Determining Whether Sex and Zygosity Modulates the Association between APOE4 and Psychosis in a Neuropathologically-Confirmed Alzheimer’s Disease Cohort
Abstract
1. Introduction
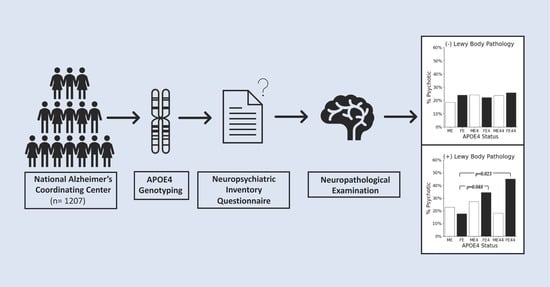
2. Materials and Methods
2.1. Data Source
2.2. Statistical Analyses
3. Results
3.1. Descriptive Statistics

3.2. LB (+) Cohort

3.3. LB (−) Cohort
3.4. Post Hoc Analysis: Neuropsychiatric Symptoms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belloy, M.E.; Napolioni, V.; Greicius, M.D. A quarter century of APOE and Alzheimer’s disease: Progress to date and the path forward. Neuron 2019, 101, 820–838. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Zhao, N.; Fu, Y.; Wang, N.; Linares, C.; Tsai, C.W.; Bu, G. ApoE4 accelerates early seeding of amyloid pathology. Neuron 2017, 96, 1024–1032. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, Y.; Yamazaki, Y.; Ren, Y.; Davis, M.D.; Liu, C.C.; Lu, W.; Wang, X.; Chen, K.; Cherukuri, Y.; et al. APOE4 exacerbates synapse loss and neurodegeneration in Alzheimer’s disease patient iPSC-derived cerebral organoids. Nat. Commun. 2020, 11, 5540. [Google Scholar] [CrossRef]
- Liu, M.; Paranjpe, M.D.; Zhou, X.; Duy, P.Q.; Goyal, M.S.; Benzinger, T.L.; Lu, J.; Wang, R.; Zhou, Y. Sex modulates the ApoE ε4 effect on brain tau deposition measured by 18F-AV-1451 PET in individuals with mild cognitive impairment. Theranostics 2019, 9, 4959. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Murray, P.S.; Kumar, S.; DeMichele-Sweet, M.A.A.; Sweet, R.A. Psychosis in Alzheimer’s disease. Biol. Psychiatry 2014, 75, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.E.; Agüera-Ortiz, L. Psychosis and dementia: Risk factor, prodrome, or cause? Int. Psychogeriatr. 2018, 30, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.E.; Ismail, Z.; Youakim, J.M.; Creese, B.; Kumar, S.; Nuñez, N.; Darby, R.R.; Di Vita, A.; D’Antonio, F.; de Lena, C.; et al. Revisiting criteria for psychosis in Alzheimer’s disease and related dementias: Toward better phenotypic classification and biomarker research. J. Alzheimer’s Dis. 2020, 73, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Ismail, Z.; Creese, B.; Aarsland, D.; Kales, H.C.; Lyketsos, C.G.; Sweet, R.A.; Ballard, C. Psychosis in Alzheimer disease—Mechanisms, genetics and therapeutic opportunities. Nat. Rev. Neurol. 2022, 18, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, G.; Marder, K.; Tang, M.; Schofield, P.W.; Chun, M.R.; Devanand, D.P.; Stern, Y.; Mayeux, R. A preliminary study of apolipoprotein E genotype and psychiatric manifestations of Alzheimer’s disease. Neurology 1996, 47, 256–259. [Google Scholar] [CrossRef]
- Borroni, B.; Grassi, M.; Agosti, C.; Costanzi, C.; Archetti, S.; Franzoni, S.; Caltagirone, C.; Di Luca, M.; Caimi, L.; Padovani, A. Genetic correlates of behavioral endophenotypes in Alzheimer disease: Role of COMT, 5-HTTLPR and APOE polymorphisms. Neurobiol. Aging 2006, 27, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.; DeMichele-Sweet, M.A.A.; Sweet, R.A. Genetics of psychosis of Alzheimer disease. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2017, 174, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Fischer, C.E.; Schweizer, T.A.; Munoz, D.G. Gender and pathology-specific effect of Apolipoprotein E genotype on psychosis in Alzheimer’s disease. Curr. Alzheimer Res. 2017, 14, 834–840. [Google Scholar] [CrossRef] [PubMed]
- McKeith, I.G.; Boeve, B.F.; Dickson, D.W.; Halliday, G.; Taylor, J.P.; Weintraub, D.; Aarsland, D.; Galvin, J.; Attems, J.; Ballard, C.G.; et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 2017, 89, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Li, F.; Sonoustoun, B.; Kondru, N.C.; Martens, Y.A.; Qiao, W.; Heckman, M.G.; Ikezu, T.C.; Li, Z.; Burgess, J.D.; et al. APOE4 exacerbates α-synuclein seeding activity and contributes to neurotoxicity in Alzheimer’s disease with Lewy body pathology. Acta Neuropathol. 2022, 143, 641–662. [Google Scholar] [CrossRef] [PubMed]
- Hyman, B.T.; Trojanowski, J.Q. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the national institute on aging and the reagan institute working group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J. Neuropathol. Exp. Neurol. 1997, 56, 1095–1097. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.N. Generalized Additive Models: An Introduction with R; Chapman and Hall/CRC: Boca Raton, FL, USA, 2006; Volume 66. [Google Scholar] [CrossRef]
- DeMichele-Sweet, M.A.A.; Lopez, O.L.; Sweet, R.A. Psychosis in Alzheimer’s disease in the national Alzheimer’s disease coordinating center uniform data set: Clinical correlates and association with apolipoprotein e. Int. J. Alzheimer’s Dis. 2011, 2011, 926597. [Google Scholar] [CrossRef] [PubMed]
- Tsuang, D.W.; Wilson, R.K.; Lopez, O.L.; Luedecking-Zimmer, E.K.; Leverenz, J.B.; DeKosky, S.T.; Kamboh, M.I.; Hamilton, R.L. Genetic association between the APOE* 4 allele and Lewy bodies in Alzheimer disease. Neurology 2005, 64, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Agüera-Ortiz, L.; Babulal, G.M.; Bruneau, M.A.; Creese, B.; D’Antonio, F.; Fischer, C.E.; Gatchel, J.R.; Ismail, Z.; Kumar, S.; McGeown, W.J.; et al. Psychosis as a treatment target in dementia: A roadmap for designing interventions. J. Alzheimer’s Dis. 2022, 88, 1203–1228. [Google Scholar] [CrossRef] [PubMed]

| E4 (−) (n = 424) | E4 (n = 594) | E44 (n = 189) | ||
|---|---|---|---|---|
| Sex | ||||
| Male | 218 (51.4%) | 334 (56.2%) | 114 (60.3%) | |
| Female | 206 (48.6%) | 260 (43.8%) | 75 (39.7%) | |
| Lewy body pathology | ||||
| LB (−) | 268 (63.2%) | 354 (59.6%) | 94 (49.7%) | |
| Males | 130 (59.6%) | 187 (56.0%) | 59 (51.8%) | |
| Females | 138 (67.0%) | 167 (64.2%) | 35 (46.7%) | |
| LB (+) | 156 (36.8%) | 240 (40.4%) | 95 (50.3%) | |
| Males | 88 (40.4%) | 147 (44.0%) | 55 (48.2%) | |
| Females | 68 (33.0%) | 93 (35.8%) | 40 (53.3%) | |
| Age | ||||
| Mean (SD) | 75 (12) | 74 (10) | 73 (8) | |
| Males | 73 (11) | 74 (10) | 73 (8) | |
| Females | 77 (12) | 75 (11) | 73 (8) | |
| Median [Min, Max] | 78 [36, 100] | 76 [44, 98] | 73 [44, 90] | |
| Males | 76 [36, 96] | 75 [47, 95] | 73 [44, 90] | |
| Females | 80 [38, 100] | 77 [44, 98] | 73 [50, 89] | |
| MMSE | ||||
| Mean (SD) | 19 (8) | 17 (9) | 18 (8) | |
| Males | 19 (8) | 17 (9) | 19 (7) | |
| Females | 19 (8) | 17 (8) | 15 (8) | |
| Median [Min, Max] | 21 [0, 30] | 19 [0, 30] | 19 [0, 30] | |
| Males | 21 [0, 30] | 19 [0, 30] | 21 [0, 30] | |
| Females | 20 [0, 30] | 19 [0, 30] | 16 [0, 30] | |
| Education | ||||
| Mean (SD) | 15 (3) | 15 (3) | 16 (3) | |
| Males | 16 (3) | 16 (3) | 16 (3) | |
| Females | 14 (3) | 14 (3) | 15 (3) | |
| Median [Min, Max] | 16 [2, 28] | 16 [3, 25] | 16 [8, 22] | |
| Males | 16 [3, 28] | 16 [6, 25] | 16 [8, 22] | |
| Females | 14 [2, 20] | 14 [3, 20] | 16 [8, 20] |
| Females | Covariates (p-value) | ||
|---|---|---|---|
| Female Homozygotes vs. Female Non-Carriers | Age | Education | MMSE |
| Psychosis | p = 0.023 OR = 4.15 95% CI [1.21, 14.2] | 0.409 | 0.034 | 0.021 |
| Female Heterozygotes vs. Female Non-Carriers | |||
| Psychosis | p = 0.048 OR = 2.37 95% CI [1.01, 5.59] | 0.287 | 0.350 | 0.016 |
| Female Homozygotes vs. Female Heterozygotes | |||
| Psychosis | p = 0.688 OR = 0.83 95% CI [0.33, 2.04] | 0.322 | 0.747 | <0.001 |
| Males | |||
| Male Homozygotes vs. Male Non-Carriers | |||
| Psychosis | p = 0.995 OR = 1.01 95% CI [0.39, 2.57] | 0.086 | 0.615 | <0.001 |
| Male Heterozygotes vs. Male Non-Carriers | |||
| Psychosis | p = 0.299 OR = 1.46 95% CI [0.71, 3.00] | 0.670 | 0.331 | <0.001 |
| Male Homozygotes vs. Male Heterozygotes | |||
| Psychosis | p = 0.373 OR = 0.68 95% CI [0.301, 1.56] | 0.718 | 0.647 | <0.001 |
| Between Sex | |||
| Male Homozygotes vs. Female Homozygotes | |||
| Psychosis | p = 0.160 OR = 2.20 95% CI [0.73, 6.61] | 0.140 | 0.457 | <0.001 |
| Male Heterozygotes vs. Female Heterozygotes | |||
| Psychosis | p = 0.148 OR = 1.60 95% CI [0.84, 3.05] | 0.474 | 0.632 | <0.001 |
| Females | Covariates (p-value) | ||
|---|---|---|---|
| Female Homozygotes vs. Female Non-Carriers | Age | Education | MMSE |
| Psychosis | p = 0.794 OR = 1.13 95% CI [0.42, 3.03] | 0.441 | 0.310 | 0.036 |
| Female Heterozygotes vs. Female Non-Carriers | |||
| Psychosis | p = 0.301 OR = 0.71 95% CI [0.38, 1.34] | 0.642 | 0.035 | 0.013 |
| Female Homozygotes vs. Female Heterozygotes | |||
| Psychosis | p = 0.388 OR = 1.55 95% CI [0.56, 4.28] | 0.143 | 0.017 | 0.071 |
| Males | |||
| Male Homozygotes vs. Male Non-Carriers | |||
| Psychosis | p = 0.209 OR = 1.75 95% CI [0.39, 2.57] | 0.575 | 0.130 | <0.001 |
| Male Heterozygotes vs. Male Non-Carriers | |||
| Psychosis | p = 0.328 OR = 1.38 95% CI [0.71, 3.00] | 0.554 | 0.010 | <0.001 |
| Male Homozygotes vs. Male Heterozygotes | |||
| Psychosis | p = 0.577 OR = 1.26 95% CI [0.301, 1.56] | 0.291 | <0.001 | <0.001 |
| Between Sex | |||
| Male Homozygotes vs. Female Homozygotes | |||
| Psychosis | p = 0.400 OR = 0.58 95% CI [0.16, 2.03] | 0.659 | 0.080 | <0.001 |
| Male Heterozygotes vs. Female Heterozygotes | |||
| Psychosis | p = 0.060 OR = 0.54 95% CI [0.29, 1.02] | 0.648 | <0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valcic, M.; Khoury, M.A.; Kim, J.; Fornazzari, L.; Churchill, N.W.; Ismail, Z.; De Luca, V.; Tsuang, D.; Schweizer, T.A.; Munoz, D.G.; et al. Determining Whether Sex and Zygosity Modulates the Association between APOE4 and Psychosis in a Neuropathologically-Confirmed Alzheimer’s Disease Cohort. Brain Sci. 2022, 12, 1266. https://doi.org/10.3390/brainsci12091266
Valcic M, Khoury MA, Kim J, Fornazzari L, Churchill NW, Ismail Z, De Luca V, Tsuang D, Schweizer TA, Munoz DG, et al. Determining Whether Sex and Zygosity Modulates the Association between APOE4 and Psychosis in a Neuropathologically-Confirmed Alzheimer’s Disease Cohort. Brain Sciences. 2022; 12(9):1266. https://doi.org/10.3390/brainsci12091266
Chicago/Turabian StyleValcic, Mila, Marc A. Khoury, Julia Kim, Luis Fornazzari, Nathan W. Churchill, Zahinoor Ismail, Vincenzo De Luca, Debby Tsuang, Tom A. Schweizer, David G. Munoz, and et al. 2022. "Determining Whether Sex and Zygosity Modulates the Association between APOE4 and Psychosis in a Neuropathologically-Confirmed Alzheimer’s Disease Cohort" Brain Sciences 12, no. 9: 1266. https://doi.org/10.3390/brainsci12091266
APA StyleValcic, M., Khoury, M. A., Kim, J., Fornazzari, L., Churchill, N. W., Ismail, Z., De Luca, V., Tsuang, D., Schweizer, T. A., Munoz, D. G., & Fischer, C. E. (2022). Determining Whether Sex and Zygosity Modulates the Association between APOE4 and Psychosis in a Neuropathologically-Confirmed Alzheimer’s Disease Cohort. Brain Sciences, 12(9), 1266. https://doi.org/10.3390/brainsci12091266