Resilience of Neural Cellularity to the Influence of Low Educational Level
Abstract
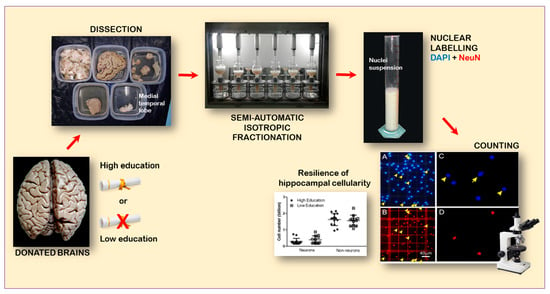
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Inclusion and Exclusion Criteria
2.3. Dissection of the MTL
2.4. Chemomecanical Dissociation
2.5. Immunocytochemistry
2.6. Statistical Analysis
3. Results
3.1. Clinical and Demographic Data of Participants with Collected Brain Samples
3.2. Brain Mass and MTL Mass Related to High and Low Educational Level
3.3. Cell Numbers (Neurons, Non-Neurons, and All Cells) Related to High and Low Educational Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stern, Y. What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 2002, 8, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y. Cognitive reserve. Neuropsychologia 2009, 47, 2015–2028. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y. Cognitive reserve: Implications for assessment and intervention. Folia Phoniat. Logopaed. 2013, 65, 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boller, B.; Mellah, S.; Ducharme-Laliberté, G.; Belleville, S. Relationships between years of education, regional grey matter volumes, and working memory-related brain activity in healthy older adults. Brain Imaging Behav. 2017, 11, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y. The concept of cognitive reserve: A catalyst for research. J. Clin. Exp. Neuropsychol. 2003, 25, 589–593. [Google Scholar] [CrossRef]
- Stern, Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2006, 20, S69–S74. [Google Scholar] [CrossRef]
- Stern, Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012, 11, 1006–1012. [Google Scholar] [CrossRef] [Green Version]
- Stern, Y.; Arenaza-Urquijo, E.M.; Bartrés-Faz, D.; Belleville, S.; Cantilon, M.; Chetelat, G.; Ewers, M.; Franzmeier, N.; Kempermann, G.; Kremen, W.S.; et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer’s Dement. 2018, 16, 1305–1311. [Google Scholar] [CrossRef]
- Suemoto, C.K.; Bertola, L.; Grinberg, L.T.; Leite, R.E.; Rodriguez, R.D.; Santana, P.H.; Pasqualucci, C.A.; Jacob-Filho, W.; Nitrini, R. Education, but not occupation, is associated with cognitive impairment: The role of cognitive reserve in a sample from a low-to-middle-income study. Alzheimer’s Dement. 2022, 18, 2079–2087. [Google Scholar] [CrossRef]
- Kim, J.P.; Seo, S.W.; Shin, H.Y.; Ye, B.S.; Yang, J.J.; Kim, C.; Kang, M.; Jeon, S.; Kim, H.J.; Lee, J.M.; et al. Effects of education on aging-related cortical thinning among cognitively normal individuals. Neurology 2015, 85, 806–812. [Google Scholar] [CrossRef]
- Foubert-Samier, A.; Catheline, G.; Amieva, H.; Dilharreguy, B.; Helmer, C.; Allard, M.; Dartigues, J.F. Education, occupation, leisure activities, and brain reserve: A population-based study. Neurobiol. Aging 2012, 33, 423.e15–423.e25. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Julkunen, V.; Paajanen, T.; Westman, E.; Wahlund, L.O.; Aitken, A.; Sobow, T.; Mecocci, P.; Tsolaki, M.; Vellas, B.; et al. Education increases reserve against Alzheimer’s disease—Evidence from structural MRI analysis. Neuroradiology 2012, 54, 929–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arenaza-Urquijo, E.M.; Landeau, B.; La Joie, R.; Mevel, K.; Mézenge, F.; Perrotin, A.; Desgranges, B.; Bartrés-Faz, D.; Eustache, F.; Chételat, G. Relationships between years of education and gray matter volume, metabolism and functional connectivity in healthy elders. Neuroimage 2013, 83, 450–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rzezak, P.; Squarzoni, P.; Duran, F.L.; de Toledo Ferraz Alves, T.; Tamashiro-Duran, J.; Bottino, C.M.; Ribeiz, S.; Lotufo, P.A.; Menezes, P.R.; Scazufca, M.; et al. Relationship between brain age-related reduction in gray matter and educational attainment. PLoS ONE 2015, 10, e0140945. [Google Scholar] [CrossRef]
- Squire, L.R.; Stark, C.E.; Clark, R.E. The medial temporal lobe. Ann. Rev. Neurosci. 2004, 27, 279–306. [Google Scholar] [CrossRef] [Green Version]
- Carlesimo, G.A. The temporal lobes and memory. Handb. Clin. Neurol. 2022, 187, 319–337. [Google Scholar]
- Zeithamova, D.; Mack, M.L.; Braunlich, K.; Davis, T.; Seger, C.A.; van Kersteren, M.T.R.; Wutz, A. Brain mechanisms of concept learning. J. Neurosci. 2019, 39, 8259–8266. [Google Scholar] [CrossRef] [Green Version]
- Stepan, J.; Dine, J.; Eder, M. Functional optical probing of the hippocampal trisynaptic circuit in vitro: Network dynamics, filter properties, and polysynaptic induction of CA1 LTP. Front. Neurosci. 2015, 9, 160. [Google Scholar] [CrossRef] [Green Version]
- Cheyne, J.E.; Montgomery, J.M. The cellular and molecular basis of in vivo synaptic plasticity in rodents. Am. J. Physiol. Cell Physiol. 2020, 318, C1264–C1283. [Google Scholar] [CrossRef]
- Ko, S.Y.; Frankland, P.W. Neurogenesis-dependent transformation of hippocampal engrams. Neurosci. Lett. 2021, 762, 136176. [Google Scholar] [CrossRef]
- Raine, P.J.; Rao, H. Volume, density, and thickness brain abnormalities in mild cognitive impairment: And ALE meta-analysis controlling for age and education. Brain Imaging Behav. 2022, 16, 2335–2352. [Google Scholar] [CrossRef] [PubMed]
- Kok, F.K.; van Leerdam, S.L.; de Lange, E.C.M. Potential mechanisms underlying resistance to dementia in non-demented individuals with Alzheimer’s disease neuropathology. J. Alzheimer’s Dis. 2022, 87, 51–81. [Google Scholar] [CrossRef] [PubMed]
- McDonough, I.M.; Festini, S.B.; Wood, M.M. Risk for Alzheimer’s disease: A review of long-term episodic memory encoding and retrieval fMRI studies. Ageing Res. Rev. 2020, 62, 101133. [Google Scholar] [CrossRef] [PubMed]
- Pollmann, S.; Schneider, W.X. Working memory and active sampling of the environment: Medial temporal contributions. Handb. Clin. Neurol. 2022, 187, 339–357. [Google Scholar] [PubMed]
- Hengstschläger, A.; Sommerland, A.; Huntley, J. What are the neural correlates of impaired awareness of social cognition and function in dementia? A systematic review. Brain Sci. 2022, 12, 1136. [Google Scholar] [CrossRef]
- Jacobs, B.; Schall, M.; Scheibel, A.B. A quantitative dendritic analysis of Wernicke’s area in humans. II. Gender, hemispheric, and environmental factors. J. Comp. Neurol. 1993, 327, 97–111. [Google Scholar] [CrossRef]
- Tang, X.; Varma, V.R.; Miller, M.I.; Carlson, M.C. Education is associated with sub-regions of the hippocampus and the amygdala vulnerable to neuropathologies of Alzheimer’s disease. Brain Struct. Funct. 2017, 222, 1469–1479. [Google Scholar] [CrossRef] [Green Version]
- Brayne, C.; Ince, P.G.; Keage, H.A.; McKeith, I.G.; Matthews, F.E.; Polvikoski, T.; Sulkava, R. Education, the brain and dementia: Neuroprotection or compensation? EClipSE Collaborative Members. Brain 2010, 133, 2210–2216. [Google Scholar] [CrossRef] [Green Version]
- Ferretti-Rebustini, R.E.D.L.; Jacob-Filho, W.; Suemoto, C.K.; Farfel, J.M.; Leite, R.E.P.; Grinberg, L.T.; Pasqualucci, C.A.; Nitrini, R. Factors associated with morphometric brain changes in cognitively normal aging. Dement. Neuropsychol. 2015, 9, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Noble, K.G.; Grieve, S.M.; Korgaonkar, M.S.; Engelhardt, L.E.; Griffith, E.Y.; Williams, L.M.; Brickman, A.M. Hippocampal volume varies with educational attainment across the lifespan. Front. Hum. Neurosci. 2012, 6, 307. [Google Scholar] [CrossRef] [Green Version]
- Rosano, C.; Aizenstein, H.J.; Newman, A.B.; Venkatraman, V.; Harris, T.; Ding, J.; Satterfield, S.; Yaffe, K. Neuroimaging differences between older adults with maintained versus declining cognition over a 10-year period. Neuroimage 2012, 62, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Perneczky, R.; Wagenpfeil, S.; Lunetta, K.L.; Cupples, L.A.; Green, R.C.; DeCarli, C.; Farrer, L.A.; Kurz, A. Education attenuates the effect of medial temporal lobe atrophy on cognitive function in Alzheimer’s disease: The MIRAGE study. J. Alzheimer’s Dis. 2009, 17, 855–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitrini, R.; Bottino, C.M.; Albala, C.; Capuñay, N.S.C.; Ketzoian, C.; Rodriguez, J.J.L.; Maestre, G.E.; Ramos-Cerqueira, A.T.A.; Caramelli, P. Prevalence of dementia in Latin America: A collaborative study of population-based cohorts. Int. Psychogeriatr. 2009, 21, 622–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caramelli, P.; Poissant, A.; Gauthier, S.; Bellavance, A.; Gauvreau, D.; Lecours, A.R.; Joanette, Y. Educational level and neuropsychological heterogeneity in dementia of the Alzheimer type. Alzheimer Dis. Assoc. Disord. 1997, 11, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chey, J.; Kim, S.E.; Kim, H. The effect of education on regional brain metabolism and its functional connectivity in an aged population utilizing positron emission tomography. Neurosci. Res. 2015, 94, 50–61. [Google Scholar] [CrossRef] [PubMed]
- López-Barroso, D.; de Schotten, M.T.; Morais, J.; Kolinsky, R.; Braga, L.W.; Guerreiro-Tauil, A.; Dehaene, S.; Cohen, L. Impact of literacy on the functional connectivity of vision and language related networks. NeuroImage 2020, 213, 116722. [Google Scholar] [CrossRef]
- Lager, A.C.J.; Torssander, J. Causal effect of education on mortality in a quasi-experiment on 1.2 million Swedes. Proc. Natl. Acad. Sci. USA 2012, 109, 8461–8466. [Google Scholar] [CrossRef] [Green Version]
- Clark, D.; Royer, H. The effect of education on adult mortality and health: Evidence from Britain. Am. Econ. Rev. 2013, 103, 2087–2120. [Google Scholar] [CrossRef] [Green Version]
- Davies, N.M.; Dickson, M.; Smith, G.D.; Van Den Berg, G.J.; Windmeijer, F. The causal effects of education on health outcomes in the UK Biobank. Nat. Hum. Behav. 2018, 2, 117–125. [Google Scholar] [CrossRef]
- Dehaene, S. Evolution of human cortical circuits for reading and arithmetic: The “neuronal recycling” hypothesis. In From Monkey Brain to Human Nrain: A Fyssen Foundation Symposium; MIT Press: Cambridge, MA, USA, 2005; pp. 133–157. [Google Scholar]
- Dehaene-Lambertz, G.; Monzalvo, K.; Dehaene, S. The emergence of the visual word form: Longitudinal evolution of category-specific ventral visual areas during reading acquisition. PLoS Biol. 2018, 16, e2004103. [Google Scholar] [CrossRef] [Green Version]
- Dehaene, S.; Cohen, L.; Morais, J.; Kolinsky, R. Illiterate to literate: Behavioural and cerebral changes induced by reading acquisition. Nat. Rev. Neurosci. 2015, 16, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Farfel, J.M.; Nitrini, R.; Suemoto, C.K.; Grinberg, L.T.; Ferretti, R.E.L.; Leite, R.E.P.; Tampellini, E.; Lima, L.; Farias, D.S.; Neves, R.C.; et al. Very low levels of education and cognitive reserve: A clincopathological study. Neurology 2013, 81, 650–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resende, E.D.P.F.; Rosen, H.J.; Chiang, K.; Staffaroni, A.M.; Allen, I.; Grinberg, L.T.; Carmona, K.C.; Guimarães, H.C.; Carvalho, V.A.; Barbosa, M.T.; et al. Primary school education may be sufficient to moderate a memory-hippocampal relationship. Front. Aging Neurosci. 2018, 10, 381. [Google Scholar] [CrossRef] [PubMed]
- Resende, E.D.P.F.; Tovar-Moll, F.F.; Ferreira, F.M.; Bramati, I.; Souza, L.C.D.; Carmona, K.C.; Guimarães, H.C.; Carvalho, V.A.; Barbosa, M.T.; Caramelli, P. White matter microstructure in illiterate and low-literate elderly Brazilians: Preliminary findings. Cognit. Behav. Neurol. 2018, 31, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Resende, E.D.P.F.; Tovar-Moll, F.F.; Ferreira, F.M.; Bramati, I.; Souza, L.C.D.; Carmona, K.C.; Guimarães, H.C.; Carvalho, V.A.; Barbosa, M.T.; Caramelli, P. Integrity of white matter structure is related to episodic memory performance in the low-educated elderly. Arq. Neuro-Psiquiatr. 2017, 75, 778–784. [Google Scholar] [CrossRef] [Green Version]
- Shankar, S.K.; Mahadevan, A. Brain banking in India: Relevance in current day practice. Indian J. Pathol. Microbiol. 2022, 65, 218–225. [Google Scholar]
- Nicoll, J.A.R.; Bloom, T.; Clarke, A.; Boche, D.; Hilton, D. BRAIN UK: Accessing NHS tissue archives for neuroscience research. Neuropathol. Appl. Neurobiol. 2022, 48, e12766. [Google Scholar] [CrossRef]
- Anestakis, D.; Petanidis, S.; Salifoglou, A.; Tsolaki, M. Clinicopathological guidelines for the management of brain and tissue banking in Greece. Res. Neurol. 2014, 2014, 121634. [Google Scholar]
- Pakkenberg, B.; Pelvig, D.; Marner, L.; Bundgaard, M.J.; Gundersen, H.J.G.; Nyengaard, J.R.; Regeur, L. Aging and the human neocortex. Exp. Gerontol. 2003, 38, 95–99. [Google Scholar] [CrossRef]
- Joelving, F.C.; Billeskov, R.; Christensen, J.R.; West, M.; Pakkenberg, B. Hippocampal neuron and glial cell numbers in Parkinson’s disease—A stereological study. Hippocampus 2006, 16, 826–833. [Google Scholar] [CrossRef]
- Kjær, M.; Fabricius, K.; Sigaard, R.K.; Pakkenberg, B. Neocortical development in brain of young children—A stereological study. Cereb. Cortex 2017, 27, 5477–5484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, J.C.; Pakkenberg, B.; Vann, S.D. Striking reduction in neurons and glial cells in anterior thalamic nuclei of older patients with Down syndrome. Neurobiol. Aging 2019, 75, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, F.A.; Carvalho, L.R.; Grinberg, L.T.; Farfel, J.M.; Ferretti, R.E.; Leite, R.E.; Jacob Filho, W.; Lent, R.; Herculano-Houzel, S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurol. 2009, 513, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Moraes, C.H.; Oliveira-Pinto, A.V.; Castro-Fonseca, E.; da Silva, C.G.; Guimaraes, D.M.; Szczupak, D.; Parente-Bruno, D.R.; Carvalho, L.R.; Polichiso, L.; Gomes, B.V.; et al. Cell number changes in Alzheimer’s disease relate to dementia, not to plaques and tangles. Brain 2013, 136, 3738–3752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira-Pinto, A.V.; Santos, R.M.; Coutinho, R.A.; Oliveira, L.M.; Santos, G.B.; Alho, A.T.; Leite, R.E.; Farfel, J.M.; Suemoto, C.K.; Grinberg, L.T.; et al. Sexual dimorphism in the human olfactory bulb: Females have more neurons and glial cells than males. PLoS ONE 2014, 9, e111733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herculano-Houzel, S.; Lent, R. Isotropic fractionator: A simple, rapid method for the quantification of total cell and neuron numbers in the brain. J. Neurosci. 2005, 25, 2518–2521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lent, R.; Azevedo, F.A.; Andrade-Moraes, C.H.; Pinto, A.V. How many neurons do you have? Some dogmas of quantitative neuroscience under revision. Eur. J. Neurosci. 2012, 35, 1–9. [Google Scholar] [CrossRef]
- Morris, J.C. Current vision and scoring rules the clinical dementia rating (CDR). Neurology 1993, 43, 2412–2414. [Google Scholar] [CrossRef]
- Jorm, A.F.; Jacomb, P.A. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): Socio-demographic correlates, reliability, validity and some norms. Psychol. Med. 1989, 19, 1015–1022. [Google Scholar] [CrossRef]
- Oliveira-Pinto, A.V.; Andrade-Moraes, C.H.; Oliveira, L.M.; Parente-Bruno, D.R.; Santos, R.M.; Coutinho, R.A.; Alho, A.T.; Leite, R.E.; Suemoto, C.K.; Grinberg, L.T.; et al. Do age and sex impact on the absolute cell numbers of human brain regions? Brain Struct. Func. 2016, 221, 3547–3559. [Google Scholar] [CrossRef]
- Azevedo, F.A.; Andrade-Moraes, C.H.; Curado, M.R.; Oliveira-Pinto, A.V.; Guimarães, D.M.; Szczupak, D.; Gomes, B.V.; Alho, A.T.L.; Polichiso, L.; Tampellini, E.; et al. Automatic isotropic fractionation for large-scale quantitative cell analysis of nervous tissue. J. Neurosci. Meth. 2013, 212, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Neves, K.; Guimarães, D.M.; Rayêe, D.; Valerio-Gomes, B.; Iack, P.M.; Lent, R.; Mota, B. The reliability of the isotropic fractionator method for counting total cells and neurons. J. Neurosci. Meth. 2019, 326, 108392. [Google Scholar] [CrossRef] [PubMed]
- Dehaene, S.; Cohen, L. Cultural recycling of cortical maps. Neuron 2007, 56, 384–398. [Google Scholar] [CrossRef] [PubMed]
- Dehaene, S.; Pegado, F.; Braga, L.W.; Ventura, P.; Filho, G.N.; Jobert, A.; Dehaene-Lambertz, G.; Kolinsky, R.; Morais, J.; Cohen, L. How learning to read changes the cortical networks for vision and language. Science 2010, 330, 1359–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cachia, A.; Roell, M.; Mangin, J.F.; Sun, Z.Y.; Jobert, A.; Braga, L.; Houde, O.; Dehaene, S.; Borst, G. How interindividual differences in brain anatomy shape reading accuracy. Brain Struct. Funct. 2018, 223, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Dehaene, S.; Dehaene-Lambertz, G. Is the brain prewired for letters? Nat. Neurosci. 2016, 19, 1192–1193. [Google Scholar] [CrossRef]
- Bento-Torres, N.V.; Bento-Torres, J.; Tomás, A.M.; Costa, V.O.; Corrêa, P.G.; Costa, C.N.; Jardim, N.Y.; Picanço-Diniz, C.W. Influence of education and age on cognitive performance in healthy older adults. Braz. J. Med. Biol. Res. 2017, 50, e5892. [Google Scholar] [CrossRef] [Green Version]
- Boldrini, M.; Fulmore, C.A.; Tartt, A.N.; Simeon, L.R.; Pavlova, I.; Poposka, V.; Rosoklija, G.B.; Stankov, A.; Arango, V.; Dwork, A.J.; et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 2018, 22, 589–599. [Google Scholar] [CrossRef] [Green Version]
- Sorrells, S.F.; Paredes, M.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I.; et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 2018, 555, 377–381. [Google Scholar] [CrossRef]
- Supekar, K.; Swigart, A.G.; Tenison, C.; Jolles, D.D.; Rosenberg-Lee, M.; Fuchs, L.; Menon, V. Neural predictors of individual differences in response to math tutoring in primary-grade school children. Proc. Natl. Acad. Sci. USA 2013, 110, 8230–8235. [Google Scholar] [CrossRef] [Green Version]
- Qin, S.; Cho, S.; Chen, T.; Rosenberg-Lee, M.; Geary, D.C.; Menon, V. Hippocampal-neocortical functional reorganization underlies children’s cognitive development. Nat. Neurosci. 2014, 17, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Menon, V. Memory and cognitive control circuits in mathematical cognition and learning. Prog. Brain Res. 2016, 227, 159–186. [Google Scholar] [PubMed] [Green Version]
- Kreiman, G.; Koch, C.; Fried, I. Category-specific visual responses of single neurons in the human medial temporal lobe. Nat. Neurosci. 2000, 3, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Kutter, E.F.; Bostroem, J.; Elger, C.E.; Mormann, F.; Nieder, A. Single neurons in the human brain encode numbers. Neuron 2018, 100, 753–761.e4. [Google Scholar] [CrossRef] [Green Version]
- Yeatman, J.D.; Dougherty, R.F.; Ben-Shachar, M.; Wandell, B.A. Development of white matter and reading skills. Proc. Natl. Acad. Sci. USA 2012, 109, E3045–E3053. [Google Scholar] [CrossRef] [Green Version]
- Fields, R.D. A new mechanism of nervous system plasticity: Activity-dependent myelination. Nat. Rev. Neurosci. 2015, 16, 756–767. [Google Scholar] [CrossRef]
- Tallal, P. The science of literacy: From the laboratory to the classroom. Proc. Natl. Acad. Sci. USA 2000, 97, 2402–2404. [Google Scholar] [CrossRef] [Green Version]
- Carreiras, M.; Seghier, M.L.; Baquero, S.; Estévez, A.; Lozano, A.; Devlin, J.T.; Price, C.J. An anatomical signature for literacy. Nature 2009, 461, 983–986. [Google Scholar] [CrossRef]


| Case | Age at Death | Schooling | Cause of Death | Time from Death to Fixation | Cerebral Weight (g) | MTL Weight(g) | Braak | CERAD | AP Diagnostic |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 62 | 8 | Acute myocardial infarction | 11 h 30 min | 1220 | 31.1 | 2 | 0 | Normal |
| 2 | 47 | 8 | Pulmonary edema | 18 h | 1582 | 39.9 | 0 | 0 | Normal |
| 3 | 49 | 8 | Pulmonary Alveolar Hemorrhage | 16 h 38 min | 1210 | 35.76 | 0 | 0 | Normal |
| 4 | 63 | 11 | Retroperitonial Hemorrhage | 12 h 10 min | 1176 | 27.6 | 1 | 0 | Normal |
| 5 | 56 | 12 | Acute Pulmonary edema | 11 h 20 min | 1298 | 35.54 | 1 | 0 | Normal |
| 6 | 64 | 11 | Acute myocardial infarction | 18 h 42 min | 1453 | 32.54 | 1 | 0 | Normal |
| 7 | 47 | 16 | Broncho-pneumonia | 13 h 30 min | 1775 | 30.84 | 1 | 0 | Normal |
| 8 | 54 | 15 | Pulmonary thromboembolism | 8 h 01 min | 1310 | 30.68 | 0 | 0 | Normal |
| 9 | 48 | 11 | Acute myocardial infarction | 16 h 07 min | 1362 | 26.14 | 2 | 0 | Normal |
| 10 | 49 | 15 | Pulmonary edema | 14 h 59 min | 1492 | 33.1 | 0 | 0 | Normal |
| 11 | 61 | 8 | Acute Pulmonary edema | 9 h 25 min | 1544 | 29.98 | 1 | 0 | Normal |
| 12 | 55 | 12 | Pulmonary thromboembolism | 18 h 55 min | 1249 | 28.48 | 0 | 0 | Normal |
| 13 | 58 | 11 | Pulmonary infarction | 12 h 45 min | 1382 | 25.76 | 1 | 0 | Normal |
| 14 | 58 | 11 | Pulmonary thromboembolism | 14 h 52 min | 1351 | 31.48 | 2 | 0 | Normal |
| 15 | 59 | 4 | Acute renal failure | 13 h 35 min | 1271 | 43.36 | 2 | 0 | Normal |
| 16 | 58 | 4 | Sepsis | 15 h 25 min | 1210 | 24.94 | 2 | 0 | Normal |
| 17 | 59 | 4 | Bilateral caseous bronchopneumonia | 10 h 18 min | 1360 | 33.7 | 1 | 0 | Normal |
| 18 | 53 | 2 | Pulmonary tuberculosis | 13 h 24 min | 1282 | 31.44 | 3 | 0 | Normal |
| 19 | 49 | 4 | Chronic Pneumopathy | 11 h | 1221 | 31.46 | 1 | 0 | Normal |
| 20 | 55 | 4 | Pulmonary edema | 12 h 19 min | 1150 | 37.58 | 1 | 1 | Normal |
| 21 | 62 | 0 | Septic shock | 17 h 57 min | 1209 | 27.28 | 1 | 0 | Normal |
| 22 | 60 | 1 | Acute myocardial infarction | 9 h 40 min | 1484 | 35.66 | 1 | A | Normal |
| 23 | 62 | 4 | Pulmonary edema | 13 h 38 min | 1238 | 24.6 | 2 | 0 | Normal |
| 24 | 62 | 4 | Hemiperitoneum | 12 h 1 min | 1232 | 28.08 | 0 | 0 | Normal |
| 25 | 64 | 4 | Broncho-pneumonia | 16 h 25 min | 1200 | 30.62 | 0 | 0 | Normal |
| 26 | 62 | 4 | Acute Pulmonary edema | 19 h 26 min | 1352 | 36.44 | 3 | 0 | Normal |
| Unadjusted Model | Model 1 * | Model 2 ** | ||||
|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
| Neurons | 0.95 (−0.07; 0.26) | 0.25 | 0.12 (−0.08; 0.32) | 0.22 | 0.12 (−0.09; 0.34) | 0.23 |
| Non-neurons | −0.08 (−0.36; 0.21) | 0.59 | −0.17 (−0.51; 0.16) | 0.30 | −0.20 (−0.55; 0.15) | 0.26 |
| Total cells | 0.20 (−0.23; 0.27) | 0.87 | −0.05 (−0.35; 0.24) | 0.71 | −0.07 (−0.38; 0.24) | 0.63 |
| Unadjusted Model | Model 1 * | Model 2 ** | ||||
|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
| Neurons | −0.003 (−0.022; 0.015) | 0.70 | −0.005 (−0.030; 0.020) | 0.70 | −0.005 (−0.030; 0.021) | 0.71 |
| Non-neurons | 0.005 (−0.027; 0.037) | 0.74 | 0.020 (−0.021; 0.060) | 0.33 | 0.022 (−0.020; 0.064 | 0.29 |
| Total cells | 0.002 (−0.026; 0.029) | 0.90 | 0.015 (−0.020; 0.050) | 0.39 | 0.018 (−0.019; 0.054) | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Morais, V.A.C.; de Oliveira-Pinto, A.V.; Mello Neto, A.F.; Freitas, J.S.; da Silva, M.M.; Suemoto, C.K.; Leite, R.P.; Grinberg, L.T.; Jacob-Filho, W.; Pasqualucci, C.; et al. Resilience of Neural Cellularity to the Influence of Low Educational Level. Brain Sci. 2023, 13, 104. https://doi.org/10.3390/brainsci13010104
de Morais VAC, de Oliveira-Pinto AV, Mello Neto AF, Freitas JS, da Silva MM, Suemoto CK, Leite RP, Grinberg LT, Jacob-Filho W, Pasqualucci C, et al. Resilience of Neural Cellularity to the Influence of Low Educational Level. Brain Sciences. 2023; 13(1):104. https://doi.org/10.3390/brainsci13010104
Chicago/Turabian Stylede Morais, Viviane A. Carvalho, Ana V. de Oliveira-Pinto, Arthur F. Mello Neto, Jaqueline S. Freitas, Magnólia M. da Silva, Claudia Kimie Suemoto, Renata P. Leite, Lea T. Grinberg, Wilson Jacob-Filho, Carlos Pasqualucci, and et al. 2023. "Resilience of Neural Cellularity to the Influence of Low Educational Level" Brain Sciences 13, no. 1: 104. https://doi.org/10.3390/brainsci13010104
APA Stylede Morais, V. A. C., de Oliveira-Pinto, A. V., Mello Neto, A. F., Freitas, J. S., da Silva, M. M., Suemoto, C. K., Leite, R. P., Grinberg, L. T., Jacob-Filho, W., Pasqualucci, C., Nitrini, R., Caramelli, P., & Lent, R. (2023). Resilience of Neural Cellularity to the Influence of Low Educational Level. Brain Sciences, 13(1), 104. https://doi.org/10.3390/brainsci13010104