Time-Frequency Analysis of Somatosensory Evoked High-Frequency (600 Hz) Oscillations as an Early Indicator of Arousal Recovery after Hypoxic-Ischemic Brain Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model and Ethics
2.2. Asphyxial CA Model and Experimental Procedures
2.3. SSEP Stimulation and Recording Protocol
2.4. Neurological Evaluation
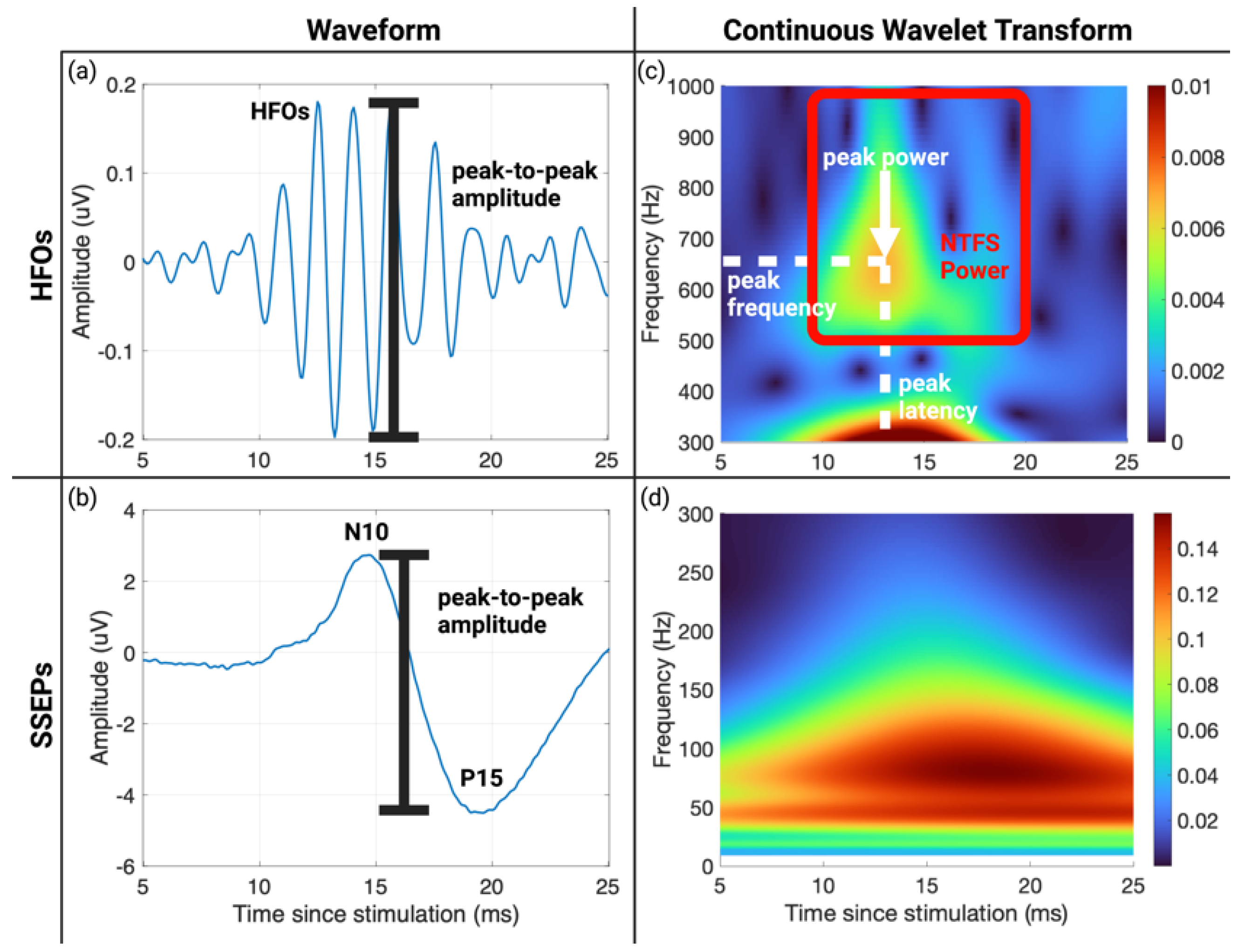
2.5. Continuous Wavelet Transform
2.6. SSEP and HFO Parameter Extraction
2.7. Statistical Analysis
3. Results
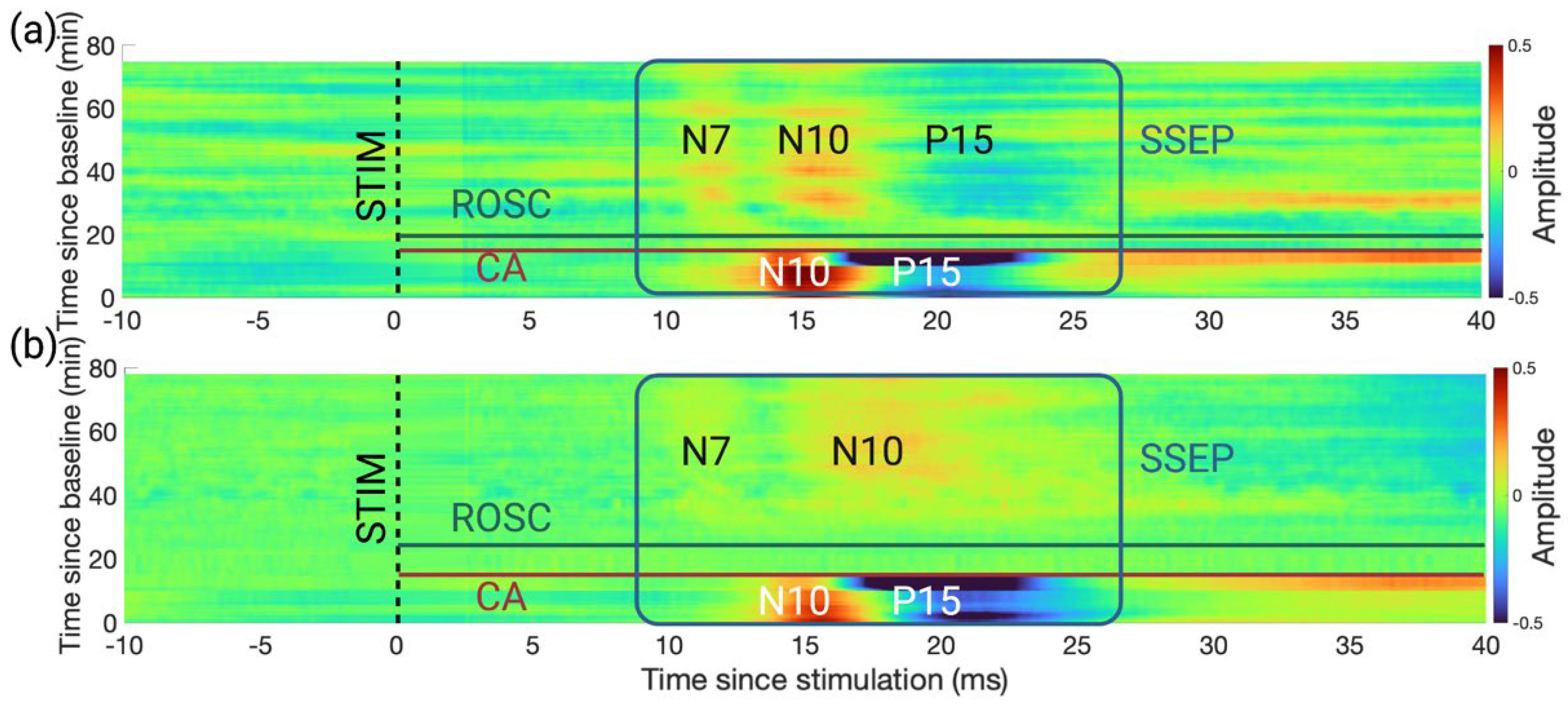
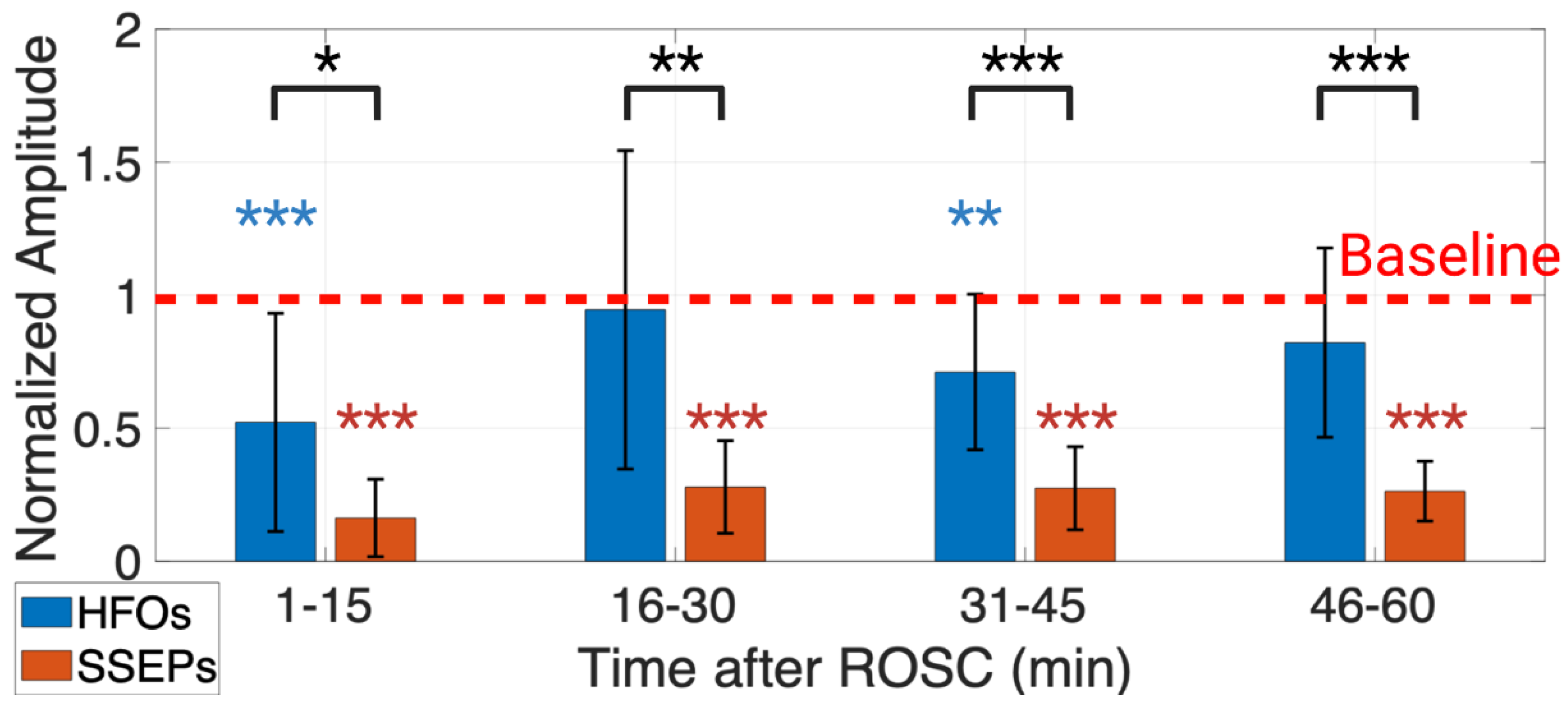
3.1. An Earlier Recovery of HFOs vs. SSEPs Shown in the Time Domain
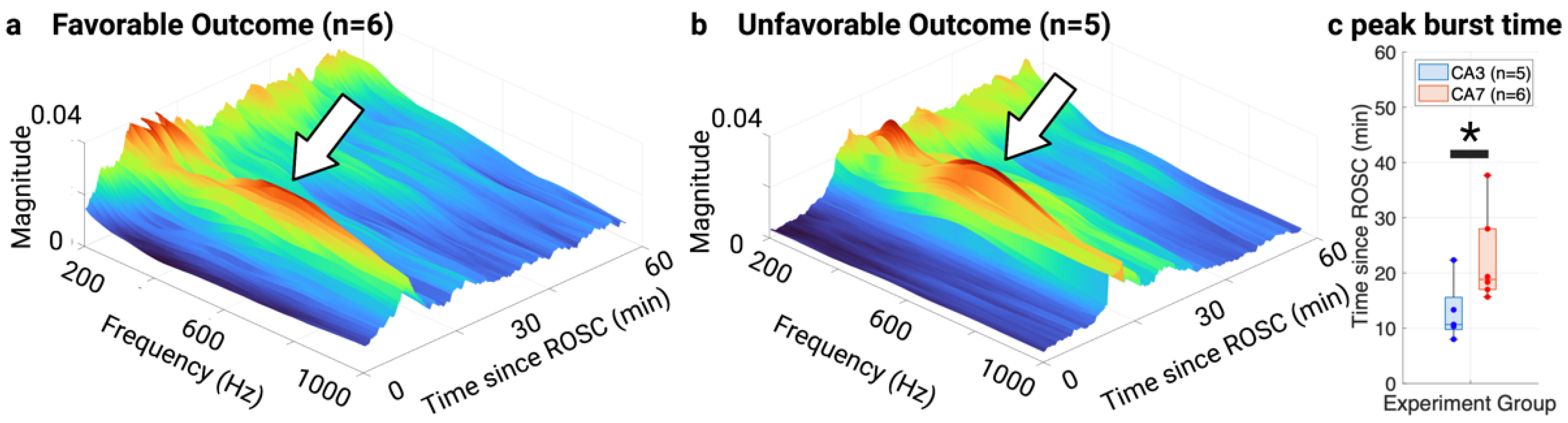
3.2. Evolution of HFO Recovery in the TF Domain
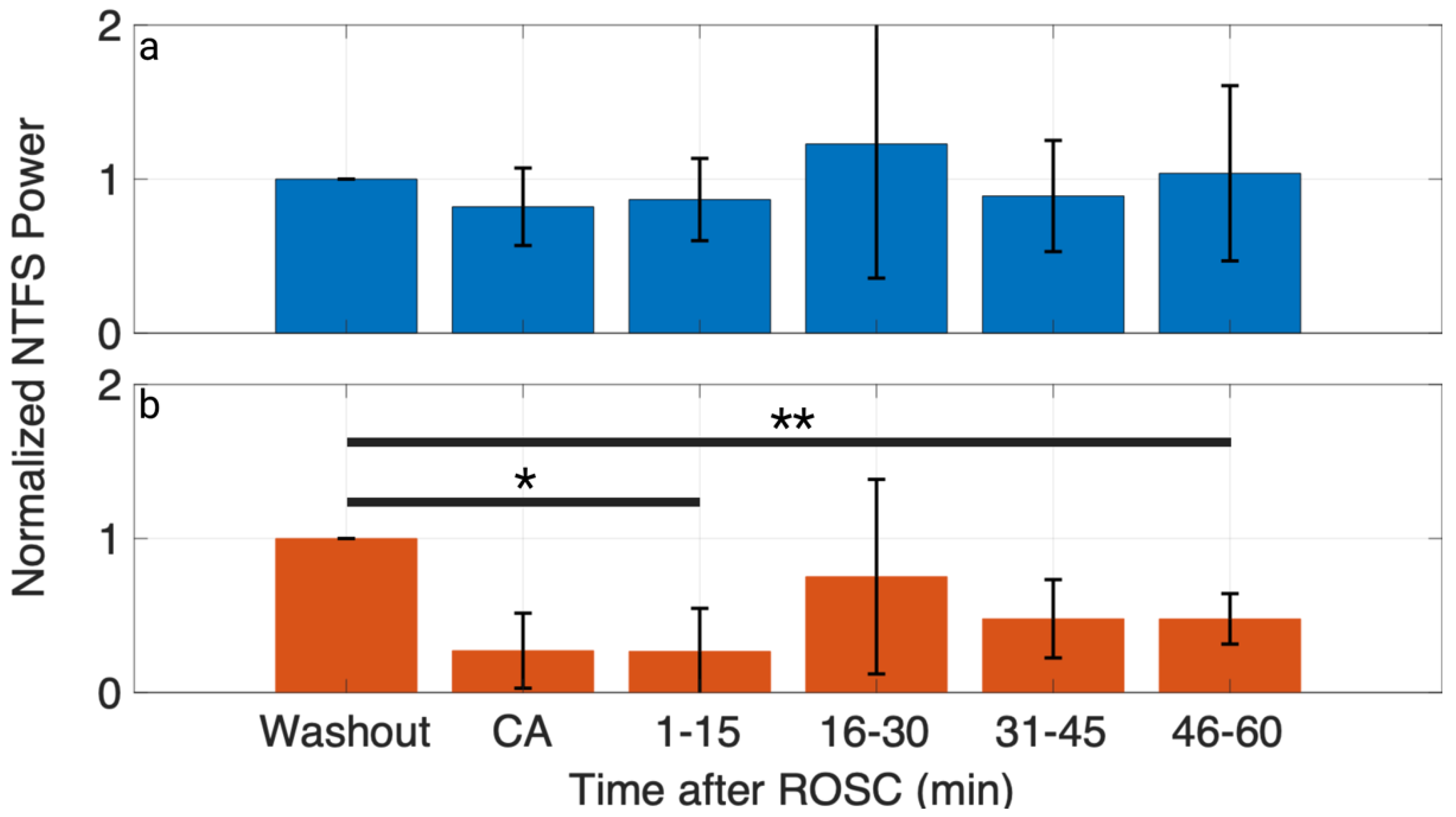
3.3. NTFS Power of HFOs Associated with Neurological Outcome
3.4. Stability of Time- and TF-Domain Measures of HFOs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Neurologic Deficit Scale
| Category | Score |
|---|---|
| General Behavior Deficit | 0–19 |
| Alerting | Normal (10), stuporous (5), comatose (0) |
| Eye opening | Open spontaneously (3), open to pain (1), absent (0) |
| Respiration | Normal (6), abnormal (3), absent (0) |
| Brainstem function | 0–21 |
| Olfaction | Present (3), absent (0) |
| Vision | Present (3), absent (0) |
| Pupillary reflex | Present (3), absent (0) |
| Corneal reflex | Present (3), absent (0) |
| Startle reflex | Present (3), absent (0) |
| Whisker stimulation | Present (3), absent (0) |
| Swallowing | Present (3), absent (0) |
| Motor Assessment | 0–6 |
| Strength | Normal (3), weak movement (1), no movement (0) (each side tested and scored separately) |
| Sensory Assessment | 0–6 |
| Pain response | Brisk withdrawal (3), weak movement (1), no movement (0) (each side tested and scored separately) |
| Motor behavior | 0–6 |
| Gait coordinate | Normal (3), abnormal (1), absent (0) |
| Balance beam walking | Normal (3), abnormal (1), absent (0) |
| Behavior | 0–12 |
| Righting reflex | Normal (3), abnormal (1), absent (0) |
| Negative geotaxis | Normal (3), abnormal (1), absent (0) |
| Visual placing | Normal (3), abnormal (1), absent (0) |
| Turning alley | Normal (3), abnormal (1), absent (0) |
| Seizures | 0–10 |
| Seizures | No seizure (10), focal seizure (5), generalized seizure (0) |
Appendix B. HFO TF Peak Latency, TF Peak Frequency, and TF Peak Power Evolution
| Parameters | CA Duration | Washout | CA | 1–15 min | 16–30 min | 31–45 min | 46–60 min | p-Value |
|---|---|---|---|---|---|---|---|---|
| Peak Latency (ms) | 3 min | 15.77 ± 4.26 | 13.70 ± 3.59 | 13.45 ± 3.68 | 12.26 ± 2.46 | 15.73 ± 3.49 | 12.59 ± 1.67 | 0.44 |
| 7 min | 16.05 ± 3.49 | 13.46 ± 2.68 | 15.70 ± 4.39 | 13.15 ± 3.67 | 12.67 ± 3.89 | 15.22 ± 3.82 | 0.18 | |
| Peak Frequency (Hz) | 3 min | 575 ± 90 | 627 ± 83 | 674 ± 143 | 612 ± 177 | 533 ± 50 | 540 ± 40 | 0.44 |
| 7 min | 563 ± 88 | 711 ± 235 | 729 ± 211 | 587 ± 107 | 573 ± 133 | 509 ± 14 | 0.18 | |
| Peak Power (normalized) | 3 min | 1.00 ± 0 | 0.69 ± 0.27 | 0.67 ± 0.23 | 1.19 ± 0.74 | 0.86 ± 0.32 | 0.98 ± 0.28 | 0.24 |
| 7 min | 1.00 ± 0 | 0.24 ± 0.23 | 0.26 ± 0.34 | 0.76 ± 0.80 | 0.56 ± 0.27 | 0.61 ± 0.23 | 0.05 |
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation 2022, 145, E153–E639. [Google Scholar] [CrossRef]
- Rossetti, A.O.; Rabinstein, A.A.; Oddo, M. Neurological Prognostication of Outcome in Patients in Coma after Cardiac Arrest. Lancet Neurol. 2016, 15, 597–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivaraju, A.; Gilmore, E.J.; Wira, C.R.; Stevens, A.; Rampal, N.; Moeller, J.J.; Greer, D.M.; Hirsch, L.J.; Gaspard, N. Prognostication of Post-Cardiac Arrest Coma: Early Clinical and Electroencephalographic Predictors of Outcome. Intensive Care Med. 2015, 41, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Elmer, J.; Torres, C.; Aufderheide, T.P.; Austin, M.A.; Callaway, C.W.; Golan, E.; Herren, H.; Jasti, J.; Kudenchuk, P.J.; Scales, D.C.; et al. Association of Early Withdrawal of Life-Sustaining Therapy for Perceived Neurological Prognosis with Mortality after Cardiac Arrest. Resuscitation 2016, 102, 127–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Town, J.A.; Churpek, M.M.; Yuen, T.C.; Huber, M.T.; Kress, J.P.; Edelson, D.P. Relationship between ICU Bed Availability, ICU Readmission, and Cardiac Arrest on the General Wards. Crit. Care Med. 2014, 42, 2037. [Google Scholar] [CrossRef] [PubMed]
- Kamps, M.J.A.; Horn, J.; Oddo, M.; Fugate, J.E.; Storm, C.; Cronberg, T.; Wijman, C.A.; Wu, O.; Binnekade, J.M.; Hoedemaekers, C.W.E. Prognostication of Neurologic Outcome in Cardiac Arrest Patients after Mild Therapeutic Hypothermia: A Meta-Analysis of the Current Literature. Intensive Care Med. 2013, 39, 1671–1682. [Google Scholar] [CrossRef]
- Panchal, A.R.; Bartos, J.A.; Cabañas, J.G.; Donnino, M.W.; Drennan, I.R.; Hirsch, K.G.; Kudenchuk, P.J.; Kurz, M.C.; Lavonas, E.J.; Morley, P.T.; et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020, 142, S366–S468. [Google Scholar] [CrossRef]
- Lachance, B.; Wang, Z.; Badjatia, N.; Jia, X. Somatosensory Evoked Potentials and Neuroprognostication after Cardiac Arrest. Neurocrit. Care 2020, 32, 847–857. [Google Scholar] [CrossRef]
- Cronberg, T.; Rundgren, M.; Westhall, E.; Englund, E.; Siemund, R.; Rosén, I.; Widner, H.; Friberg, H. Neuron-Specific Enolase Correlates with Other Prognostic Markers after Cardiac Arrest. Neurology 2011, 77, 623–630. [Google Scholar] [CrossRef]
- Geocadin, R.G.; Peberdy, M.A.; Lazar, R.M. Poor Survival after Cardiac Arrest Resuscitation: A Self-Fulfilling Prophecy or Biologic Destiny? Crit. Care Med. 2012, 40, 979–980. [Google Scholar] [CrossRef]
- Dezfulian, C.; Shiva, S.; Alekseyenko, A.; Pendyal, A.; Beiser, D.G.; Munasinghe, J.P.; Anderson, S.A.; Chesley, C.F.; vanden Hoek, T.L.; Gladwin, M.T. Nitrite Therapy After Cardiac Arrest Reduces Reactive Oxygen Species Generation, Improves Cardiac and Neurological Function, and Enhances Survival via Reversible Inhibition of Mitochondrial Complex I. Circulation 2009, 120, 897–905. [Google Scholar] [CrossRef] [Green Version]
- Webb, A.C.; Samuels, O.B. Reversible Brain Death after Cardiopulmonary Arrest and Induced Hypothermia. Crit. Care Med. 2011, 39, 1538–1542. [Google Scholar] [CrossRef] [PubMed]
- Zandbergen, E.G.J.; Koelman, J.H.T.M.; de Haan, R.J.; Hijdra, A. SSEPs and Prognosis in Postanoxic Coma. Neurology 2006, 67, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Madhok, J.; Maybhate, A.; Xiong, W.; Koenig, M.A.; Geocadin, R.G.; Jia, X.; Thakor, N.V. Quantitative Assessment of Somatosensory-Evoked Potentials after Cardiac Arrest in Rats: Prognostication of Functional Outcomes. Crit. Care Med. 2010, 38, 1709–1717. [Google Scholar] [CrossRef] [Green Version]
- Zanatta, P.; Linassi, F.; Mazzarolo, A.P.; Aricò, M.; Bosco, E.; Bendini, M.; Sorbara, C.; Ori, C.; Carron, M.; Scarpa, B. Pain-Related Somato Sensory Evoked Potentials: A Potential New Tool to Improve the Prognostic Prediction of Coma after Cardiac Arrest. Crit. Care 2015, 19, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Muthuswamy, J.; Kimura, T.; Ding, M.C.; Geocadin, R.; Hanley, D.F.; Thakor, N.V. Vulnerability of the Thalamic Somatosensory Pathway after Prolonged Global Hypoxic–Ischemic Injury. Neuroscience 2002, 115, 917–929. [Google Scholar] [CrossRef]
- Steriade, M.; McCormick, D.A.; Sejnowski, T.J. Thalamocortical Oscillations in the Sleeping and Aroused Brain. Science 1993, 262, 679–685. [Google Scholar] [CrossRef]
- McCormick, D.A.; Bal, T. Sleep and Arousal: Thalamocortical Mechanisms. Annu. Rev. Neurosci. 1997, 20, 185–215. [Google Scholar] [CrossRef] [Green Version]
- Castro-Alamancos, M.A.; Oldford, E. Cortical Sensory Suppression during Arousal Is Due to the Activity-Dependent Depression of Thalamocortical Synapses. J. Physiol. 2002, 541, 319–331. [Google Scholar] [CrossRef]
- Herrera, C.G.; Cadavieco, M.C.; Jego, S.; Ponomarenko, A.; Korotkova, T.; Adamantidis, A. Hypothalamic Feedforward Inhibition of Thalamocortical Network Controls Arousal and Consciousness. Nat. Neurosci. 2015, 19, 290–298. [Google Scholar] [CrossRef]
- Ozaki, I.; Hashimoto, I. Exploring the Physiology and Function of High-Frequency Oscillations (HFOs) from the Somatosensory Cortex. Clin. Neurophysiol. 2011, 122, 1908–1923. [Google Scholar] [CrossRef] [PubMed]
- Gobbelé, R.; Waberski, T.D.; Simon, H.; Peters, E.; Klostermann, F.; Curio, G.; Buchner, H. Different Origins of Low- and High-Frequency Components (600 Hz) of Human Somatosensory Evoked Potentials. Clin. Neurophysiol. 2004, 115, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Ozaki, I.; Hashimoto, I. Impulse Propagation along Thalamocortical Fibers Can Be Detected Magnetically Outside the Human Brain. J. Neurosci. 2008, 28, 12535–12538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, H.; Leyba, L.; Bartolo, A.; Wang, Y.; Okada, Y.C. Synchronized Spikes of Thalamocortical Axonal Terminals and Cortical Neurons Are Detectable Outside the Pig Brain with MEG. J. Neurophysiol. 2002, 87, 626–630. [Google Scholar] [CrossRef]
- Götz, T.; Huonker, R.; Witte, O.W.; Haueisen, J. Thalamocortical Impulse Propagation and Information Transfer in EEG and MEG. J. Clin. Neurophysiol. 2014, 31, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Endisch, C.; Waterstraat, G.; Storm, C.; Ploner, C.J.; Curio, G.; Leithner, C. Cortical Somatosensory Evoked High-Frequency (600 Hz) Oscillations Predict Absence of Severe Hypoxic Encephalopathy after Resuscitation. Clin. Neurophysiol. 2016, 127, 2561–2569. [Google Scholar] [CrossRef]
- Yamada, T.; Kameyama, S.; Fuchigami, Y.; Nakazumi, Y.; Dickins, Q.S.; Kimura, J. Changes of Short Latency Somatosensory Evoked Potential in Sleep. Electroencephalogr. Clin. Neurophysiol. 1988, 70, 126–136. [Google Scholar] [CrossRef]
- Halboni, P.; Kaminski, R.; Gobbelé, R.; Züchner, S.; Waberski, T.D.; Herrmann, C.S.; Töpper, R.; Buchner, H. Sleep Stage Dependant Changes of the High-Frequency Part of the Somatosensory Evoked Potentials at the Thalamus and Cortex. Clin. Neurophysiol. 2000, 111, 2277–2284. [Google Scholar] [CrossRef]
- Gobbelé, R.; Waberski, T.D.; Kuelkens, S.; Sturm, W.; Curio, G.; Buchner, H. Thalamic and Cortical High-Frequency (600 Hz) Somatosensory-Evoked Potential (SEP) Components Are Modulated by Slight Arousal Changes in Awake Subjects. Exp. Brain Res. 2000, 133, 506–513. [Google Scholar] [CrossRef]
- Restuccia, D.; Coppola, G. Auditory Stimulation Enhances Thalamic Somatosensory High-Frequency Oscillations in Healthy Humans: A Neurophysiological Marker of Cross-Sensory Sensitization? Eur. J. Neurosci. 2015, 41, 1079–1085. [Google Scholar] [CrossRef]
- Endisch, C.; Storm, C.; Ploner, C.J.; Leithner, C. Amplitudes of SSEP and Outcome in Cardiac Arrest Survivors. Neurology 2015, 85, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Taoufik, E.; Probert, L. Ischemic Neuronal Damage. Curr. Pharm. Des. 2008, 14, 3565–3573. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Bezerianos, A.; Zhang, H.; Jia, X.; Thakor, N.V. Exploring High-Frequency Oscillation as a Marker of Brain Ischemia Using S-Transform. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBC’10, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 6099–6102. [Google Scholar] [CrossRef]
- Braun, J.C.; Hanley, D.F.; Thakor, N.V. Detection of Neurological Injury Using Time-Frequency Analysis of the Somatosensory Evoked Potential. Electroencephalogr. Clin. Neurophysiol. Evoked Potentials Sect. 1996, 100, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.G.; Yang, J.L.; Chan, S.C.; Luk, K.D.K.; Hu, Y. Time-Frequency Component Analysis of Somatosensory Evoked Potentials in Rats. Biomed. Eng. Online 2009, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Liu, H.; Luk, K.D. Time-Frequency Analysis of Somatosensory Evoked Potentials for Intraoperative Spinal Cord Monitoring. J. Clin. Neurophysiol. 2011, 28, 504–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geocadin, R.G.; Muthuswamy, J.; Sherman, D.L.; Thakor, N.V.; Hanley, D.F. Early Electrophysiological and Histologic Changes after Global Cerebral Ischemia in Rats. Mov. Disord. 2000, 15, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Miao, P.; Modi, H.R.; Garikapati, S.; Koehler, R.C.; Thakor, N.V. Therapeutic Hypothermia Promotes Cerebral Blood Flow Recovery and Brain Homeostasis after Resuscitation from Cardiac Arrest in a Rat Model. J. Cereb. Blood Flow Metab. 2019, 39, 1961–1973. [Google Scholar] [CrossRef]
- Guo, Y.; Cho, S.M.; Wei, Z.; Wang, Q.; Modi, H.R.; Gharibani, P.; Lu, H.; Thakor, N.V.; Geocadin, R.G. Early Thalamocortical Reperfusion Leads to Neurologic Recovery in a Rodent Cardiac Arrest Model. Neurocrit. Care 2022, 37, 60–72. [Google Scholar] [CrossRef]
- Geocadin, R.G.; Ghodadra, R.; Kimura, T.; Lei, H.; Sherman, D.L.; Hanley, D.F.; Thakor, N.V. A Novel Quantitative EEG Injury Measure of Global Cerebral Ischemia. Clin. Neurophysiol. 2000, 111, 1779–1787. [Google Scholar] [CrossRef]
- Xiong, W.; Koenig, M.A.; Madhok, J.; Jia, X.; Puttgen, H.A.; Thakor, N.V.; Geocadin, R.G. Evolution of Somatosensory Evoked Potentials after Cardiac Arrest Induced Hypoxic–Ischemic Injury. Resuscitation 2010, 81, 893–897. [Google Scholar] [CrossRef] [Green Version]
- Katz, L.; Ebmeyer, U.; Safar, P.; Radovsky, A.; Neumar, R. Outcome Model of Asphyxial Cardiac Arrest in Rats. J. Cereb. Blood Flow Metab. 1995, 15, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Koenig, M.A.; Jia, X.; Kang, X.; Velasquez, A.; Thakor, N.V.; Geocadin, R.G. Intraventricular Orexin-A Improves Arousal and Early EEG Entropy in Rats after Cardiac Arrest. Brain Res. 2009, 1255, 153–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguiar-Conraria, L.; Soares, M.J. The Continuous Wavelet Transform: Moving Beyond Uni- and Bivariate Analysis. J. Econ. Surv. 2014, 28, 344–375. [Google Scholar] [CrossRef]
- Cohen, M.X. A Better Way to Define and Describe Morlet Wavelets for Time-Frequency Analysis. Neuroimage 2019, 199, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Insola, A.; Mazzone, P.; Scarnati, E.; Restuccia, D.; Valeriani, M. Contribution of Different Somatosensory Afferent Input to Subcortical Somatosensory Evoked Potentials in Humans. Clin. Neurophysiol. 2021, 132, 2357–2364. [Google Scholar] [CrossRef] [PubMed]
- Teolis, A. Computational Signal Processing with Wavelets. In Computational Signal Processing with Wavelets; Birkhäuser: Boston, MA, USA, 1998. [Google Scholar] [CrossRef]
- Lilly, J.M.; Olhede, S.C. Higher-Order Properties of Analytic Wavelets. IEEE Trans. Signal Process. 2009, 57, 146–160. [Google Scholar] [CrossRef] [Green Version]
- Burnos, S.; Fedele, T.; Schmid, O.; Krayenbühl, N.; Sarnthein, J. Detectability of the Somatosensory Evoked High Frequency Oscillation (HFO) Co-Recorded by Scalp EEG and ECoG under Propofol. Neuroimage Clin. 2016, 10, 318–325. [Google Scholar] [CrossRef] [Green Version]
- MathWorks Multiple Comparison of Estimated Marginal Means (R2022b). Available online: https://www.mathworks.com/help/stats/repeatedmeasuresmodel.multcompare.html (accessed on 7 December 2022).
- Putzu, A.; Valtorta, S.; di Grigoli, G.; Haenggi, M.; Belloli, S.; Malgaroli, A.; Gemma, M.; Landoni, G.; Beretta, L.; Moresco, R.M. Regional Differences in Cerebral Glucose Metabolism After Cardiac Arrest and Resuscitation in Rats Using [18F]FDG Positron Emission Tomography and Autoradiography. Neurocrit. Care 2018, 28, 370–378. [Google Scholar] [CrossRef]
- Sieber, F.E.; Palmon, S.C.; Traystman, R.J.; Martin, L.J. Global Incomplete Cerebral Ischemia Produces Predominantly Cortical Neuronal Injury. Stroke 1995, 26, 2091–2095. [Google Scholar] [CrossRef]
- Zhang, D.; Jia, X.; Ding, H.; Ye, D.; Thakor, N.V. Application of Tsallis Entropy to EEG: Quantifying the Presence of Burst Suppression after Asphyxial Cardiac Arrest in Rats. IEEE Trans. Biomed. Eng. 2010, 57, 867–874. [Google Scholar] [CrossRef] [Green Version]
- Steriade, M.; Amzica, F.; Contreras, D. Cortical and Thalamic Cellular Correlates of Electroencephalographic Burst-Suppression. Electroencephalogr. Clin. Neurophysiol. 1994, 90, 1–16. [Google Scholar] [CrossRef]
- Japaridze, N.; Muthuraman, M.; Reinicke, C.; Moeller, F.; Anwar, A.R.; Mideksa, K.G.; Pressler, R.; Deuschl, G.; Stephani, U.; Siniatchkin, M. Neuronal Networks during Burst Suppression as Revealed by Source Analysis. PLoS ONE 2015, 10, e0123807. [Google Scholar] [CrossRef] [Green Version]
- Ming, Q.; Liou, J.Y.; Yang, F.; Li, J.; Chu, C.; Zhou, Q.; Wu, D.; Xu, S.; Luo, P.; Liang, J.; et al. Isoflurane-Induced Burst Suppression Is a Thalamus-Modulated, Focal-Onset Rhythm with Persistent Local Asynchrony and Variable Propagation Patterns in Rats. Front. Syst. Neurosci. 2021, 14, 599781. [Google Scholar] [CrossRef]
- Sander, T.H.; Zavala-Fernandez, H.; Burghoff, M.; Uchikawa, Y.; Trahms, L. Extracting High Frequency Oscillatory Brain Signals from Magnetoencephalographic Recordings. IFAC-PapersOnLine 2015, 48, 453–458. [Google Scholar] [CrossRef]
- Hu, Y.; Luk, K.D.K.; Lu, W.W.; Leong, J.C.Y. Application of Time–Frequency Analysis to Somatosensory Evoked Potential for Intraoperative Spinal Cord Monitoring. J. Neurol. Neurosurg. Psychiatry 2003, 74, 82–87. [Google Scholar] [CrossRef]



| Characteristics | CA3 (n = 5) | CA7 (n = 6) | p-Value |
|---|---|---|---|
| Weight (g) | 414 [30.75] | 414 [74] | 0.64 |
| NDS at 4 h post-ROSC | 80 [0.5] | 58.5 [9] | 0.004 |
| NDS at 24 h post-ROSC | 80.00 [0] | 77 [27.75] | 0.016 |
| NDS at 48 h post-ROSC | 80.00 [0] | 78 [78.5] | 0.21 |
| Parameters | Washout | CA | Post-ROSC |
|---|---|---|---|
| Peak-to-peak-amplitude | 48% ± 10% | 42% ± 12% | 59% ± 16% |
| TF peak power | 47% ± 9% | 41% ± 12% | 60% ± 16% |
| NTFS power | 35% ± 7% | 33% ± 8% | 55% ± 15% |
| p-Value | 0.007 | 0.16 | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, Z.; Guo, Y.; Gharibani, P.; Slepyan, A.; Routkevitch, D.; Bezerianos, A.; Geocadin, R.G.; Thakor, N.V. Time-Frequency Analysis of Somatosensory Evoked High-Frequency (600 Hz) Oscillations as an Early Indicator of Arousal Recovery after Hypoxic-Ischemic Brain Injury. Brain Sci. 2023, 13, 2. https://doi.org/10.3390/brainsci13010002
Ou Z, Guo Y, Gharibani P, Slepyan A, Routkevitch D, Bezerianos A, Geocadin RG, Thakor NV. Time-Frequency Analysis of Somatosensory Evoked High-Frequency (600 Hz) Oscillations as an Early Indicator of Arousal Recovery after Hypoxic-Ischemic Brain Injury. Brain Sciences. 2023; 13(1):2. https://doi.org/10.3390/brainsci13010002
Chicago/Turabian StyleOu, Ze, Yu Guo, Payam Gharibani, Ariel Slepyan, Denis Routkevitch, Anastasios Bezerianos, Romergryko G. Geocadin, and Nitish V. Thakor. 2023. "Time-Frequency Analysis of Somatosensory Evoked High-Frequency (600 Hz) Oscillations as an Early Indicator of Arousal Recovery after Hypoxic-Ischemic Brain Injury" Brain Sciences 13, no. 1: 2. https://doi.org/10.3390/brainsci13010002





