Reduced Sleep Amount and Increased Sleep Latency in Prisoners: A Pilot Study in an Italian Jail
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Materials
2.3. Procedure
2.4. Statistical Analyses
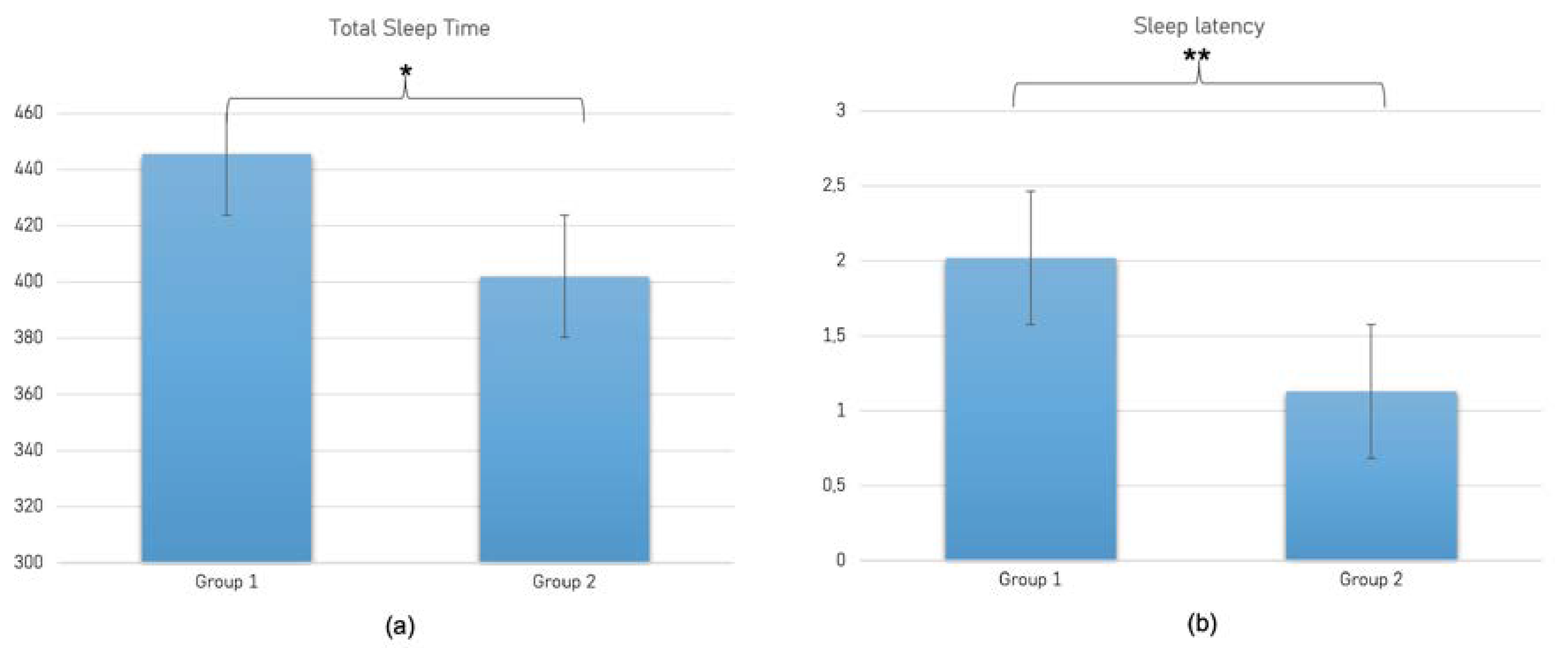
3. Results
3.1. Correlation Analysis
3.2. ANOVA Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, J.M. Sleep function: An evolutionary perspective. Lancet Neurol. 2022, 21, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Couyoumdjian, A.; Sdoia, S.; Tempesta, D.; Curcio, G.; Rastellini, E.; De Gennaro, L.; Ferrara, M. The effects of sleep and sleep deprivation on task-switching performance. J. Sleep Res. 2010, 19, 64–70. [Google Scholar] [CrossRef]
- Killgore, W.D. Effects of sleep deprivation on cognition. Prog. Brain Res. 2010, 185, 105–129. [Google Scholar] [CrossRef]
- McCoy, J.G.; Strecker, R.E. The cognitive cost of sleep lost. Neurobiol. Learn. Mem. 2011, 96, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Tempesta, D.; Couyoumdjian, A.; Curcio, G.; Moroni, F.; Marzano, C.; De Gennaro, L.; Ferrara, M. Lack of sleep affects the evaluation of emotional stimuli. Brain Res. Bull. 2010, 82, 104–108. [Google Scholar]
- Tempesta, D.; Socci, V.; De Gennaro, L.; Ferrara, M. Sleep and emotional processing. Sleep Med. Rev. 2018, 40, 183–195. [Google Scholar] [CrossRef]
- Peigneux, P.; Laureys, S.; Delbeuck, X.; Maquet, P. Sleeping brain, learning brain. The role of sleep for memory systems. Neuroreport 2001, 12, A111–A124. [Google Scholar] [CrossRef] [PubMed]
- Curcio, G.; Ferrara, M.; De Gennaro, L. Sleep loss, learning capacity and academic performance. Sleep Med. Rev. 2006, 10, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Pilcher, J.J.; Morris, D.M.; Donnelly, J.; Feigl, H.B. Interactions between sleep habits and self-control. Front. Hum. Neurosci. 2015, 9, 284. [Google Scholar] [CrossRef] [PubMed]
- Krause, A.J.; Ben Simon, E.; Mander, B.A.; Greer, S.M.; Saletin, J.M.; Goldstein-Piekarski, A.N.; Walker, M.P. The sleep-deprived human brain. Nat. Rev. Neurosci. 2017, 18, 404–418. [Google Scholar] [CrossRef]
- Kucharczyk, E.R.; Morgan, K.; Hall, A.P. The occupational impact of sleep quality and insomnia symptoms. Sleep Med. Rev. 2012, 16, 547–559. [Google Scholar] [CrossRef]
- Itani, O.; Jike, M.; Watanabe, N.; Kaneita, Y. Short sleep duration and health outcomes: A systematic review, meta-analysis, and meta-regression. Sleep Med. 2017, 32, 246–256. [Google Scholar] [CrossRef]
- Irwin, M.R. Why Sleep Is Important for Health: A Psychoneuroimmunology Perspective. Annu. Rev. Psychol. 2015, 66, 143–172. [Google Scholar] [CrossRef]
- Pilcher, J.J.; Ginter, D.R.; Sadowsky, B. Sleep quality versus sleep quantity: Relationships between sleep and measures of health, well-being and sleepiness in college students. J. Psychosom. Res. 1997, 42, 583–596. [Google Scholar] [CrossRef]
- Mellman, T.A. Sleep and Anxiety Disorders. Psychiatr. Clin. N. Am. 2006, 29, 1047–1058. [Google Scholar] [CrossRef]
- Koffel, E.; Watson, D. The two-factor structure of sleep complaints and its relation to depression and anxiety. J. Abnorm. Psychol. 2009, 118, 183–194. [Google Scholar] [CrossRef]
- Dewa, L.H.; Kyle, S.D.; Hassan, L.; Shaw, J.; Senior, J. Prevalence, associated factors and management of insomnia in prison populations: An integrative review. Sleep Med. Rev. 2015, 24, 13–27. [Google Scholar] [CrossRef]
- Barker, L.F.; Ireland, J.L.; Chu, S.; Ireland, C.A. Sleep and its association with aggression among prisoners: Quantity or quality? Int. J. Law Psychiatry 2016, 47, 115–121. [Google Scholar] [CrossRef]
- Morris, N.P.; Holliday, J.R.; Binder, R.L. Litigation Over Sleep Deprivation in U.S. Jails and Prisons. Psychiatr. Serv. 2021, 72, 1237–1239. [Google Scholar] [CrossRef]
- Elger, B.S.; Sekera, E. Prospective evaluation of insomnia in prison using the Pittsburgh Sleep Quality Index: Which are the factors predicting insomnia? Int. J. Psychiatry Clin. Pract. 2009, 13, 206–217. [Google Scholar] [CrossRef]
- Elger, B.S. Prevalence, types and possible causes of insomnia in a Swiss remand prison. Eur. J. Epidemiol. 2004, 19, 665–677. [Google Scholar] [CrossRef]
- Socci, V.; Rossi, R.; Talevi, D.; Crecini, C.; Tempesta, D.; Pacitti, F. Sleep, stress and trauma. J. Psychopathol. 2020, 26, 92–98. [Google Scholar]
- Tempesta, D.; Curcio, G.; De Gennaro, L.; Ferrara, M. Long-Term Impact of Earthquakes on Sleep Quality. PLoS ONE 2013, 8, e55936. [Google Scholar] [CrossRef]
- Wang, S.-J.; Rushiti, F.; Sejdiu, X.; Pacolli, S.; Gashi, B.; Salihu, F.; Modvig, J. Survivors of war in northern Kosovo (III): The role of anger and hatred in pain and PTSD and their interactive effects on career outcome, quality of sleep and suicide ideation. Confl. Health 2012, 6, 4. [Google Scholar] [CrossRef]
- Papadimitriou, G.N.; Linkowski, P. Sleep disturbance in anxiety disorders. Int. Rev. Psychiatry 2005, 17, 229–236. [Google Scholar] [CrossRef]
- Sheppard, N.; Hogan, L. Prevalence of insomnia and poor sleep quality in the prison population: A systematic review. J. Sleep Res. 2022, 31, e13677. [Google Scholar] [CrossRef]
- D’Aurizio, G.; Caldarola, A.; Ninniri, M.; Avvantaggiato, M.; Curcio, G. Sleep Quality and Psychological Status in a Group of Italian Prisoners. Int. J. Environ. Res. Public Health 2020, 17, 4224. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Ball, R.; Ranieri, W.F. Comparison of Beck Depression Inventories-IA and-II in Psychiatric Outpatients. J. Pers. Assess. 1996, 67, 588–597. [Google Scholar] [CrossRef]
- Sica, C.; Ghisi, M. The Italian versions of the beck anxiety inventory and the beck depression inventory-II: Psychometric properties and discriminant power. In Leading-Edge Psychological Tests and Testing Research; Lange, M.A., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2007; pp. 27–50. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Garbin, M.G. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evalua-tion. Clin. Psychol. Rev. 1988, 8, 77–100. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.E. Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1970. [Google Scholar]
- Pedrabissi, L.; Santinello, M. Verifica della validità dello STAI forma Y di Spielberger [Verification of the validity of the STAI, form, Y., by Spielberger]. Giunti Organ. Spec. 1989, 191–192, 11–14. [Google Scholar]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Curcio, G.G.; Tempesta, D.; Scarlata, S.; Marzano, C.; Moroni, F.; Rossini, P.M.; Ferrara, M.; De Gennaro, L. Validity of the Italian Version of the Pittsburgh Sleep Quality Index (PSQI). Neurol. Sci. 2013, 34, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.M. Insomnia: Psychological Assessment and Management; Guilford Press: New York, NY, USA, 1993. [Google Scholar]
- Castronovo, V.; Galbiati, A.; Marelli, S.; Brombin, C.; Cugnata, F.; Giarolli, L.; Anelli, M.M.; Rinaldi, F.; Ferini-Strambi, L. Validation study of the Italian version of the Insomnia Severity Index (ISI). Neurol. Sci. 2016, 37, 1517–1524. [Google Scholar] [CrossRef]
- Morin, C.M.; Belleville, G.; Bélanger, L.; Ivers, H. The Insomnia Severity Index: Psychometric Indicators to Detect Insomnia Cases and Evaluate Treatment Response. Sleep 2011, 34, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Værøy, H. Depression, Anxiety, and History of Substance Abuse Among Norwegian Inmates in Preventive Detention: Reasons to Worry? BMC Psychiatry 2011, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Dewa, L.H.; Hassan, L.; Shaw, J.; Senior, J. Design of a treatment pathway for insomnia in prison settings in England: A modified Delphi study. BMJ Open 2018, 8, e022406. [Google Scholar] [CrossRef]
- Goudard, A.; Lalande, L.; Bertin, C.; Sautereau, M.; Le Borgne, M.; Cabelguenne, D. Sleep Disorders and Therapeutic Man-agement: A Survey in a French Population of Prisoners. J. Correct. Health Care 2017, 23, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Carli, V.; Roy, A.; Bevilacqua, L.; Maggi, S.; Cesaro, C.; Sarchiapone, M. Insomnia and suicidal behaviour in prisoners. Psychiatry Res. 2011, 185, 141–144. [Google Scholar] [CrossRef]


| BDI-AS | BDI-C | BDI Total Score | STAI Y1 | STAI Y2 | PSQI Tot | ISI Severity | ISI Impact | ISI Satisfaction | ISI Total Score | Latency | Efficiency | TST | WASO | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| BDI-AS | ||||||||||||||||||||||||||||
| BDI-C | 0.58 | <0.001 | ||||||||||||||||||||||||||
| BDI total score | 0.933 | <0.001 | 0.82 | <0.001 | ||||||||||||||||||||||||
| STAI Y1 | 0.518 | <0.001 | 0.213 | 0.102 | 0.462 | <0.001 | ||||||||||||||||||||||
| STAI Y2 | 0.464 | <0.001 | 0.228 | 0.079 | 0.44 | <0.001 | 0.718 | <0.001 | ||||||||||||||||||||
| PSQI tot | 0.385 | 0.002 | 0.151 | 0.251 | 0.331 | 0.01 | 0.607 | <0.001 | 0.62 | <0.001 | ||||||||||||||||||
| ISI severity | 0.102 | 0.438 | 0.193 | 0.14 | 0.188 | 0.15 | 0.385 | 0.002 | 0.424 | 0.001 | 0.499 | <0.001 | ||||||||||||||||
| ISI impact | 0.168 | 0.201 | 0.166 | 0.204 | 0.207 | 0.113 | 0.31 | 0.016 | 0.311 | 0.015 | 0.455 | <0.001 | 0.65 | <0.001 | ||||||||||||||
| ISI satisfaction | 0.258 | 0.047 | 0.224 | 0.085 | 0.299 | 0.02 | 0.475 | <0.001 | 0.471 | <0.001 | 0.479 | <0.001 | 0.732 | <0.001 | 0.729 | <0.001 | ||||||||||||
| ISI total score | 0.193 | 0.14 | 0.218 | 0.095 | 0.257 | 0.048 | 0.437 | <0.001 | 0.452 | <0.001 | 0.535 | <0.001 | 0.9 | <0.001 | 0.872 | <0.001 | 0.914 | <0.001 | ||||||||||
| Latency | 0.195 | 0.135 | 0.004 | 0.977 | 0.137 | 0.298 | 0.413 | 0.001 | 0.297 | 0.021 | 0.265 | 0.041 | 0.238 | 0.068 | 0.017 | 0.9 | 0.253 | 0.051 | 0.197 | 0.132 | ||||||||
| Efficiency | 0.128 | 0.33 | 0.271 | 0.037 | 0.2 | 0.126 | 0.089 | 0.501 | −0.01 | 0.942 | 0.045 | 0.734 | 0.084 | 0.524 | 0.079 | 0.548 | 0.07 | 0.595 | 0.087 | 0.509 | −0.211 | 0.106 | ||||||
| TST | 0.1 | 0.447 | 0.264 | 0.042 | 0.201 | 0.124 | 0.295 | 0.022 | 0.156 | 0.233 | 0.127 | 0.333 | 0.142 | 0.278 | 0.017 | 0.896 | 0.252 | 0.052 | 0.157 | 0.232 | 0.058 | 0.66 | 0.311 | 0.015 | ||||
| WASO | −0.119 | 0.363 | −0.104 | 0.431 | −0.111 | 0.398 | 0.022 | 0.866 | 0.057 | 0.666 | −0.024 | 0.853 | 0.007 | 0.96 | −0.112 | 0.393 | 0.027 | 0.84 | −0.025 | 0.848 | 0.182 | 0.164 | −0.84 | 0.001 | 0.204 | 0.118 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Aurizio, G.; Tosti, B.; Tempesta, D.; Avvantaggiato, L.; Splendiani, A.; Sacco, S.; Mandolesi, L.; Curcio, G. Reduced Sleep Amount and Increased Sleep Latency in Prisoners: A Pilot Study in an Italian Jail. Brain Sci. 2023, 13, 132. https://doi.org/10.3390/brainsci13010132
D’Aurizio G, Tosti B, Tempesta D, Avvantaggiato L, Splendiani A, Sacco S, Mandolesi L, Curcio G. Reduced Sleep Amount and Increased Sleep Latency in Prisoners: A Pilot Study in an Italian Jail. Brain Sciences. 2023; 13(1):132. https://doi.org/10.3390/brainsci13010132
Chicago/Turabian StyleD’Aurizio, Giulia, Beatrice Tosti, Daniela Tempesta, Lucia Avvantaggiato, Alessandra Splendiani, Simona Sacco, Laura Mandolesi, and Giuseppe Curcio. 2023. "Reduced Sleep Amount and Increased Sleep Latency in Prisoners: A Pilot Study in an Italian Jail" Brain Sciences 13, no. 1: 132. https://doi.org/10.3390/brainsci13010132
APA StyleD’Aurizio, G., Tosti, B., Tempesta, D., Avvantaggiato, L., Splendiani, A., Sacco, S., Mandolesi, L., & Curcio, G. (2023). Reduced Sleep Amount and Increased Sleep Latency in Prisoners: A Pilot Study in an Italian Jail. Brain Sciences, 13(1), 132. https://doi.org/10.3390/brainsci13010132







