, 659–665. [Google Scholar] [CrossRef]
- Pell, G.S.; Roth, Y.; Zangen, A. Modulation of Cortical Excitability Induced by Repetitive Transcranial Magnetic Stimulation: Influence of Timing and Geometrical Parameters and Underlying Mechanisms. Prog. Neurobiol. 2011, 93, 59–98. [Google Scholar] [CrossRef]
- Laycock, R.; Crewther, D.P.; Fitzgerald, P.B.; Crewther, S.G. Evidence for Fast Signals and Later Processing in Human V1/V2 and V5/MT+: A TMS Study of Motion Perception. J. Neurophysiol. 2007, 98, 1253–1262. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Xie, C.-M.; Zhang, M.; Shi, Y.; Song, R.; Lu, X.; Zhang, H.; Li, K.; Wang, B.; et al. Task-Related Functional Magnetic Resonance Imaging-Based Neuronavigation for the Treatment of Depression by Individualized Repetitive Transcranial Magnetic Stimulation of the Visual Cortex. Sci. China Life Sci. 2021, 64, 96–106. [Google Scholar] [CrossRef]
- Valero-Cabré, A.; Amengual, J.L.; Stengel, C.; Pascual-Leone, A.; Coubard, O.A. Transcranial Magnetic Stimulation in Basic and Clinical Neuroscience: A Comprehensive Review of Fundamental Principles and Novel Insights. Neurosci. Biobehav. Rev. 2017, 83, 381–404. [Google Scholar] [CrossRef]
- Silvanto, J.; Cattaneo, Z. Transcranial Magnetic Stimulation Reveals the Content of Visual Short-Term Memory in the Visual Cortex. Neuroimage 2010, 50, 1683. [Google Scholar] [CrossRef]
- Peters, M.A.K.; Fesi, J.; Amendi, N.; Knotts, J.D.; Lau, H.; Ro, T. Transcranial Magnetic Stimulation to Visual Cortex Induces Suboptimal Introspection. Cortex 2017, 93, 119–132. [Google Scholar] [CrossRef]
- Koivisto, M.; Leino, K.; Pekkarinen, A.; Karttunen, J.; Railo, H.; Hurme, M. Transcranial Magnetic Stimulation (TMS)-Induced Blindsight of Orientation Is Degraded Conscious Vision. Neuroscience 2021, 475, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Laycock, R.; Crewther, D.P.; Fitzgerald, P.B.; Crewther, S.G. TMS Disruption of V5/MT+ Indicates a Role for the Dorsal Stream in Word Recognition. Exp. Brain Res. 2009, 197, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A.; Avanzini, G.; Bestmann, S.; Berardelli, A.; Brewer, C.; Canli, T.; Cantello, R.; et al. Safety, Ethical Considerations, and Application Guidelines for the Use of Transcranial Magnetic Stimulation in Clinical Practice and Research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef]
- Borckardt, J.J.; Nahas, Z.; Koola, J.; George, M.S. Estimating Resting Motor Thresholds in Transcranial Magnetic Stimulation Research and Practice: A Computer Simulation Evaluation of Best Methods. J. ECT 2006, 22, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.J.; Lauer, A.; Lench, D.H.; Hanlon, C.A. Visual Attention Affects the Amplitude of the Transcranial Magnetic Stimulation-Associated Motor-Evoked Potential: A Preliminary Study with Clinical Utility. J. Psychiatr. Pract. 2018, 24, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Nikolov, P.; Hassan, S.S.; Schnitzler, A.; Groiss, S.J. Influence of High Pass Filter Settings on Motor Evoked Potentials. Front. Neurosci. 2021, 15, 485. [Google Scholar] [CrossRef]
- Ah Sen, C.B.; Fassett, H.J.; El-Sayes, J.; Turco, C.V.; Hameer, M.M.; Nelson, A.J. Active and Resting Motor Threshold Are Efficiently Obtained with Adaptive Threshold Hunting. PLoS ONE 2017, 12, e0186007. [Google Scholar] [CrossRef]
- Duque, J.; Greenhouse, I.; Labruna, L.; Ivry, R.B. Physiological Markers of Motor Inhibition during Human Behavior. Trends Neurosci. 2017, 40, 219–236. [Google Scholar] [CrossRef]
- Gazdzinski, S.P.; Binder, M.; Bortkiewicz, A.; Baran, P.; Dziuda, Ł. Effects of All-Night Driving on Selective Attention in Professional Truck Drivers: A Preliminary Functional Magnetic Resonance Study. Energies 2021, 14, 5409. [Google Scholar] [CrossRef]
- Rademaker, R.L.; van de Ven, V.G.; Tong, F.; Sack, A.T. The Impact of Early Visual Cortex Transcranial Magnetic Stimulation on Visual Working Memory Precision and Guess Rate. PLoS ONE 2017, 12, e0175230. [Google Scholar] [CrossRef] [Green Version]
- Van Lamsweerde, A.E.; Johnson, J.S. Assessing the Effect of Early Visual Cortex Transcranial Magnetic Stimulation on Working Memory Consolidation. J. Cogn. Neurosci. 2017, 29, 1226–1238. [Google Scholar] [CrossRef] [PubMed]
- Heuer, A.; Schubö, A.; Crawford, J.D. Different Cortical Mechanisms for Spatial vs. Feature-Based Attentional Selection in Visual Working Memory. Front. Hum. Neurosci. 2016, 10, 415. [Google Scholar] [CrossRef] [PubMed]
- Bona, S.; Herbert, A.; Toneatto, C.; Silvanto, J.; Cattaneo, Z. The Causal Role of the Lateral Occipital Complex in Visual Mirror Symmetry Detection and Grouping: An FMRI-Guided TMS Study. Cortex 2014, 51, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Mullin, C.R.; Steeves, J.K.E. TMS to the Lateral Occipital Cortex Disrupts Object Processing but Facilitates Scene Processing. J. Cogn. Neurosci. 2011, 23, 4174–4184. [Google Scholar] [CrossRef]
- Hwang, J.H.; Kim, S.H.; Park, C.S.; Bang, S.A.; Kim, S.E. Acute High-Frequency RTMS of the Left Dorsolateral Prefrontal Cortex and Attentional Control in Healthy Young Men. Brain Res. 2010, 1329, 152–158. [Google Scholar] [CrossRef]
- Maeda, F.; Keenan, J.P.; Tormos, J.M.; Topka, H.; Pascual-Leone, A. Interindividual Variability of the Modulatory Effects of Repetitive Transcranial Magnetic Stimulation on Cortical Excitability. Exp. Brain Res. 2000, 133, 425–430. [Google Scholar] [CrossRef]
- Gamboa, O.L.; Antal, A.; Moliadze, V.; Paulus, W. Simply Longer Is Not Better: Reversal of Theta Burst After-Effect with Prolonged Stimulation. Exp. Brain Res. 2010, 204, 181–187. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasprzycka, W.; Naurecka, M.L.; Sierakowski, B.M.; Putko, P.; Mierczyk, Z.; Chabik, G.; Dec, S.; Gaździński, S.; Rola, R. Recognition and Processing of Visual Information after Neuronavigated Transcranial Magnetic Stimulation Session. Brain Sci. 2022, 12, 1241. https://doi.org/10.3390/brainsci12091241
Kasprzycka W, Naurecka ML, Sierakowski BM, Putko P, Mierczyk Z, Chabik G, Dec S, Gaździński S, Rola R. Recognition and Processing of Visual Information after Neuronavigated Transcranial Magnetic Stimulation Session. Brain Sciences. 2022; 12(9):1241. https://doi.org/10.3390/brainsci12091241
Chicago/Turabian StyleKasprzycka, Wiktoria, Magdalena Ligia Naurecka, Bartosz Michał Sierakowski, Paulina Putko, Zygmunt Mierczyk, Grzegorz Chabik, Stanisław Dec, Stefan Gaździński, and Rafał Rola. 2022. "Recognition and Processing of Visual Information after Neuronavigated Transcranial Magnetic Stimulation Session" Brain Sciences 12, no. 9: 1241. https://doi.org/10.3390/brainsci12091241
APA StyleKasprzycka, W., Naurecka, M. L., Sierakowski, B. M., Putko, P., Mierczyk, Z., Chabik, G., Dec, S., Gaździński, S., & Rola, R. (2022). Recognition and Processing of Visual Information after Neuronavigated Transcranial Magnetic Stimulation Session. Brain Sciences, 12(9), 1241. https://doi.org/10.3390/brainsci12091241






