Giant Non-Functioning Pituitary Adenomas: Treatment Considerations
Abstract
1. Introduction
2. Clinical Features
3. Goals of Surgery
- Maximal safe tumor removal to grant the relief of mass effect signs;
- preservation of normal neurologic functions;
- decompression of the pituitary gland to improve or preserve the residual hormonal function.
4. Surgical Techniques
4.1. Transsphenoidal Approaches
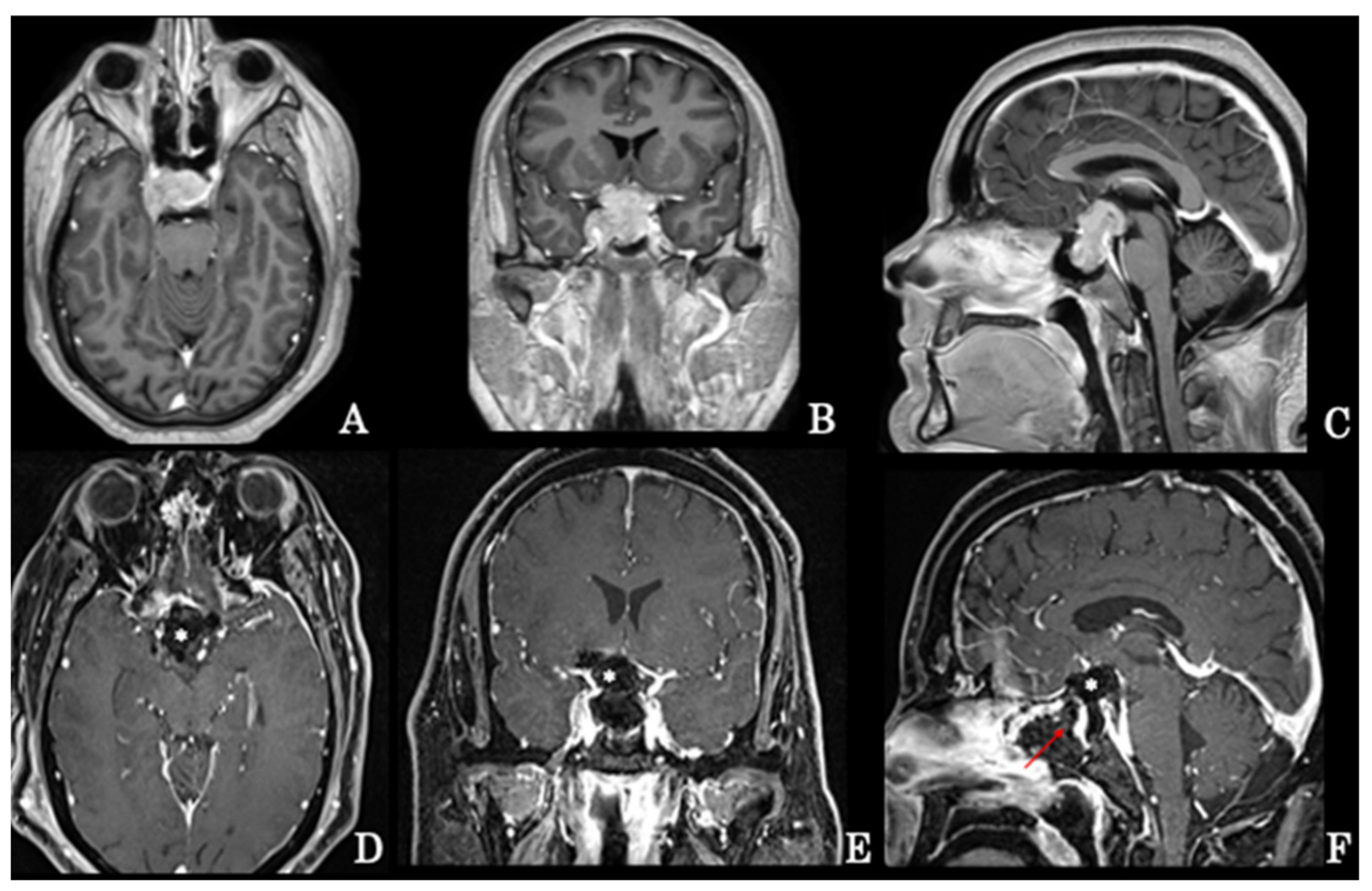
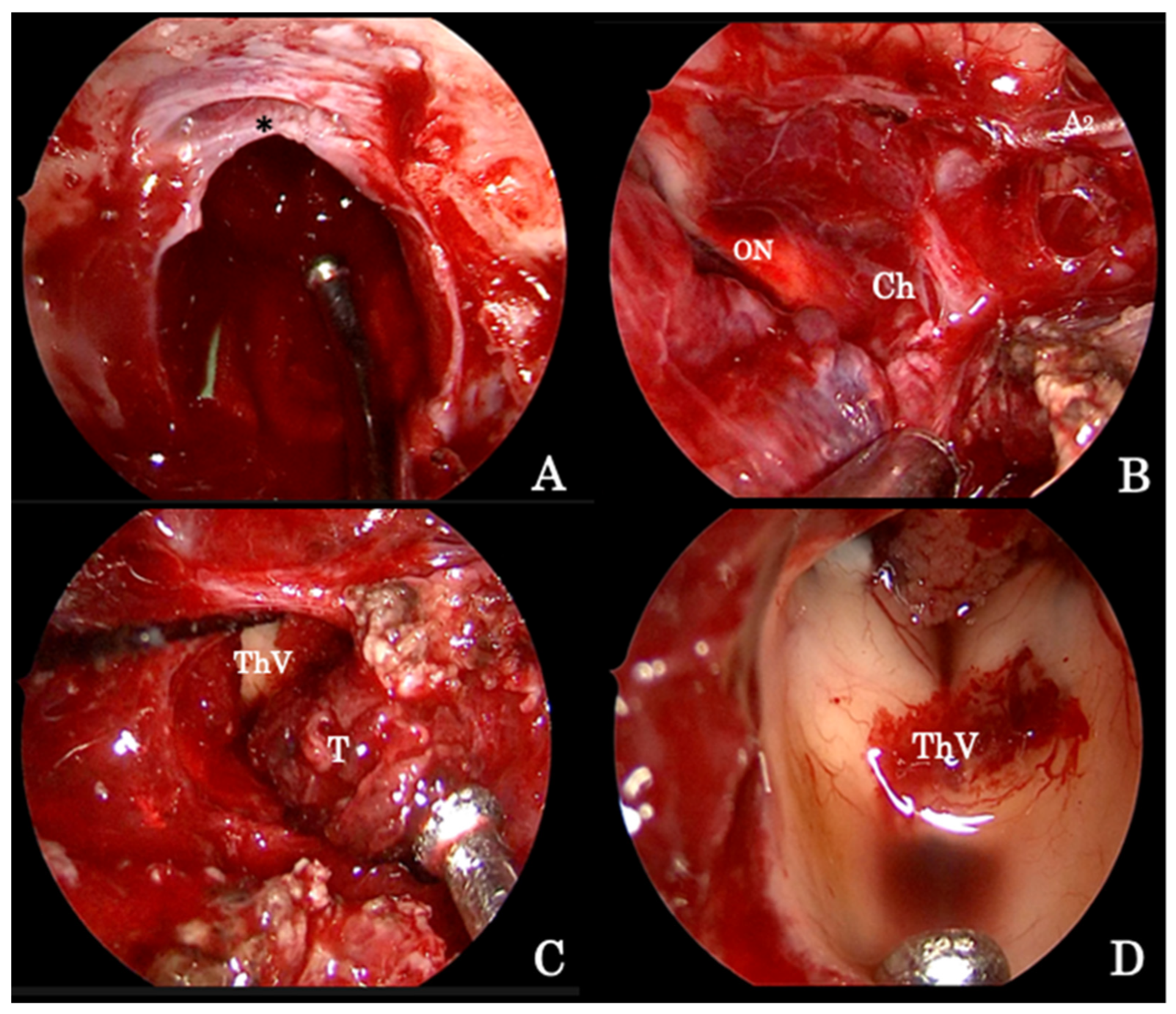
4.1.1. Endoscopic Endonasal Approach
Suprasellar Extension
Parasellar Extension
4.2. Reconstruction Technique
4.3. Transcranial Approaches
4.4. Special Considerations
5. Treatment Considerations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ezzat, S.; Asa, S.L.; Couldwell, W.T.; Barr, C.E.; Dodge, W.E.; Vance, M.L.; McCutcheon, I.E. The prevalence of pituitary adenomas: A systematic review. Cancer 2004, 101, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.F.; Rixhon, M.; Adam, C.; Dempegioti, A.; Tichomirowa, M.A.; Beckers, A. High prevalence of pituitary adenomas: A cross-sectional study in the province of Liege, Belgium. J. Clin. Endocrinol. Metab. 2006, 91, 4769–4775. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.; Karavitaki, N.; Wass, J.A. Prevalence of pituitary adenomas: A community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin. Endocrinol. 2010, 72, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Symon, L.; Jakubowski, J.; Kendall, B. Surgical treatment of giant pituitary adenomas. J. Neurol. Neurosurg. Psychiatry 1979, 42, 973–982. [Google Scholar] [CrossRef]
- Goel, A.; Nadkarni, T.; Muzumdar, D.; Desai, K.; Phalke, U.; Sharma, P. Giant pituitary tumors: A study based on surgical treatment of 118 cases. Surg. Neurol. 2004, 61, 436–445; discussion 445–436. [Google Scholar] [CrossRef]
- Agrawal, A.; Cincu, R.; Goel, A. Current concepts and controversies in the management of non-functioning giant pituitary macroadenomas. Clin. Neurol. Neurosurg. 2007, 109, 645–650. [Google Scholar] [CrossRef]
- Cappabianca, P.; Cavallo, L.M.; Solari, D.; de Divitiis, O.; Chiaramonte, C.; Esposito, F. Size does not matter. The intrigue of giant adenomas: A true surgical challenge. Acta Neurochir. 2014, 156, 2217–2220. [Google Scholar] [CrossRef]
- Cappabianca, P.; Cavallo, L.M.; de Divitiis, O.; de Angelis, M.; Chiaramonte, C.; Solari, D. Endoscopic endonasal extended approaches for the management of large pituitary adenomas. Neurosurg. Clin. N. Am. 2015, 26, 323–331. [Google Scholar] [CrossRef]
- Solari, D.; Pivonello, R.; Caggiano, C.; Guadagno, E.; Chiaramonte, C.; Miccoli, G.; Cavallo, L.M.; De Caro, M.D.B.; Colao, A.; Cappabianca, P. Pituitary adenomas: What are the key features? What are the current treatments? Where is the future taking us? World Neurosurg. 2019, 127, 695–709. [Google Scholar] [CrossRef]
- Cappabianca, P.; Cavallo, L.M.; de Divitiis, O.; Esposito, F. Pituitary surgery. In Endocrinology, 6th ed.; DeGroot, L.J., Jameson, J.L., Eds.; Saunders-Elsevier: Philadelphia, PA, USA, 2010; pp. 358–376. [Google Scholar]
- Asa, S.L.; Casar-Borota, O.; Chanson, P.; Delgrange, E.; Earls, P.; Ezzat, S.; Grossman, A.; Ikeda, H.; Inoshita, N.; Karavitaki, N.; et al. From pituitary adenoma to pituitary neuroendocrine tumor (PitNET): An International Pituitary Pathology Club proposal. Endocr. Relat. Cancer 2017, 24, C5–C8. [Google Scholar] [CrossRef]
- Ebersold, M.J.; Quast, L.M.; Laws, E.R., Jr.; Scheithauer, B.; Randall, R.V. Long-term results in transsphenoidal removal of nonfunctioning pituitary adenomas. J. Neurosurg. 1986, 64, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Laws, E.R., Jr.; Thapar, K. Pituitary surgery. Endocrinol. Metab. Clin. N. Am. 1999, 28, 119–131. [Google Scholar] [CrossRef]
- Wilson, C.B. Role of surgery in the management of pituitary tumors. Neurosurg. Clin. N. Am. 1990, 1, 139–159. [Google Scholar] [CrossRef]
- Elias, W.J.; Laws, E.R. Transsphenoidal approach to lesion of the sella. In Schmideck & Sweet Operative Neurosurgical Technique. Indications, Methods and Results, 4th ed.; Schmideck, H.H., Ed.; WB Saunders: Philadelphia, PA, USA, 2000; pp. 373–384. [Google Scholar]
- Hadad, G.; Bassagasteguy, L.; Carrau, R.L.; Mataza, J.C.; Kassam, A.; Snyderman, C.H.; Mintz, A. A novel reconstructive technique after endoscopic expanded endonasal approaches: Vascular pedicle nasoseptal flap. Laryngoscope 2006, 116, 1882–1886. [Google Scholar] [CrossRef]
- Kassam, A.B.; Carrau, R.L.; Snyderman, C.H.; Thomas, A.; Vescan, A.; Prevedello, D.; Mintz, A.; Gardner, P. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery 2008, 63 (Suppl. 1), ONS44–ONS52; discussion ONS52–ONS53. [Google Scholar] [CrossRef]
- Cavallo, L.M.; Solari, D.; Somma, T.; Cappabianca, P. The 3F (Fat, Flap, and Flash) technique for skull base reconstruction after endoscopic endonasal suprasellar approach. World Neurosurg. 2019, 126, 439–446. [Google Scholar] [CrossRef]
- de Notaris, M.; Solari, D.; Cavallo, L.M.; D’Enza, A.I.; Enseñat, J.; Berenguer, J.; Ferrer, E.; Prats-Galino, A.; Cappabianca, P. The “suprasellar notch,” or the tuberculum sellae as seen from below: Definition, features, and clinical implications from an endoscopic endonasal perspective. J. Neurosurg. 2012, 116, 622–629. [Google Scholar] [CrossRef]
- Cappabianca, P.; Cavallo, L.M.; Esposito, F.; De Divitiis, O.; Messina, A.; De Divitiis, E. Extended endoscopic endonasal approach to the midline skull base: The evolving role of transsphenoidal surgery. Adv. Tech. Stand. Neurosurg. 2008, 33, 151–199. [Google Scholar]
- Kitano, M.; Taneda, M.; Shimono, T.; Nakao, Y. Extended transsphenoidal approach for surgical management of pituitary adenomas invading the cavernous sinus. J. Neurosurg. 2008, 108, 26–36. [Google Scholar] [CrossRef]
- Laws, E.R.; Kanter, A.S.; Jane, J.A.; Dumont, A.S. Extended transsphenoidal approach. J. Neurosurg. 2005, 102, 825–827; discussion 827–828. [Google Scholar] [CrossRef]
- de Divitiis, E.; Cavallo, L.M.; Cappabianca, P.; Esposito, F. Extended endoscopic endonasal transsphenoidal approach for the removal of suprasellar tumors: Part 2. Neurosurgery 2007, 60, 46–58; discussion 58–59. [Google Scholar] [CrossRef]
- Frank, G.; Pasquini, E. Endoscopic endonasal cavernous sinus surgery, with special reference to pituitary adenomas. Front. Horm. Res. 2006, 34, 64–82. [Google Scholar]
- Micko, A.S.; Wohrer, A.; Wolfsberger, S.; Knosp, E. Invasion of the cavernous sinus space in pituitary adenomas: Endoscopic verification and its correlation with an MRI-based classification. J. Neurosurg. 2015, 122, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Kassam, A.B.; Vescan, A.D.; Carrau, R.L.; Prevedello, D.M.; Gardner, P.; Mintz, A.H.; Snyderman, C.H.; Rhoton, A.L. Expanded endonasal approach: Vidian canal as a landmark to the petrous internal carotid artery. J. Neurosurg. 2008, 108, 177–183. [Google Scholar] [CrossRef]
- Battaglia, P.; Turri-Zanoni, M.; Dallan, I.; Gallo, S.; Sica, E.; Padoan, G.; Castelnuovo, P. Endoscopic endonasal transpterygoid transmaxillary approach to the infratemporal and upper parapharyngeal tumors. Otolaryngol. Head Neck Surg. 2014, 150, 696–702. [Google Scholar] [CrossRef]
- Zada, G.; Lin, N.; Laws, E.R., Jr. Patterns of extrasellar extension in growth hormone-secreting and nonfunctional pituitary macroadenomas. Neurosurg. Focus 2010, 29, E4. [Google Scholar] [CrossRef]
- Fernandez-Miranda, J.C.; Zwagerman, N.T.; Abhinav, K.; Lieber, S.; Wang, E.W.; Snyderman, C.H.; Gardner, P.A. Cavernous sinus compartments from the endoscopic endonasal approach: Anatomical considerations and surgical relevance to adenoma surgery. J. Neurosurg. 2018, 129, 430–441. [Google Scholar] [CrossRef]
- Cavallo, L.M.; Messina, A.; Esposito, F.; de Divitiis, O.; Dal Fabbro, M.; de Divitiis, E.; Cappabianca, P. Skull base reconstruction in the extended endoscopic transsphenoidal approach for suprasellar lesions. J. Neurosurg. 2007, 107, 713–720. [Google Scholar] [CrossRef]
- Kassam, A.B.; Prevedello, D.M.; Carrau, R.L.; Snyderman, C.H.; Thomas, A.; Gardner, P.; Zanation, A.; Duz, B.; Stefko, S.T.; Byers, K.; et al. Endoscopic endonasal skull base surgery: Analysis of complications in the authors’ initial 800 patients. J. Neurosurg. 2011, 114, 1544–1568. [Google Scholar] [CrossRef]
- Laws, E.R. Complications of transsphenoidal surgery. In Management of Pituitary Adenomas and Related Lesions with Emphasis on Transsphenoidal Microsurgery; Appleton-Century-Crofts: New York, NY, USA, 1982. [Google Scholar]
- Cappabianca, P.; Cavallo, L.M.; Colao, A.; de Divitiis, E. Surgical complications associated with the endoscopic endonasal transsphenoidal approach for pituitary adenomas. J. Neurosurg. 2002, 97, 293–298. [Google Scholar] [CrossRef]
- Leng, L.Z.; Brown, S.; Anand, V.K.; Schwartz, T.H. “Gasket-seal” watertight closure in minimal-access endoscopic cranial base surgery. Neurosurgery 2008, 62 (Suppl. 2), ONSE342–ONSE343; discussion ONSE343. [Google Scholar] [CrossRef] [PubMed]
- Tabaee, A.; Anand, V.K.; Brown, S.M.; Lin, J.W.; Schwartz, T.H. Algorithm for reconstruction after endoscopic pituitary and skull base surgery. Laryngoscope 2007, 117, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Gardner, P.A.; Kassam, A.B.; Snyderman, C.H.; Carrau, R.L.; Mintz, A.H.; Grahovac, S.; Stefko, S. Outcomes following endoscopic, expanded endonasal resection of suprasellar craniopharyngiomas: A case series. J. Neurosurg. 2008, 109, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Gardner, P.A.; Kassam, A.B.; Thomas, A.; Snyderman, C.H.; Carrau, R.L.; Mintz, A.H.; Prevedello, D.M. Endoscopic endonasal resection of anterior cranial base meningiomas. Neurosurgery 2008, 63, 36–52; discussion 52–34. [Google Scholar] [CrossRef]
- Koutourousiou, M.; Gardner, P.A.; Fernandez-Miranda, J.C.; Paluzzi, A.; Wang, E.W.; Snyderman, C.H. Endoscopic endonasal surgery for giant pituitary adenomas: Advantages and limitations. J. Neurosurg. 2013, 118, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Juraschka, K.; Khan, O.H.; Godoy, B.L.; Monsalves, E.; Kilian, A.; Krischek, B.; Ghare, A.; Vescan, A.; Gentili, F.; Zadeh, G. Endoscopic endonasal transsphenoidal approach to large and giant pituitary adenomas: Institutional experience and predictors of extent of resection. J. Neurosurg. 2014, 121, 75–83. [Google Scholar] [CrossRef]
- Esposito, F.; Dusick, J.R.; Fatemi, N.; Kelly, D.F. Graded repair of cranial base defects and cerebrospinal fluid leaks in transsphenoidal surgery. Oper. Neurosurg. 2007, 60 (Suppl. 2), 295–303; discussion 303–294. [Google Scholar] [CrossRef]
- Gondim, J.A.; Almeida, J.P.; Albuquerque, L.A.; Gomes, E.F.; Schops, M. Giant pituitary adenomas: Surgical outcomes of 50 cases operated on by the endonasal endoscopic approach. World Neurosurg. 2014, 82, e281–e290. [Google Scholar] [CrossRef]
- Luginbuhl, A.J.; Campbell, P.G.; Evans, J.; Rosen, M. Endoscopic repair of high-flow cranial base defects using a bilayer button. Laryngoscope 2010, 120, 876–880. [Google Scholar] [CrossRef]
- Dolenc, V.V. Transcranial epidural approach to pituitary tumors extending beyond the sella. Neurosurgery 1997, 41, 542–550; discussion 551–542. [Google Scholar]
- Jane, J.A.; Thapar, K.; Kaptain, G.J.; Maartens, N.; Laws, E.R. Pituitary surgery: Transsphenoidal approach. Neurosurgery 2002, 51, 435–442; discussion 442–434. [Google Scholar] [CrossRef] [PubMed]
- Laws, E.R.; Jane, J.A., Jr. Pituitary tumors—Long-term outcomes and expectations. Clin. Neurosurg. 2001, 48, 306–319. [Google Scholar] [PubMed]
- McLaughlin, N.; Eisenberg, A.A.; Cohan, P.; Chaloner, C.B.; Kelly, D.F. Value of endoscopy for maximizing tumor removal in endonasal transsphenoidal pituitary adenoma surgery. J. Neurosurg. 2013, 118, 613–620. [Google Scholar] [CrossRef]
- Laws, E.R.; Lanzino, G. Transsphenoidal Surgery; Elsevier Health Sciences: Amsterdam, The Netherlands, 2010. [Google Scholar]
- de Paiva Neto, M.A.; Vandergrift, A.; Fatemi, N.; Gorgulho, A.A.; DeSalles, A.A.; Cohan, P.; Wang, C.; Swerdloff, R.; Kelly, D.F. Endonasal transsphenoidal surgery and multimodality treatment for giant pituitary adenomas. Clin. Endocrinol. 2010, 72, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Zada, G.; Du, R.; Laws, E.R., Jr. Defining the “edge of the envelope”: Patient selection in treating complex sellar-based neoplasms via transsphenoidal versus open craniotomy. J. Neurosurg. 2011, 114, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Solari, D.; D’Avella, E.; Bove, I.; Cappabianca, P.; Cavallo, L.M. Extended endonasal approaches for pituitary adenomas. J. Neurosurg. Sci. 2021, 65, 160–168. [Google Scholar] [CrossRef]
- Zada, G.; Terterov, S.; Russin, J.; Clavijo, L.; Giannotta, S. Cerebral vasospasm and concurrent left ventricular outflow tract obstruction: Requirement for modification of hyperdynamic therapy regimen. Neurocrit. Care 2010, 12, 265–268. [Google Scholar] [CrossRef]
- Puri, A.S.; Zada, G.; Zarzour, H.; Laws, E.; Frerichs, K. Cerebral vasospasm after transsphenoidal resection of pituitary macroadenomas: Report of 3 cases and review of the literature. Neurosurgery 2012, 71, 173–180; discussion 180–171. [Google Scholar] [CrossRef]
- Suero Molina, E.; Di Somma, A.; Stummer, W.; Briganti, F.; Cavallo, L.M. Clinical vasospasm after an extended endoscopic endonasal approach for recurrent pituitary adenoma: Illustrative case and systematic review of the literature. World Neurosurg. 2019, 128, 29–36. [Google Scholar] [CrossRef]
- Camp, P.E.; Paxton, H.D.; Buchan, G.C.; Gahbauer, H. Vasospasm after trans-sphenoidal hypophysectomy. Neurosurgery 1980, 7, 382–386. [Google Scholar] [CrossRef]
- Kasliwal, M.K.; Srivastava, R.; Sinha, S.; Kale, S.S.; Sharma, B.S. Vasospasm after transsphenoidal pituitary surgery: A case report and review of the literature. Neurol. India 2008, 56, 81–83. [Google Scholar] [PubMed]
- Molyneux, A.J.; Kerr, R.S.; Yu, L.-M.; Clarke, M.; Sneade, M.; Yarnold, J.; Sandercock, P. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005, 366, 809–817. [Google Scholar] [PubMed]
- Shimon, I. Giant prolactinomas. Neuroendocrinology 2019, 109, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Moraes, A.B.; Silva, C.M.; Vieira Neto, L.; Gadelha, M.R. Giant prolactinomas: The therapeutic approach. Clin. Endocrinol. 2013, 79, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, S.; Cavallo, L.M.; Esposito, F.; Stagno, V.; Corriero, O.V.; Cappabianca, P. Extended endoscopic endonasal approach for selected pituitary adenomas: Early experience. J. Neurosurg. 2011, 114, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Frank, G.; Pasquini, E.; Farneti, G.; Mazzatenta, D.; Sciarretta, V.; Grasso, V.; Fustini, M.F. The endoscopic versus the traditional approach in pituitary surgery. Neuroendocrinology 2006, 83, 240–248. [Google Scholar] [CrossRef]
- Youssef, A.S.; Agazzi, S.; van Loveren, H.R. Transcranial surgery for pituitary adenomas. Neurosurgery 2005, 57 (Suppl. 1), 168–175. discussion 168–175. [Google Scholar] [CrossRef]
- Zada, G.; Laws, E.R., Jr. Simultaneous transsphenoidal and intraventricular endoscopic approaches for macroadenomas with extensive suprasellar extension: Surgery from below, above, or both? World Neurosurg. 2010, 74, 109–110. [Google Scholar] [CrossRef]
- Cappabianca, P.; Cavallo, L.M.; de Divitiis, E. Endoscopic endonasal transsphenoidal surgery. Neurosurgery 2004, 55, 933–940. discussion 940–941. [Google Scholar] [CrossRef]
- Jane, J.A., Jr.; Laws, E.R., Jr. Surgical treatment of pituitary adenomas. In Endotext; De Groot, L.J., Chrousos, G., Dungan, K., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Laws, E.R.; Lanzino, G. (Eds.) Transsphenoidal Surgery; Saunders-Elsevier: Philadelphia, PA, USA, 2010. [Google Scholar]
- Cusimano, M.D.; Kan, P.; Nassiri, F.; Anderson, J.; Goguen, J.; Vanek, I.; Smyth, H.S.; Fenton, R.; Muller, P.J.; Kovacs, K. Outcomes of surgically treated giant pituitary tumours. Can. J. Neurol. Sci. 2012, 39, 446–457. [Google Scholar] [CrossRef]
- de Divitiis, E.; Cappabianca, P.; Cavallo, L.M. Endoscopic transsphenoidal approach: Adaptability of the procedure to different sellar lesions. Neurosurgery 2002, 51, 699–705; discussion 705–707. [Google Scholar] [CrossRef]
- Elshazly, K.; Kshettry, V.R.; Farrell, C.J.; Nyquist, G.; Rosen, M.; Evans, J.J. Clinical outcomes after endoscopic endonasal resection of giant pituitary adenomas. World Neurosurg. 2018, 114, e447–e456. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.; Deogaonkar, K.; Desai, A. Fatal postoperative ‘pituitary apoplexy’: Its cause and management. Br. J. Neurosurg. 1995, 9, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.U.; Pandey, P.; Mahapatra, A.K. Post operative ‘pituitary apoplexy’ in giant pituitary adenomas: A series of cases. Neurol. India 2005, 53, 326–328. [Google Scholar] [PubMed]
- Han, S.; Gao, W.; Jing, Z.; Wang, Y.; Wu, A. How to deal with giant pituitary adenomas: Transsphenoidal or transcranial, simultaneous or two-staged? J. Neurooncol. 2017, 132, 313–321. [Google Scholar] [CrossRef]
- Leung, G.K.; Law, H.Y.; Hung, K.N.; Fan, Y.W.; Lui, W.M. Combined simultaneous transcranial and transsphenoidal resection of large-to-giant pituitary adenomas. Acta Neurochir. 2011, 153, 1401–1408; discussion 1408. [Google Scholar] [CrossRef]
- Alleyne, C.H.; Barrow, D.L.; Oyesiku, N.M. Combined transsphenoidal and pterional craniotomy approach to giant pituitary tumors. Surg. Neurol. 2002, 57, 380–390; discussion 390. [Google Scholar] [CrossRef]
- Leung, G.K.; Yuen, M.M.; Chow, W.S.; Tse, P.Y.; Lui, W.M. An endoscopic modification of the simultaneous ‘above and below’ approach to large pituitary adenomas. Pituitary 2012, 15, 237–241. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solari, D.; Cavallo, L.M.; Graziadio, C.; Corvino, S.; Bove, I.; Esposito, F.; Cappabianca, P. Giant Non-Functioning Pituitary Adenomas: Treatment Considerations. Brain Sci. 2022, 12, 1256. https://doi.org/10.3390/brainsci12091256
Solari D, Cavallo LM, Graziadio C, Corvino S, Bove I, Esposito F, Cappabianca P. Giant Non-Functioning Pituitary Adenomas: Treatment Considerations. Brain Sciences. 2022; 12(9):1256. https://doi.org/10.3390/brainsci12091256
Chicago/Turabian StyleSolari, Domenico, Luigi Maria Cavallo, Chiara Graziadio, Sergio Corvino, Ilaria Bove, Felice Esposito, and Paolo Cappabianca. 2022. "Giant Non-Functioning Pituitary Adenomas: Treatment Considerations" Brain Sciences 12, no. 9: 1256. https://doi.org/10.3390/brainsci12091256
APA StyleSolari, D., Cavallo, L. M., Graziadio, C., Corvino, S., Bove, I., Esposito, F., & Cappabianca, P. (2022). Giant Non-Functioning Pituitary Adenomas: Treatment Considerations. Brain Sciences, 12(9), 1256. https://doi.org/10.3390/brainsci12091256






