Subthreshold Oscillating Waves in Neural Tissue Propagate by Volume Conduction and Generate Interference
Abstract
:1. Introduction
2. Results
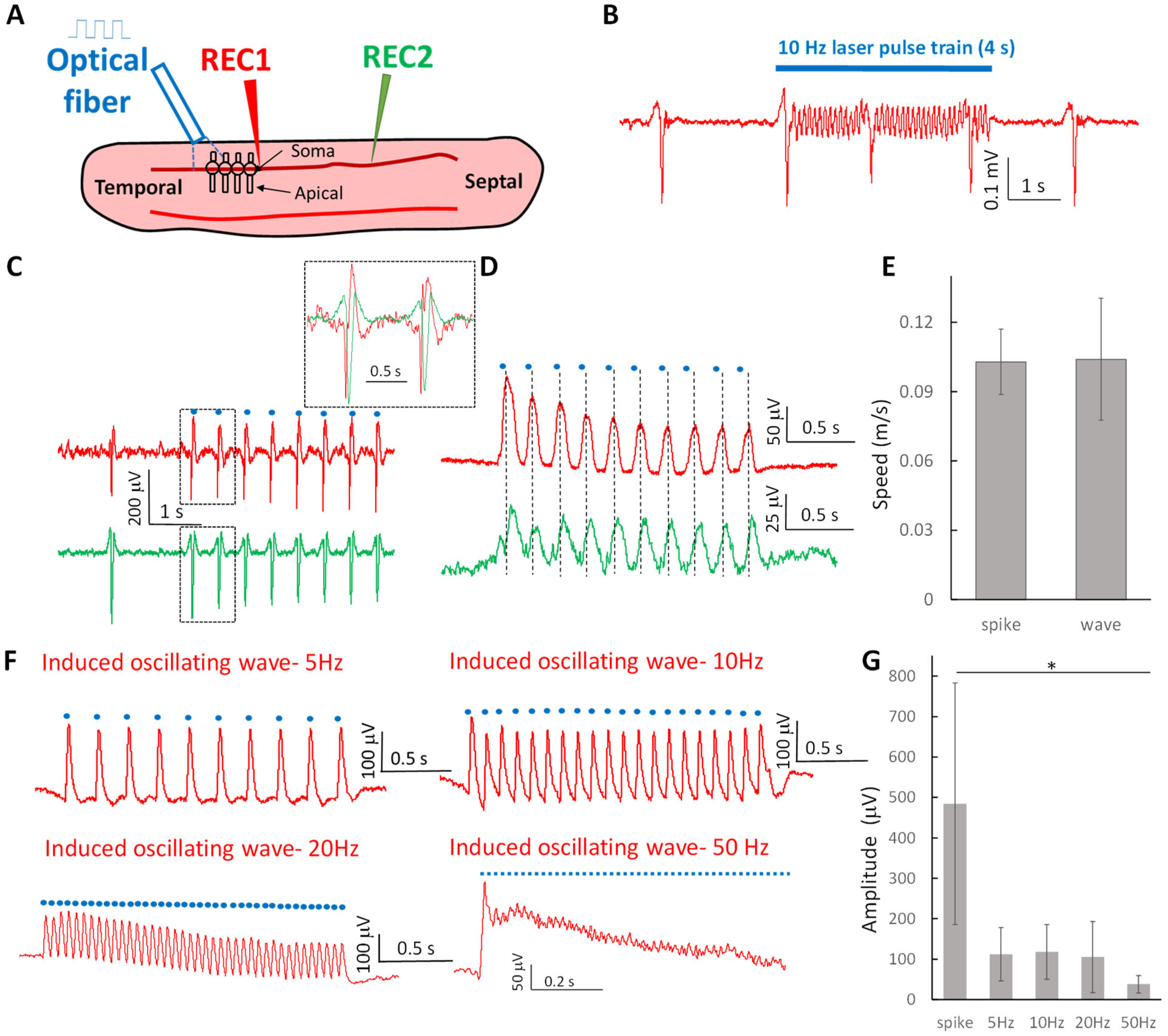
2.1. Optogenetic Stimulation Can Trigger Propagating Subthreshold Oscillating Waves at Various Frequencies and Suprathreshold Neural Spikes in the Hippocampal Slice
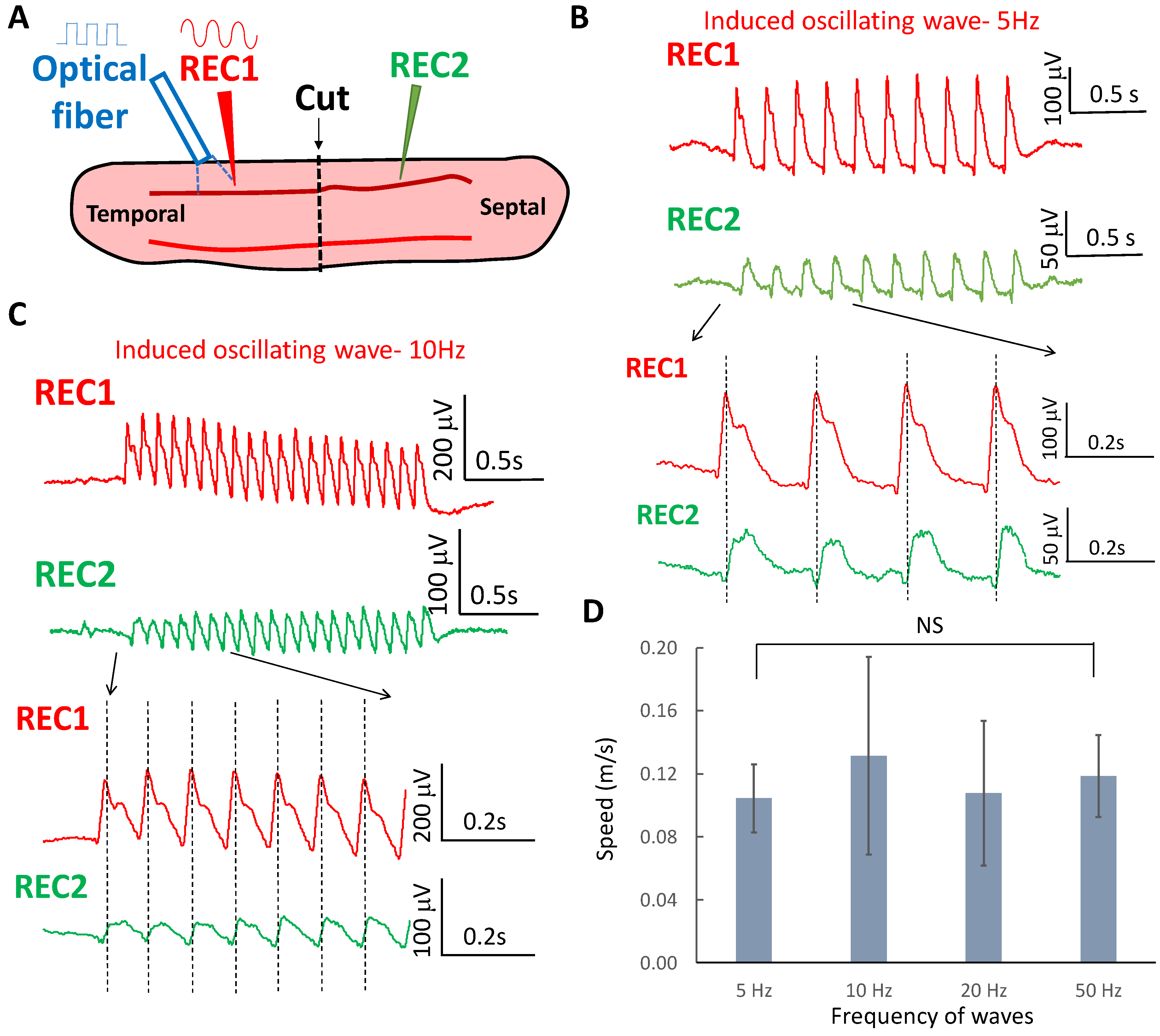
2.2. Oscillating Wave Propagate by Volume Conduction through Electric Field Coupling
2.3. Propagating Oscillating Waves Generate Very Low Amplitude Electric Fields
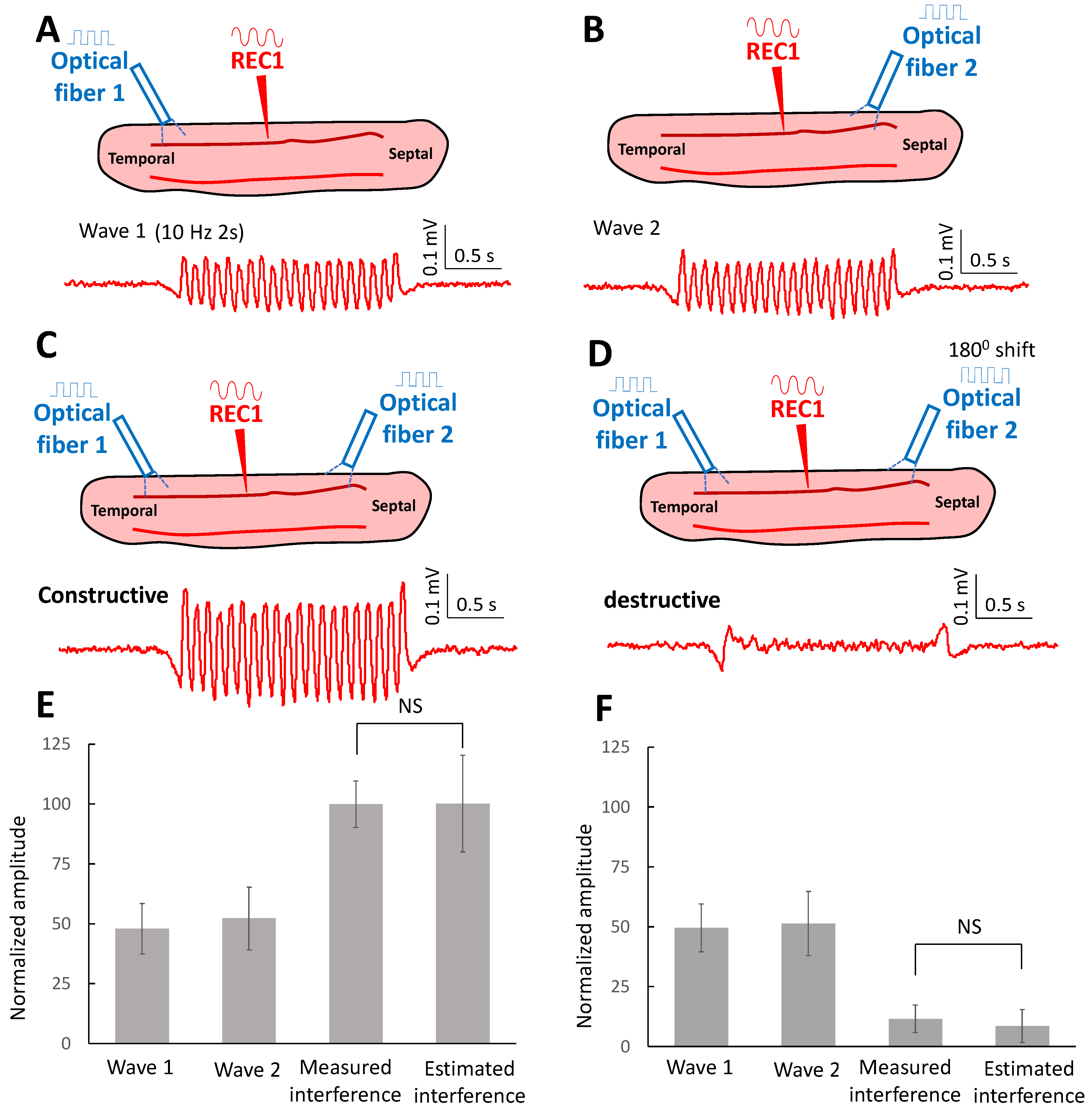
2.4. Oscillating Waves Interfere with Each Other Constructively and Destructively
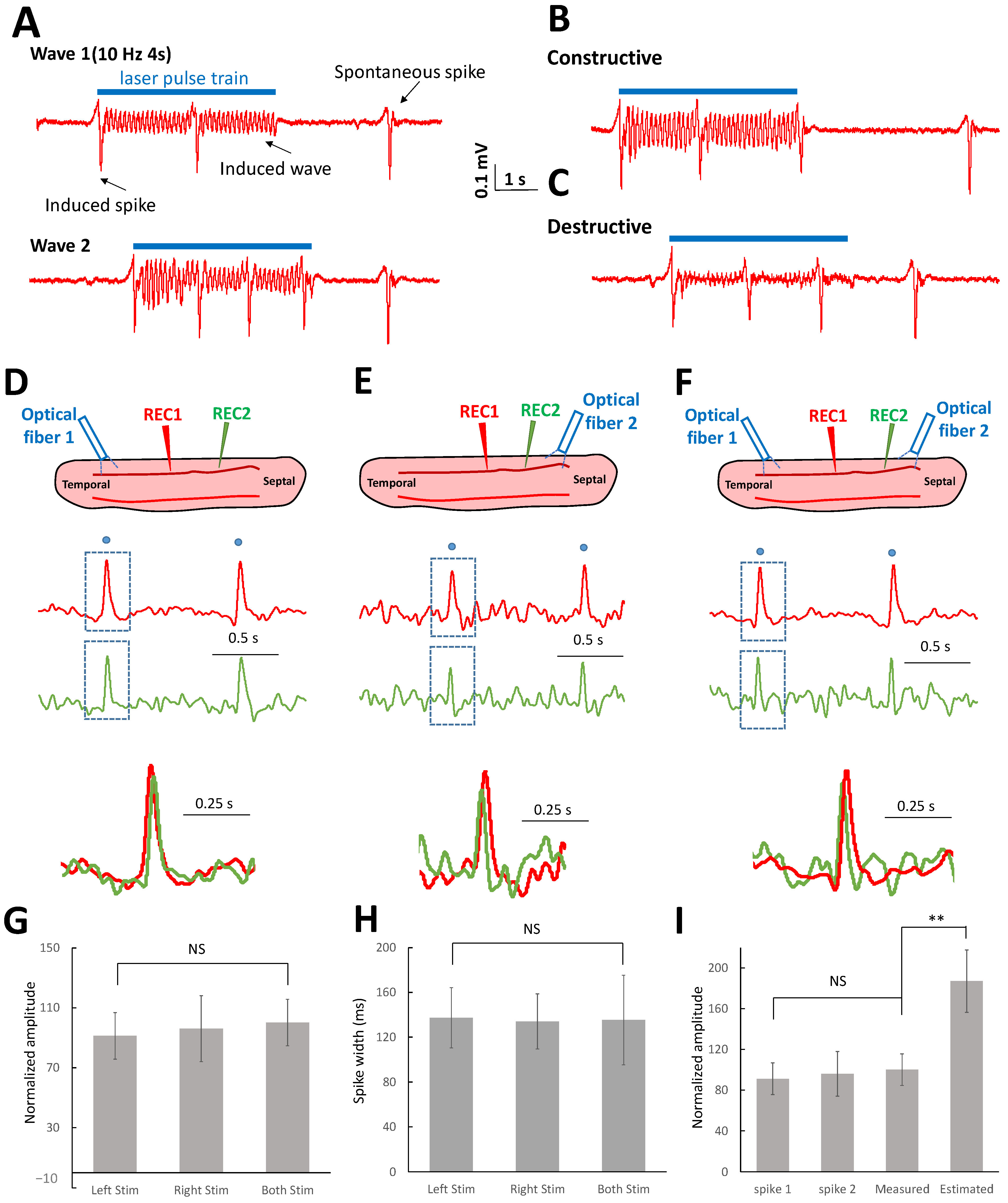
2.5. Suprathreshold Neural Spikes Collide When Encountering Other Spikes
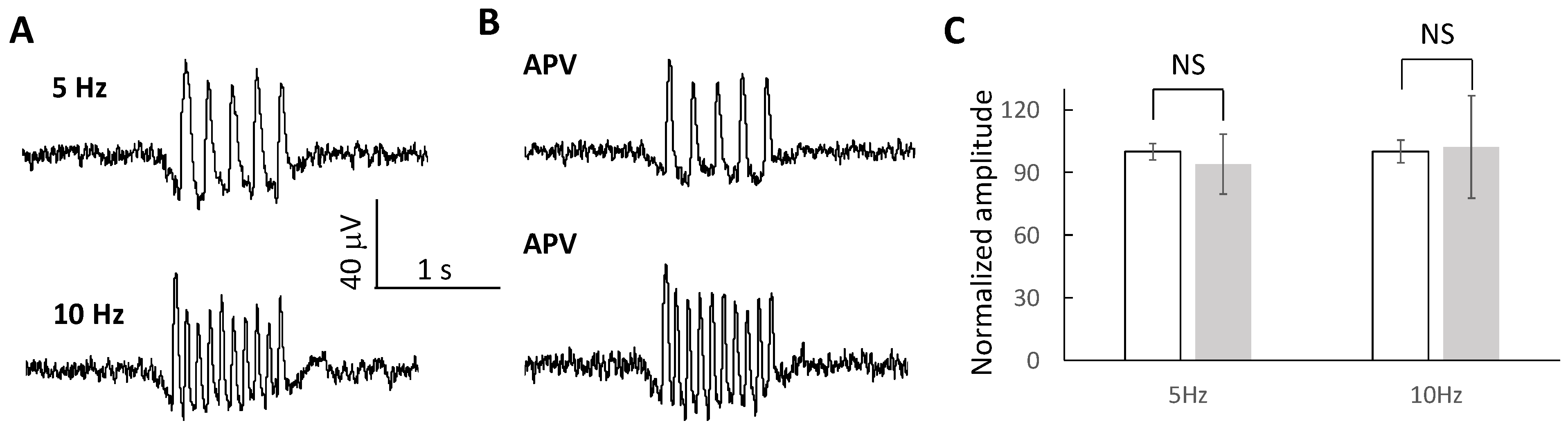
2.6. Oscillating Waves Are Independent of NMDA Receptors
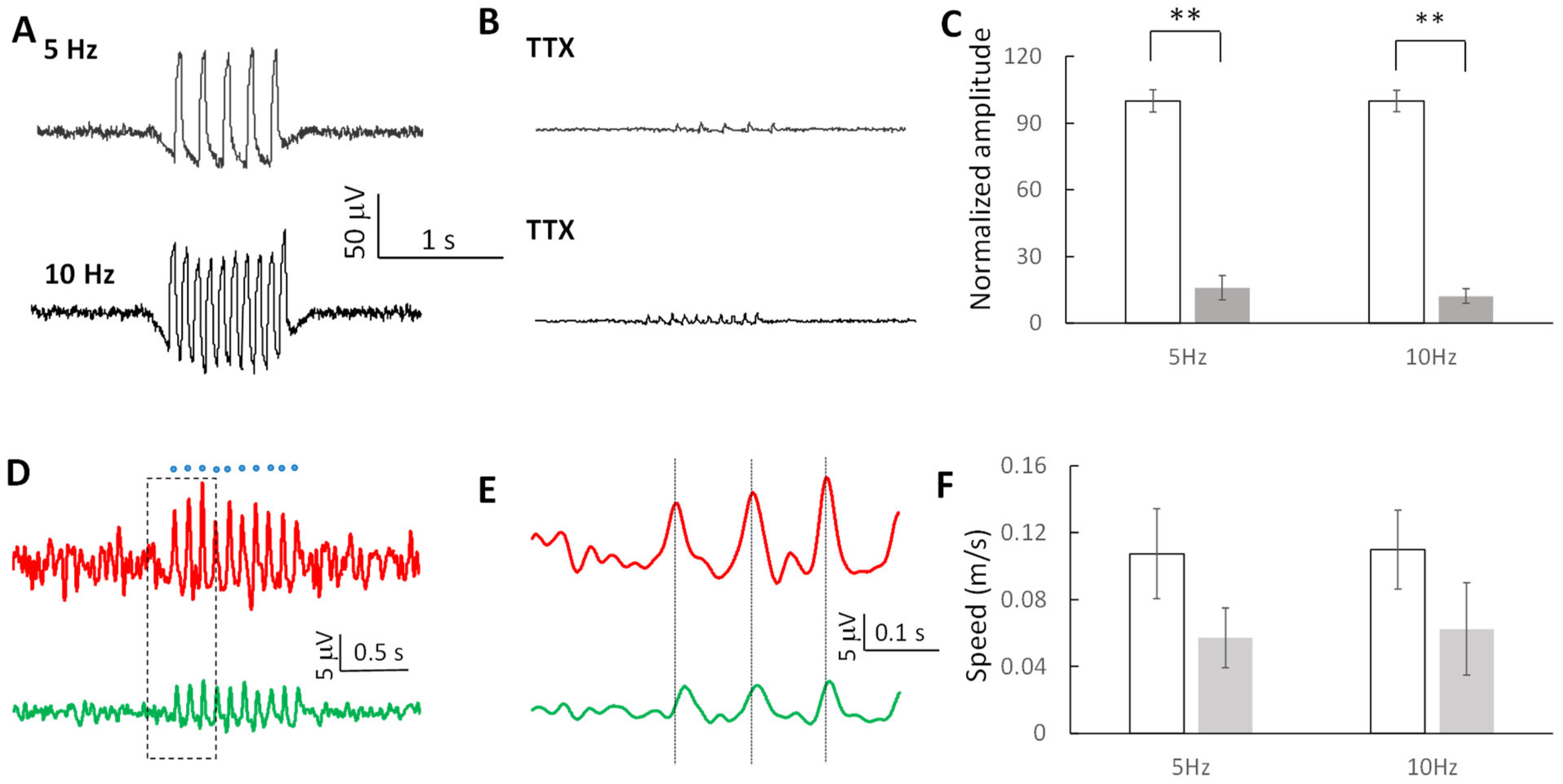
2.7. Subthreshold Oscillating Waves Are Modulated by Sodium Channels
3. Discussion
4. Methods
4.1. Animals
4.2. In Vitro Hippocampal Slice Preparation and Recordings
4.3. Optogenetic Stimulation
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steriade, M.; Nunez, A.; Amzica, F. A novel slow (less than 1 Hz) oscillation of neocortical neurons in vivo—Depolarizing and hyperpolarizing components. J. Neurosci. 1993, 13, 3252–3265. [Google Scholar] [CrossRef] [Green Version]
- Massimini, M. The sleep slow oscillation as a traveling wave. J. Neurosci. 2004, 24, 6862–6870. [Google Scholar] [CrossRef] [Green Version]
- Isomura, Y.; Sirota, A.; Özen, S.; Montgomery, S.; Mizuseki, K.; Henze, D.A.; Buzsáki, G. Integration and segregation of activity in entorhinal-hippocampal subregions by neocortical slow oscillations. Neuron 2006, 52, 871–882. [Google Scholar] [CrossRef] [Green Version]
- Wolansky, T.; Clement, E.A.; Peters, S.R.; Palczak, M.A.; Dickson, C.T. Hippocampal slow oscillation: A novel EEG state and its coordination with ongoing neocortical activity. J. Neurosci. 2006, 26, 6213–6229. [Google Scholar] [CrossRef] [Green Version]
- Patel, J.; Fujisawa, S.; Berényi, A.; Royer, S.; Buzsáki, G. Traveling Theta Waves along the Entire Septotemporal Axis of the Hippocampus. Neuron 2012, 75, 410–417. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Jacobs, J. Traveling theta waves in the human hippocampus. J. Neurosci. 2015, 35, 12477–12487. [Google Scholar] [CrossRef] [Green Version]
- Marshall, L.; Helgadóttir, H.; Mölle, M.; Born, J. Boosting slow oscillations during sleep potentiates memory. Nature 2006, 444, 610–613. [Google Scholar] [CrossRef]
- Colgin, L.L. Mechanisms and Functions of Theta Rhythms. Annu. Rev. Neurosci. 2013, 36, 295–312. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Watrous, A.J.; Patel, A.; Jacobs, J. Theta and Alpha Oscillations Are Traveling Waves in the Human Neocortex. Neuron 2018, 98, 1269–1281.e4. [Google Scholar] [CrossRef] [Green Version]
- Lubenov, E.V.; Siapas, A.G. Hippocampal theta oscillations are travelling waves. Nature 2009, 459, 534–539. [Google Scholar] [CrossRef]
- Kleen, J.K.; Chung, J.E.; Sellers, K.K.; Zhou, J.; Triplett, M.; Lee, K.; Tooker, A.; Haque, R.; Chang, E.F. Bidirectional propagation of low frequency oscillations over the human hippocampal surface. Nat. Commun. 2021, 12, 2764. [Google Scholar] [CrossRef]
- Llinás, R.; Yarom, Y. Oscillatory properties of guinea-pig inferior olivary neurones and their pharmacological modulation: An in vitro study. J. Physiol. 1986, 376, 163–182. [Google Scholar] [CrossRef]
- Alonso, A.; Llinas, R.R. Subthreshold Na+-dependent theta like rhythmicity in stellate cells of entorhinal cortex layer II. Nature 1989, 342, 175–177. [Google Scholar] [CrossRef]
- Schmitz, D.; Gloveli, T.; Behr, J.; Dugladze, T.; Heinemann, U. Subthreshold membrane potential oscillations in neurons of deep layers of the entorhinal cortex. Neuroscience 1998, 85, 999–1004. [Google Scholar] [CrossRef]
- Bilkey, D.K.; Heinemann, U. Intrinsic theta-frequency membrane potential oscillations in layer III/V perirhinal cortex neurons of the rat. Hippocampus 1999, 518, 510–518. [Google Scholar] [CrossRef]
- Gutfreund, Y.; Yarom, Y.; Segev, I. Subthreshold oscillations and resonant frequency in guinea-pig cortical neurons: Physiology and modelling. J. Physiol. 1995, 483, 621–640. [Google Scholar] [CrossRef]
- Llinás, R.R.; Grace, A.A.; Yarom, Y. In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-hz frequency range. Proc. Natl. Acad. Sci. USA 1991, 88, 897–901. [Google Scholar] [CrossRef] [Green Version]
- Desmaisons, D.; Vincent, J.D.; Lledo, P.M. Control of action potential timing by intrinsic subthreshold oscillations in olfactory bulb output neurons. J. Neurosci. 1999, 19, 10727–10737. [Google Scholar] [CrossRef] [Green Version]
- Manis, P.B.; Molitor, S.C.; Wu, H. Subthreshold oscillations generated by TTX-sensitive sodium currents in dorsal cochlear nucleus pyramidal cells. Exp. Brain Res. 2003, 153, 443–451. [Google Scholar] [CrossRef]
- Leung, L.W.S.; Yim, C.Y.C. Intrinsic membrane potential oscillations in hippocampal neurons in vitro. Brain Res. 1991, 553, 261–274. [Google Scholar] [CrossRef]
- García-Muñoz, A.; Barrio, L.C.; Buño, W. Membrane potential oscillations in CA1 hippocampal pyramidal neurons in vitro: Intrinsic rhythms and fluctuations entrained by sinusoidal injected current. Exp. Brain Res. 1993, 97, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Kamondi, A.; Acsády, L.; Wang, X.J.; Buzsáki, G. Theta oscillations in somata and dendrites of hippocampal pyramidal cells in vivo: Activity-dependent phase-precession of action potentials. Hippocampus 1998, 8, 244–261. [Google Scholar] [CrossRef]
- Bland, B.H.; Konopacki, J.; Dyck, R. Heterogeneity among hippocampal pyramidal neurons revealed by their relation to theta-band oscillation and synchrony. Exp. Neurol. 2005, 195, 458–474. [Google Scholar] [CrossRef]
- Lee, S.H.; Urbano, F.J.; Garcia-Rill, E. The Critical Role of Intrinsic Membrane Oscillations. NeuroSignals 2019, 26, 66–76. [Google Scholar] [CrossRef]
- Lampl, I.; Yarom, Y. Subthreshold oscillations of the membrane potential: A functional synchronizing and timing device. J. Neurophysiol. 1993, 70, 2181–2186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stiefel, K.M.; Fellous, J.M.; Thomas, P.J.; Sejnowski, T.J. Intrinsic subthreshold oscillations extend the influence of inhibitory synaptic inputs on cortical pyramidal neurons. Eur. J. Neurosci. 2010, 31, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Izhikevich, E.M. Resonance and selective communication via bursts in neurons having subthreshold oscillations. BioSystems 2002, 67, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.; Lee, H.; Martell, A.; van Drongelen, W. Resonance in neocortical neurons and networks. Eur. J. Neurosci. 2012, 36, 3698–3708. [Google Scholar] [CrossRef]
- Longtin, A.; Hinzer, K. Encoding with Bursting, Subthreshold Oscillations, and Noise in Mammalian Cold Receptors. Neural Comput. 1996, 8, 215–255. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.W.S.; Yim, C.Y. Intracellular records of theta rhythm in hippocampal CA1 cells of the rat. Brain Res. 1986, 367, 323–327. [Google Scholar] [CrossRef]
- Vertes, R.P.; Kocsis, B. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience 1997, 81, 893–926. [Google Scholar] [PubMed]
- Piatkevich, K.D.; Bensussen, S.; Tseng, H.A.; Shroff, S.N.; Lopez-Huerta, V.G.; Park, D.; Jung, E.E.; Shemesh, O.A.; Straub, C.; Gritton, H.J.; et al. Population imaging of neural activity in awake behaving mice. Nature 2019, 574, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.; Adam, Y.; Weinstein, E.N.; Williams, E.; Williams, K.; Parot, V.; Kavokine, N.; Liberles, S.; Madisen, L.; Zeng, H.; et al. Genetically targeted all-optical electrophysiology with a transgenic cre-dependent optopatch mouse. J. Neurosci. 2016, 36, 11059–11073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Vives, M.V.; McCormick, D.A. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat. Neurosci. 2000, 3, 1027–1034. [Google Scholar] [CrossRef]
- Rebollo, B.; Telenczuk, B.; Navarro-Guzman, A.; Destexhe, A.; Sanchez-Vives, M.V. Modulation of intercolumnar synchronization by endogenous electric fields in cerebral cortex. Sci. Adv. 2021, 7, eabc7772. [Google Scholar] [CrossRef]
- Chiang, C.-C.; Shivacharan, R.S.; Wei, X.; Gonzalez-Reyes, L.E.; Durand, D.M. Slow periodic activity in the longitudinal hippocampal slice can self-propagate non-synaptically by a mechanism consistent with ephaptic coupling. J. Physiol. 2019, 597, 249–269. [Google Scholar] [CrossRef]
- Qiu, C.; Shivacharan, R.S.; Zhang, M.; Durand, D.M. Can neural activity propagate by endogenous electrical field? J. Neurosci. 2015, 35, 15800–15811. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Ladas, T.P.; Qiu, C.; Shivacharan, R.S.; Gonzalez-Reyes, L.E.; Durand, D.M. Propagation of epileptiform activity can be independent of synaptic transmission, gap junctions, or diffusion and is consistent with electrical field transmission. J. Neurosci. 2014, 34, 1409–1419. [Google Scholar] [CrossRef] [Green Version]
- Shivacharan, R.S.; Chiang, C.C.; Wei, X.; Subramanian, M.; Couturier, N.H.; Pakalapati, N.; Durand, D.M. Neural recruitment by ephaptic coupling in epilepsy. Epilepsia 2021, 62, 1505–1517. [Google Scholar] [CrossRef]
- Vöröslakos, M.; Takeuchi, Y.; Brinyiczki, K.; Zombori, T.; Oliva, A.; Fernández-Ruiz, A.; Kozák, G.; Kincses, Z.T.; Iványi, B.; Buzsáki, G.; et al. Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nat. Commun. 2018, 9, 483. [Google Scholar] [CrossRef]
- Kjelstrup, K.B.; Solstad, T.; Brun, V.H.; Hafting, T.; Leutgeb, S.; Witter, M.P.; Moser, E.I.; Moser, M.B. Finite scale of spatial representation in the hippocampus. Science 2008, 321, 140–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strange, B.A.; Witter, M.P.; Lein, E.S.; Moser, E.I. Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci. 2014, 15, 655–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunec, I.K.; Bellana, B.; Ozubko, J.D.; Man, V.; Robin, J.; Liu, Z.X.; Grady, C.; Rosenbaum, R.S.; Winocur, G.; Barense, M.D.; et al. Multiple scales of representation along the hippocampal anteroposterior axis in humans. Curr. Biol. 2018, 28, 2129–2135.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Saleh, M.; Penn, R.D.; Hatsopoulos, N.G. Propagating waves in human motor cortex. Front. Hum. Neurosci. 2011, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Mohan, U.R.; Zhang, H.; Jacobs, J. The direction and timing of theta and alpha traveling wave modulate human memory processing. bioRxiv. in press. Available online: https://www.biorxiv.org/content/10.1101/2022.02.07.479466v1 (accessed on 10 February 2022).
- Subramanian, M.; Chiang, C.C.; Couturier, N.H.; Durand, D.M. Theta waves, neural spikes and seizures can propagate by ephaptic coupling in vivo. Exp. Neurol. 2022, 354, 114109. [Google Scholar] [CrossRef]
- Sanhueza, M.; Bacigalupo, J. Intrinsic subthreshold oscillations of the membrane potential in pyramidal neurons of the olfactory amygdala. Eur. J. Neurosci. 2005, 22, 1618–1626. [Google Scholar] [CrossRef]
- Shivacharan, R.S.; Chiang, C.-C.; Zhang, M.; Gonzalez-Reyes, L.E.; Durand, D.M. Self-propagating, non-synaptic epileptiform activity recruits neurons by endogenous electric fields. Exp. Neurol. 2019, 317, 119–128. [Google Scholar] [CrossRef]
- Miller, J.; Berger, B.; Sauseng, P. Anodal transcranial direct current stimulation (tDCS) increases frontal-midline theta activity in the human EEG: A preliminary investigation of non-invasive stimulation. Neurosci. Lett. 2015, 588, 114–119. [Google Scholar] [CrossRef]
- Kim, J.; Jang, K.I.; Roh, D.; Kim, H.; Kim, D.H. A direct comparison of the electrophysiological effects of transcranial direct and alternating current stimulation in healthy subjects. Brain Res. 2020, 1747, 147065. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, A.A.; Lafon, B.; Friedman, D.; Dayan, M.; Wang, X.; Bikson, M.; Doyle, W.K.; Devinsky, O.; Parra, L.C. Measurements and models of electric fields in the in vivo human brain during transcranial electric stimulation. Elife 2017, 6, e18834. [Google Scholar] [CrossRef] [PubMed]
- Crill, W.E. Persistent sodium current in mammalian central neurons. Annu. Rev. Physiol. 1996, 58, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Hafting, T.; Fyhn, M.; Molden, S.; Moser, M.B.; Moser, E.I. Microstructure of a spatial map in the entorhinal cortex. Nature 2005, 436, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Burgess, N.; Barry, C.; Keefe, J.O.; Neuroscience, C. An oscillatory interference model of grid cell firing. Hippocampus 2007, 17, 801–812. [Google Scholar] [CrossRef] [Green Version]
- Bush, D.; Burgess, N. A hybrid oscillatory interference/continuous attractor network model of grid cell firing. J. Neurosci. 2014, 34, 5065–5079. [Google Scholar] [CrossRef] [Green Version]
- O’Keefe, J.; Burgess, N. Dual phase and rate coding in hippocampal place cells: Theoretical significance and relationship to entorhinal grid cells. Hippocampus 2005, 15, 853–866. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Perez, A.; Budvytyte, R.; Mosgaard, L.D.; Nissen, S.; Heimburg, T. Penetration of action potentials during collision in the median and lateral giant axons of invertebrates. Phys. Rev. X 2014, 4, 031047. [Google Scholar] [CrossRef] [Green Version]
- Yizhar, O.; Fenno, L.E.; Davidson, T.J.; Mogri, M.; Deisseroth, K. Optogenetics in Neural Systems. Neuron 2011, 71, 9–34. [Google Scholar]
- Kastanenka, K.V.; Hou, S.S.; Shakerdge, N.; Logan, R.; Feng, D.; Wegmann, S.; Chopra, V.; Hawkes, J.M.; Chen, X.; Bacskai, B.J. Optogenetic restoration of disrupted slow oscillations halts amyloid deposition and restores calcium homeostasis in an animal model of Alzheimer’s disease. PLoS ONE 2017, 12, e0170275. [Google Scholar] [CrossRef] [Green Version]
- Etter, G.; van der Veldt, S.; Manseau, F.; Zarrinkoub, I.; Trillaud-Doppia, E.; Williams, S. Optogenetic gamma stimulation rescues memory impairments in an Alzheimer’s disease mouse model. Nat. Commun. 2019, 10, 5322. [Google Scholar] [CrossRef] [Green Version]
- Kilias, A.; Canales, A.; Froriep, U.P.; Park, S.; Egert, U.; Anikeeva, P. Optogenetic entrainment of neural oscillations with hybrid fiber probes. J. Neural Eng. 2018, 15, 056006. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, C.-C.; Durand, D.M. Subthreshold Oscillating Waves in Neural Tissue Propagate by Volume Conduction and Generate Interference. Brain Sci. 2023, 13, 74. https://doi.org/10.3390/brainsci13010074
Chiang C-C, Durand DM. Subthreshold Oscillating Waves in Neural Tissue Propagate by Volume Conduction and Generate Interference. Brain Sciences. 2023; 13(1):74. https://doi.org/10.3390/brainsci13010074
Chicago/Turabian StyleChiang, Chia-Chu, and Dominique M. Durand. 2023. "Subthreshold Oscillating Waves in Neural Tissue Propagate by Volume Conduction and Generate Interference" Brain Sciences 13, no. 1: 74. https://doi.org/10.3390/brainsci13010074
APA StyleChiang, C.-C., & Durand, D. M. (2023). Subthreshold Oscillating Waves in Neural Tissue Propagate by Volume Conduction and Generate Interference. Brain Sciences, 13(1), 74. https://doi.org/10.3390/brainsci13010074




