Relationship among Connectivity of the Frontal Aslant Tract, Executive Functions, and Speech and Language Impairment in Children with Childhood Apraxia of Speech
Abstract
1. Introduction
1.1. Executive Functions
1.2. Neuroanatomical Correlates of CAS
1.3. Aim of the Study
2. Materials and Methods
2.1. Participants
2.2. Procedures and Measures
2.2.1. Clinical Assessment
2.2.2. Speech and Language Assessment
2.2.3. Executive Function Assessment
- Draw a Circle (FE-PS 2–6; [80]). The child is asked to inhibit the continuous motor response: the task requires tracing a circle with a finger on a white sheet of paper, adapting the execution speed to the examiner’s request.
- Day and Night Stroop (FE-PS 2–6; [80]). The test involves the inhibition of the verbal response by suppressing a preponderant response prompted by a stimulus. The inhibition concerns the ability to block an automatic response and to manage the conflict between two response operations associated with the same stimulus. Both time and accuracy are measured.
- Flanker Task (FE-PS 2–6; [80]). The test assesses interference management: The child must indicate the direction of the central stimulus in the presence of interfering stimuli, which can be oriented either in the same direction as the target (congruence) or in the opposite direction (incongruence). Both time and accuracy are measured.
- Dimensional Change Card Sort (FE-PS 2–6; [80]). This test, which recalls the paradigm of the Dimensional Change Card Sort (DCCS), assesses the capacity for cognitive flexibility, inhibitory control, and working memory: The child must classify a series of cards, first by color, then by shape, and finally according to the color if the card has a black border and according to the shape if the card does not have a black border.
- Keep Truck (FE-PS 2–6; [80]) The test aims to evaluate the organization of information in working memory: The child is shown images belonging to five categories. Before the beginning of the test, the child is asked to pay attention to a particular category. A series of six images belonging to different categories are then shown, and the child must name them out loud. At the end of each series, the child is asked to remember the last image belonging to the designated category.
- Spin the Pots (BAFE, [81]. A visual research task that evaluates the visual-spatial working memory: The examiner places a red token under each of the eight pots arranged on a tray. The tray is then covered with a cloth and rotated. The child is asked to remove the cloth from the tray and find, one at a time, the tokens placed under each pot.
2.2.4. Imaging Protocols
2.2.5. MRI Analysis and Postprocessing
2.2.6. Statistical analyses
3. Results
3.1. Speech Profile
3.2. Language Profile
| Language Assessment Protocol of CAS Group | % <5° Percentile/ <−1.65 z Score | Assessment Measures |
|---|---|---|
| Expressive Grammar | 87% | Grid for the Analysis of Spontaneous Speech (GASS [90]) |
| Receptive Grammar | 20% | TCGB, Test di Comprensione Grammaticale per Bambini (Grammar comprehension test for children) [91] TROG-2 Test for Reception of Grammar, Version 2. [92] |
| Receptive vocabulary | 10% | Test Fonolessicale (TFL [93],) and/or Peabody Picture Vocabulary Test (PPVT-R [94]), depending on the child’s age and on the severity of the disorder |
| Expressive vocabulary | 17% | Test Fonolessicale (TFL [93] and/or One-Word Picture Vocabulary Test [95] depending on the child’s age and on the severity of the disorder |
3.3. EF Profile
3.4. FAT Reconstruction, Analysis, and Relations with Speech and Language Profile
3.5. Moderation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ASHA—American Speech-Language-Hearing Association. Technical Report on Childhood Apraxia of Speech; American Speech-Language-Hearing Association: Rockville, MD, USA, 2007. [Google Scholar]
- Shriberg, L.D.; Kwiatkowski, J.; Mabie, H.L. Estimates of the Prevalence of Motor Speech Disorders in Children with Idiopathic Speech Delay. Clin. Linguist Phon. 2019, 33, 679–706. [Google Scholar] [CrossRef] [PubMed]
- Maassen, B.; Nijland, L.; Terband, H. Developmental Models of Childhood Apraxia of Speech. In Speech Motor Control. New Developments in Basic and Applied Research; Maassen, B., van Lieshout, P., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 243–258. [Google Scholar]
- Lewis, B.A.; Freebairn, L.A.; Hansen, A.J.; Iyengar, S.K.; Taylor, H.G. School-Age Follow-up of Children with Childhood Apraxia of Speech. Lang. Speech Hear. Serv. Sch. 2004, 35, 122–140. [Google Scholar] [CrossRef] [PubMed]
- Chilosi, A.M.; Lorenzini, I.; Fiori, S.; Graziosi, V.; Rossi, G.; Pasquariello, R.; Cipriani, P.; Cioni, G. Behavioral and Neurobiological Correlates of Childhood Apraxia of Speech in Italian Children. Brain Lang. 2015, 150, 177–185. [Google Scholar] [CrossRef]
- Chilosi, A.M.; Podda, I.; Ricca, I.; Comparini, A.; Franchi, B.; Fiori, S.; Pasquariello, R.; Casalini, C.; Cipriani, P.; Santorelli, F.M. Differences and Commonalities in Children with Childhood Apraxia of Speech and Comorbid Neurodevelopmental Disorders: A Multidimensional Perspective. J. Pers. Med. 2022, 12, 313. [Google Scholar] [CrossRef]
- Shriberg, L.D.; Lohmeier, H.L.; Strand, E.A.; Jakielski, K.J. Encoding, Memory, and Transcoding Deficits in Childhood Apraxia of Speech. Clin. Linguist. Phon. 2012, 26, 445–482. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.A.; Avrich, A.A.; Freebairn, L.A.; Taylor, H.G.; Iyengar, S.K.; Stein, C.M. Subtyping Children With Speech Sound Disorders by Endophenotypes. Top. Lang. Disord. 2011, 31, 112–127. [Google Scholar] [CrossRef]
- Kenney, M.K.; Barac-Cikoja, D.; Finnegan, K.; Jeffries, N.; Ludlow, C.L. Speech Perception and Short Term Memory Deficits in Persistent Developmental Speech Disorder. Brain Lang. 2006, 96, 178–190. [Google Scholar] [CrossRef][Green Version]
- Lewis, B.A.; Freebairn, L.A.; Taylor, H.G. Academic Outcomes in Children with Histories of Speech Sound Disorders. J. Commun. Disord. 2000, 33, 11–30. [Google Scholar] [CrossRef]
- Lewis, B.A.; Shriberg, L.D.; Freebairn, L.A.; Hansen, A.J.; Stein, C.M.; Taylor, H.G.; Iyengar, S.K. The Genetic Bases of Speech Sound Disorders: Evidence from Spoken and Written Language. J. Speech Lang. Hear. Res. 2006, 49, 1294–1312. [Google Scholar] [CrossRef]
- Smith, S.D.; Pennington, B.F.; Boada, R.; Shriberg, L.D. Linkage of Speech Sound Disorder to Reading Disability Loci. J. Child. Psychol. Psychiatry 2005, 46, 1057–1066. [Google Scholar] [CrossRef]
- Bombonato, C.; Casalini, C.; Pecini, C.; Angelucci, G.; Vicari, S.; Podda, I.; Cipriani, P.; Chilosi, A.M.; Menghini, D. Implicit Learning in Children with Childhood Apraxia of Speech. Res. Dev. Disabil. 2022, 122, 104170. [Google Scholar] [CrossRef] [PubMed]
- Iuzzini-Seigel, J. Procedural Learning, Grammar, and Motor Skills in Children With Childhood Apraxia of Speech, Speech Sound Disorder, and Typically Developing Speech. J. Speech Lang. Hear. Res. 2021, 64, 1081–1103. [Google Scholar] [CrossRef] [PubMed]
- Nijland, L.; Terband, H.; Maassen, B. Cognitive Functions in Childhood Apraxia of Speech. J. Speech Lang. Hear. Res. 2015, 58, 550–565. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.; Magimairaj, B.; Finney, M. Working Memory and Specific Language Impairment: An Update on the Relation and Perspectives on Assessment and Treatment. Am. J. Speech-Lang. Pathol./Am. Speech-Lang.-Hear. Assoc. 2009, 19, 78–94. [Google Scholar] [CrossRef]
- Duinmeijer, I.; de Jong, J.; Scheper, A. Narrative Abilities, Memory and Attention in Children with a Specific Language Impairment. Int. J. Lang. Commun. Disord. 2012, 47, 542–555. [Google Scholar] [CrossRef]
- Henry, L.A.; Messer, D.J.; Nash, G. Executive Functioning in Children with Specific Language Impairment: Executive Functioning and SLI. J. Child Psychol. Psychiatry 2012, 53, 37–45. [Google Scholar] [CrossRef]
- Bishop, D.V.M.; Norbury, C.F. Executive Functions in Children with Communication Impairments, in Relation to Autistic Symptomatology. 2: Response Inhibition. Autism 2005, 9, 29–43. [Google Scholar] [CrossRef]
- Marton, K. Visuo-Spatial Processing and Executive Functions in Children with Specific Language Impairment. Int. J. Lang. Commun. Disord. 2008, 43, 181–200. [Google Scholar] [CrossRef]
- Diamond, A. Executive Functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef]
- Diamond, A.; Ling, D.S. Conclusions about Interventions, Programs, and Approaches for Improving Executive Functions That Appear Justified and Those That, despite Much Hype, Do Not. Dev. Cogn. Neurosci. 2016, 18, 34–48. [Google Scholar] [CrossRef]
- Baddeley, A. Working Memory: Theories, Models, and Controversies. Annu. Rev. Psychol. 2012, 63, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Kane, M.J.; Engle, R.W. The Role of Prefrontal Cortex in Working-Memory Capacity, Executive Attention, and General Fluid Intelligence: An Individual-Differences Perspective. Psychon. Bull. Rev. 2002, 9, 637–671. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.P.; Miyake, A. Unity and Diversity of Executive Functions: Individual Differences as a Window on Cognitive Structure. Cortex 2017, 86, 186–204. [Google Scholar] [CrossRef] [PubMed]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The Unity and Diversity of Executive Functions and Their Contributions to Complex “Frontal Lobe” Tasks: A Latent Variable Analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef]
- Zelazo, P.D.; Qu, L.; Müller, U. Hot and Cool Aspects of Executive Function: Relations in Early Development. In Young Children’s Cognitive Development: Interrelationships among Executive Functioning, Working Memory, Verbal Ability, and Theory of Mind; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 2005; pp. 71–93. ISBN 978-0-8058-4906-6. [Google Scholar]
- Zelazo, P.D.; Müller, U. Executive Function in Typical and Atypical Development. In Handbook of Childhood Cognitive Development; Goswami, U., Ed.; Blackwell: Oxford, UK, 2002; pp. 445–469. [Google Scholar]
- Baddeley, A. Working Memory: Looking Back and Looking Forward. Nat. Rev. Neurosci. 2003, 4, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Baddeley, A.D.; Hitch, G.J. Developments in the Concept of Working Memory. Neuropsychology 1994, 8, 485–493. [Google Scholar] [CrossRef]
- Smith, E.E.; Jonides, J. Storage and Executive Processes in the Frontal Lobes. Science 1999, 283, 1657–1661. [Google Scholar] [CrossRef]
- Hughes, C.; Ensor, R.; Wilson, A.; Graham, A. Tracking Executive Function Across the Transition to School: A Latent Variable Approach. Dev. Neuropsychol. 2009, 35, 20–36. [Google Scholar] [CrossRef]
- Lehto, J.E.; Juujärvi, P.; Kooistra, L.; Pulkkinen, L. Dimensions of Executive Functioning: Evidence from Children. Br. J. Dev. Psychol. 2003, 21, 59–80. [Google Scholar] [CrossRef]
- Somerville, L.H.; Casey, B.J. Developmental Neurobiology of Cognitive Control and Motivational Systems. Curr. Opin. Neurobiol. 2010, 20, 236–241. [Google Scholar] [CrossRef]
- Gilbert, S.J.; Burgess, P.W. Executive Function. Curr. Biol. 2008, 18, R110–R114. [Google Scholar] [CrossRef] [PubMed]
- La Corte, E.; Eldahaby, D.; Greco, E.; Aquino, D.; Bertolini, G.; Levi, V.; Ottenhausen, M.; Demichelis, G.; Romito, L.M.; Acerbi, F.; et al. The Frontal Aslant Tract: A Systematic Review for Neurosurgical Applications. Front. Neurol. 2021, 12, 641586. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, P.; Dick, A.S. Broca and Wernicke Are Dead, or Moving Past the Classic Model of Language Neurobiology. Brain Lang. 2016, 162, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Ardila, A.; Lopez, M.V. Transcortical Motor Aphasia: One or Two Aphasias? Brain Lang. 1984, 22, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, P.; Gracco, V.L. Contribution of the Pre-SMA to the Production of Words and Non-Speech Oral Motor Gestures, as Revealed by Repetitive Transcranial Magnetic Stimulation (RTMS). Brain Res. 2009, 1268, 112–124. [Google Scholar] [CrossRef]
- Dick, A.S.; Garic, D.; Graziano, P.; Tremblay, P. The Frontal Aslant Tract (FAT) and Its Role in Speech, Language and Executive Function. Cortex 2019, 111, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Dick, A.S.; Mok, E.H.; Raja Beharelle, A.; Goldin-Meadow, S.; Small, S.L. Frontal and Temporal Contributions to Understanding the Iconic Co-Speech Gestures That Accompany Speech. Hum. Brain Mapp. 2014, 35, 900–917. [Google Scholar] [CrossRef]
- Katzev, M.; Tüscher, O.; Hennig, J.; Weiller, C.; Kaller, C.P. Revisiting the Functional Specialization of Left Inferior Frontal Gyrus in Phonological and Semantic Fluency: The Crucial Role of Task Demands and Individual Ability. J. Neurosci. 2013, 33, 7837–7845. [Google Scholar] [CrossRef]
- Pedersen, P.M.; Jørgensen, H.S.; Nakayama, H.; Raaschou, H.O.; Olsen, T.S. Aphasia in Acute Stroke: Incidence, Determinants, and Recovery. Ann. Neurol. 1995, 38, 659–666. [Google Scholar] [CrossRef]
- Rogić, M.; Deletis, V.; Fernández-Conejero, I. Inducing Transient Language Disruptions by Mapping of Broca’s Area with Modified Patterned Repetitive Transcranial Magnetic Stimulation Protocol. J. Neurosurg. 2014, 120, 1033–1041. [Google Scholar] [CrossRef]
- Deletis, V.; Rogić, M.; Fernández-Conejero, I.; Gabarrós, A.; Jerončić, A. Neurophysiologic Markers in Laryngeal Muscles Indicate Functional Anatomy of Laryngeal Primary Motor Cortex and Premotor Cortex in the Caudal Opercular Part of Inferior Frontal Gyrus. Clin. Neurophysiol. 2014, 125, 1912–1922. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, P.; Gracco, V.L. On the Selection of Words and Oral Motor Responses: Evidence of a Response-Independent Fronto-Parietal Network. Cortex 2010, 46, 15–28. [Google Scholar] [CrossRef]
- Tremblay, P.; Small, S.L. Motor Response Selection in Overt Sentence Production: A Functional MRI Study. Front. Psychol. 2011, 2, 253. [Google Scholar] [CrossRef]
- Bannur, U.; Rajshekhar, V. Post Operative Supplementary Motor Area Syndrome: Clinical Features and Outcome. Br. J. Neurosurg. 2000, 14, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Chivukula, S.; Pikul, B.K.; Black, K.L.; Pouratian, N.; Bookheimer, S.Y. Contralateral Functional Reorganization of the Speech Supplementary Motor Area Following Neurosurgical Tumor Resection. Brain Lang. 2018, 183, 41–46. [Google Scholar] [CrossRef]
- Kemerdere, R.; de Champfleur, N.M.; Deverdun, J.; Cochereau, J.; Moritz-Gasser, S.; Herbet, G.; Duffau, H. Role of the Left Frontal Aslant Tract in Stuttering: A Brain Stimulation and Tractographic Study. J. Neurol. 2016, 263, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Neef, N.E.; Bütfering, C.; Anwander, A.; Friederici, A.D.; Paulus, W.; Sommer, M. Left Posterior-Dorsal Area 44 Couples with Parietal Areas to Promote Speech Fluency, While Right Area 44 Activity Promotes the Stopping of Motor Responses. Neuroimage 2016, 142, 628–644. [Google Scholar] [CrossRef] [PubMed]
- Neef, N.E.; Anwander, A.; Bütfering, C.; Schmidt-Samoa, C.; Friederici, A.D.; Paulus, W.; Sommer, M. Structural Connectivity of Right Frontal Hyperactive Areas Scales with Stuttering Severity. Brain 2018, 141, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Alario, F.-X.; Chainay, H.; Lehericy, S.; Cohen, L. The Role of the Supplementary Motor Area (SMA) in Word Production. Brain Res. 2006, 1076, 129–143. [Google Scholar] [CrossRef]
- Smirni, D.; Turriziani, P.; Mangano, G.R.; Bracco, M.; Oliveri, M.; Cipolotti, L. Modulating Phonemic Fluency Performance in Healthy Subjects with Transcranial Magnetic Stimulation over the Left or Right Lateral Frontal Cortex. Neuropsychologia 2017, 102, 109–115. [Google Scholar] [CrossRef]
- Mandelli, M.L.; Caverzasi, E.; Binney, R.J.; Henry, M.L.; Lobach, I.; Block, N.; Amirbekian, B.; Dronkers, N.; Miller, B.L.; Henry, R.G.; et al. Frontal White Matter Tracts Sustaining Speech Production in Primary Progressive Aphasia. J. Neurosci. 2014, 34, 9754–9767. [Google Scholar] [CrossRef] [PubMed]
- Vassal, F.; Boutet, C.; Lemaire, J.-J.; Nuti, C. New Insights into the Functional Significance of the Frontal Aslant Tract: An Anatomo-Functional Study Using Intraoperative Electrical Stimulations Combined with Diffusion Tensor Imaging-Based Fiber Tracking. Br. J. Neurosurg. 2014, 28, 685–687. [Google Scholar] [CrossRef] [PubMed]
- Corrivetti, F.; de Schotten, M.T.; Poisson, I.; Froelich, S.; Descoteaux, M.; Rheault, F.; Mandonnet, E. Dissociating Motor-Speech from Lexico-Semantic Systems in the Left Frontal Lobe: Insight from a Series of 17 Awake Intraoperative Mappings in Glioma Patients. Brain Struct. Funct. 2019, 224, 1151–1165. [Google Scholar] [CrossRef] [PubMed]
- Aron, A.R. The Neural Basis of Inhibition in Cognitive Control. Neuroscientist 2007, 13, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Aron, A.R.; Fletcher, P.C.; Bullmore, E.T.; Sahakian, B.J.; Robbins, T.W. Stop-Signal Inhibition Disrupted by Damage to Right Inferior Frontal Gyrus in Humans. Nat. Neurosci. 2003, 6, 115–116. [Google Scholar] [CrossRef]
- Aron, A.R.; Robbins, T.W.; Poldrack, R.A. Inhibition and the Right Inferior Frontal Cortex. Trends Cogn. Sci. 2004, 8, 170–177. [Google Scholar] [CrossRef]
- Favre, E.; Ballanger, B.; Thobois, S.; Broussolle, E.; Boulinguez, P. Deep Brain Stimulation of the Subthalamic Nucleus, but Not Dopaminergic Medication, Improves Proactive Inhibitory Control of Movement Initiation in Parkinson’s Disease. Neurotherapeutics 2013, 10, 154–167. [Google Scholar] [CrossRef]
- Jahanshahi, M. Effects of Deep Brain Stimulation of the Subthalamic Nucleus on Inhibitory and Executive Control over Prepotent Responses in Parkinson’s Disease. Front. Syst. Neurosci. 2013, 7, 118. [Google Scholar] [CrossRef]
- Obeso, I.; Wilkinson, L.; Casabona, E.; Speekenbrink, M.; Luisa Bringas, M.; Álvarez, M.; Álvarez, L.; Pavón, N.; Rodríguez-Oroz, M.C.; Macías, R.; et al. The Subthalamic Nucleus and Inhibitory Control: Impact of Subthalamotomy in Parkinson’s Disease. Brain 2014, 137, 1470–1480. [Google Scholar] [CrossRef]
- van Wouwe, N.C.; Pallavaram, S.; Phibbs, F.T.; Martinez-Ramirez, D.; Neimat, J.S.; Dawant, B.M.; D’Haese, P.F.; Kanoff, K.E.; van den Wildenberg, W.P.M.; Okun, M.S.; et al. Focused Stimulation of Dorsal Subthalamic Nucleus Improves Reactive Inhibitory Control of Action Impulses. Neuropsychologia 2017, 99, 37–47. [Google Scholar] [CrossRef]
- Boehler, C.N.; Appelbaum, L.G.; Krebs, R.M.; Hopf, J.M.; Woldorff, M.G. Pinning down Response Inhibition in the Brain--Conjunction Analyses of the Stop-Signal Task. Neuroimage 2010, 52, 1621–1632. [Google Scholar] [CrossRef]
- Wessel, J.R.; Aron, A.R. On the Globality of Motor Suppression: Unexpected Events and Their Influence on Behavior and Cognition. Neuron 2017, 93, 259–280. [Google Scholar] [CrossRef]
- Cañas, A.; Juncadella, M.; Lau, R.; Gabarrós, A.; Hernández, M. Working Memory Deficits After Lesions Involving the Supplementary Motor Area. Front. Psychol. 2018, 9, 765. [Google Scholar] [CrossRef]
- Fiori, S.; Guzzetta, A.; Mitra, J.; Pannek, K.; Pasquariello, R.; Cipriani, P.; Tosetti, M.; Cioni, G.; Rose, S.E.; Chilosi, A. Neuroanatomical Correlates of Childhood Apraxia of Speech: A Connectomic Approach. Neuroimage Clin. 2016, 12, 894–901. [Google Scholar] [CrossRef]
- Ashtari, M.; Lencz, T.; Zuffante, P.; Bilder, R.; Clarke, T.; Diamond, A.; Kane, J.; Szeszko, P. Left Middle Temporal Gyrus Activation during a Phonemic Discrimination Task. Neuroreport 2004, 15, 389–393. [Google Scholar] [CrossRef]
- Guenther, F.H.; Hickok, G. Role of the Auditory System in Speech Production. Handb. Clin. Neurol. 2015, 129, 161–175. [Google Scholar] [CrossRef]
- Kadis, D.S.; Goshulak, D.; Namasivayam, A.; Pukonen, M.; Kroll, R.; De Nil, L.F.; Pang, E.W.; Lerch, J.P. Cortical Thickness in Children Receiving Intensive Therapy for Idiopathic Apraxia of Speech. Brain Topogr. 2014, 27, 240–247. [Google Scholar] [CrossRef]
- Preston, J.L.; Molfese, P.J.; Mencl, W.E.; Frost, S.J.; Hoeft, F.; Fulbright, R.K.; Landi, N.; Grigorenko, E.L.; Seki, A.; Felsenfeld, S.; et al. Structural Brain Differences in School-Age Children with Residual Speech Sound Errors. Brain Lang. 2014, 128, 25–33. [Google Scholar] [CrossRef]
- Tkach, J.A.; Chen, X.; Freebairn, L.A.; Schmithorst, V.J.; Holland, S.K.; Lewis, B.A. Neural Correlates of Phonological Processing in Speech Sound Disorder: A Functional Magnetic Resonance Imaging Study. Brain Lang. 2011, 119, 42–49. [Google Scholar] [CrossRef][Green Version]
- Grande, M.; Meffert, E.; Schoenberger, E.; Jung, S.; Frauenrath, T.; Huber, W.; Hussmann, K.; Moormann, M.; Heim, S. From a Concept to a Word in a Syntactically Complete Sentence: An FMRI Study on Spontaneous Language Production in an Overt Picture Description Task. Neuroimage 2012, 61, 702–714. [Google Scholar] [CrossRef]
- Cavanna, A.E.; Trimble, M.R. The Precuneus: A Review of Its Functional Anatomy and Behavioural Correlates. Brain 2006, 129, 564–583. [Google Scholar] [CrossRef] [PubMed]
- Liégeois, F.J.; Morgan, A.T. Neural Bases of Childhood Speech Disorders: Lateralization and Plasticity for Speech Functions during Development. Neurosci. Biobehav. Rev. 2012, 36, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Shriberg, L.D.; Potter, N.L.; Strand, E.A. Prevalence and Phenotype of Childhood Apraxia of Speech in Youth with Galactosemia. J. Speech Lang. Hear. Res. 2011, 54, 487–519. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. Wechsler Preschool and Primary Scale of Intelligence, 4th ed.; The Psychological Corporation: San Antonio, TX, USA, (WPPSI—III); 2012. [Google Scholar]
- McLeod, S.; Harrison, L.J.; McCormack, J. The Intelligibility in Context Scale: Validity and Reliability of a Subjective Rating Measure. J. Speech Lang. Hear. Res. 2012, 55, 648–656. [Google Scholar] [CrossRef]
- Usai, M.C.; Viterbori, P.; Gandolfi, E.; Traverso, L. FE-PS 2-6: Batteria per la Valutazione delle Funzioni Esecutive in età Prescolare; Edizioni Centro Studi Erickson: Roma, Italy, 2017; ISBN 978-88-590-1310-5. [Google Scholar]
- Valeri, G.; Stievano, P.; Ferretti, M.; Mariani, E.; Pieretti, M. BAFE Batteria per l’Assessment Delle Funzioni Esecutive in Età Prescolare; Hogrefe Editore: Florence, Italy, 2015. [Google Scholar]
- Conti, E.; Retico, A.; Palumbo, L.; Spera, G.; Bosco, P.; Biagi, L.; Fiori, S.; Tosetti, M.; Cipriani, P.; Cioni, G.; et al. Autism Spectrum Disorder and Childhood Apraxia of Speech: Early Language-Related Hallmarks across Structural MRI Study. J. Pers. Med. 2020, 10, 275. [Google Scholar] [CrossRef]
- Fischl, B. FreeSurfer. Neuroimage 2012, 62, 774–781. [Google Scholar] [CrossRef]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.J.; Woolrich, M.W.; Smith, S.M. FSL. Neuroimage 2012, 62, 782–790. [Google Scholar] [CrossRef]
- Tournier, J.-D.; Calamante, F.; Gadian, D.G.; Connelly, A. Direct Estimation of the Fiber Orientation Density Function from Diffusion-Weighted MRI Data Using Spherical Deconvolution. Neuroimage 2004, 23, 1176–1185. [Google Scholar] [CrossRef]
- Biagi, L.; Lenzi, S.; Cipriano, E.; Fiori, S.; Bosco, P.; Cristofani, P.; Astrea, G.; Pini, A.; Cioni, G.; Mercuri, E.; et al. Neural Substrates of Neuropsychological Profiles in Dystrophynopathies: A Pilot Study of Diffusion Tractography Imaging. PLoS ONE 2021, 16, e0250420. [Google Scholar] [CrossRef]
- Horbruegger, M.; Loewe, K.; Kaufmann, J.; Wagner, M.; Schippling, S.; Pawlitzki, M.; Schoenfeld, M.A. Anatomically Constrained Tractography Facilitates Biologically Plausible Fiber Reconstruction of the Optic Radiation in Multiple Sclerosis. NeuroImage Clin. 2019, 22, 101740. [Google Scholar] [CrossRef]
- Broce, I.; Bernal, B.; Altman, N.; Tremblay, P.; Dick, A.S. Fiber Tracking of the Frontal Aslant Tract and Subcomponents of the Arcuate Fasciculus in 5–8-Year-Olds: Relation to Speech and Language Function. Brain Lang. 2015, 149, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: Third Edition: A Regression-Based Approach. Available online: https://www.guilford.com/books/Introduction-to-Mediation-Moderation-and-Conditional-Process-Analysis/Andrew-Hayes/9781462549030 (accessed on 13 December 2022).
- Chilosi, A.M.; Comparini, A.; Scusa, M.F.; Orazini, L.; Forli, F.; Cipriani, P.; Berrettini, S. A Longitudinal Study of Lexical and Grammar Development in Deaf Italian Children Provided With Early Cochlear Implantation. Ear Hear. 2013, 34, e28. [Google Scholar] [CrossRef] [PubMed]
- Chilosi, A.M.; Cipriani, P. Tcgb-Test Di Comprensione Grammaticale Per Bambini; Edizioni del Cerro; Ottobre: Rovaniemi, Finland, 2005; ISBN 88-8216-234-6. [Google Scholar]
- Bishop, D.V.M. TROG-2: Test for Reception of Grammar, Version 2: Manuale/Dorothy M. V. Bishop; Adattamento Italiano a Cura di Silvana Suraniti, Raffaele Ferri e Vincenzo Neri; Giunti, O.S., Ed.; Università degli Studi di Padova: Firenze, Italy, 2009; ISBN 978-88-09-06320-4. [Google Scholar]
- Vicari, S.; Luigi, M.; Alessandra, L. TFL Test Fono-Lessicale; Erickson: Trento, Italy, 2007. [Google Scholar]
- Dunn, L.M.; Pizzoli, C.; Tressoldi, P.E. Peabody Picture Vocabulary Test-Revised (PPVT-R); Omega Edizioni: Torino, Italy, 2000. [Google Scholar]
- Brizzolara, D. Test Di Vocabolario Figurato. In Technical Report of the Research Project 500.4/62.1/1134 Supported by a Grant from the Italian Department of Health to IRCCS Stella Maris; Università di Pisa: Pisa, Italy, 1989. [Google Scholar]
- Hertrich, I.; Dietrich, S.; Ackermann, H. The Role of the Supplementary Motor Area for Speech and Language Processing. Neurosci. Biobehav. Rev. 2016, 68, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Iuzzini-Seigel, J. Motor Performance in Children With Childhood Apraxia of Speech and Speech Sound Disorders. J. Speech Lang. Hear. Res. 2019, 62, 3220–3233. [Google Scholar] [CrossRef] [PubMed]
- Baddeley, A. Working Memory, Thought, and Action; OUP Oxford: Oxford, UK, 2007; ISBN 978-0-19-100496-4. [Google Scholar]
- Morris, N.; Jones, D.M. Memory Updating in Working Memory: The Role of the Central Executive. Br. J. Psychol. 1990, 81, 111–121. [Google Scholar] [CrossRef]
- Kim, H.-J.; Choi, S.Y.; Ha, J.-W. Speech-Motor Program/Programming in Children with Childhood Apraxia of Speech, Children with Articulatory and Phonological Disorders and Typically Developing Children. Commun. Sci. Disord. 2015, 20, 60–71. [Google Scholar] [CrossRef]
- Case, J.; Grigos, M.I. Articulatory Control in Childhood Apraxia of Speech in a Novel Word-Learning Task. J. Speech Lang. Hear. Res. 2016, 59, 1253–1268. [Google Scholar] [CrossRef]
- Edeal, D.M.; Gildersleeve-Neumann, C.E. The Importance of Production Frequency in Therapy for Childhood Apraxia of Speech. Am. J. Speech Lang. Pathol. 2011, 20, 95–110. [Google Scholar] [CrossRef]
- Maas, E.; Gildersleeve-Neumann, C.; Jakielski, K.J.; Stoeckel, R. Motor-Based Intervention Protocols in Treatment of Childhood Apraxia of Speech (CAS). Curr. Dev. Disord. Rep. 2014, 1, 197–206. [Google Scholar] [CrossRef]
- Thomas, D.C.; McCabe, P.; Ballard, K.J. Rapid Syllable Transitions (ReST) Treatment for Childhood Apraxia of Speech: The Effect of Lower Dose-Frequency. J. Commun. Disord. 2014, 51, 29–42. [Google Scholar] [CrossRef]
- Spaulding, T.J. Investigating Mechanisms of Suppression in Preschool Children With Specific Language Impairment. J. Speech Lang. Hear. Res. 2010, 53, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Marton, K.; Schwartz, R.G. Working Memory Capacity and Language Processes in Children With Specific Language Impairment. J. Speech Lang. Hear. Res. JSLHR 2003, 46, 1138–1153. [Google Scholar] [CrossRef] [PubMed]
- Archibald, L.M.D.; Gathercole, S.E. Short-Term and Working Memory in Specific Language Impairment. Int. J. Lang. Commun. Disord. 2006, 41, 675–693. [Google Scholar] [CrossRef]
- Im-Bolter, N.; Johnson, J.; Pascual-Leone, J. Processing Limitations in Children with Specific Language Impairment: The Role of Executive Function. Child Dev. 2006, 77, 1822–1841. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Giesbrecht, G.F.; Müller, U.; McInerney, R.J.; Kerns, K.A. A Latent Variable Approach to Determining the Structure of Executive Function in Preschool Children. J. Cogn. Dev. 2012, 13, 395–423. [Google Scholar] [CrossRef]
- Rossi, E.; Cheng, H.; Kroll, J.F.; Diaz, M.T.; Newman, S.D. Changes in White-Matter Connectivity in Late Second Language Learners: Evidence from Diffusion Tensor Imaging. Front. Psychol. 2017, 8, 2040. [Google Scholar] [CrossRef]
- Takebayashi, T.; Marumoto, K.; Takahashi, K.; Domen, K. Differences in Neural Pathways Are Related to the Short- or Long-Term Benefits of Constraint-Induced Movement Therapy in Patients with Chronic Stroke and Hemiparesis: A Pilot Cohort Study. Top. Stroke Rehabil. 2018, 25, 203–208. [Google Scholar] [CrossRef]
- Tataranno, M.L.; Claessens, N.H.P.; Moeskops, P.; Toet, M.C.; Kersbergen, K.J.; Buonocore, G.; Išgum, I.; Leemans, A.; Counsell, S.; Groenendaal, F.; et al. Changes in Brain Morphology and Microstructure in Relation to Early Brain Activity in Extremely Preterm Infants. Pediatr. Res. 2018, 83, 834–842. [Google Scholar] [CrossRef]
- Fiori, S.; Pannek, K.; Podda, I.; Cipriani, P.; Lorenzoni, V.; Franchi, B.; Pasquariello, R.; Guzzetta, A.; Cioni, G.; Chilosi, A. Neural Changes Induced by a Speech Motor Treatment in Childhood Apraxia of Speech: A Case Series. J. Child Neurol. 2021, 36, 958–967. [Google Scholar] [CrossRef]
- Catani, M.; Mesulam, M.M.; Jakobsen, E.; Malik, F.; Martersteck, A.; Wieneke, C.; Thompson, C.K.; Thiebaut de Schotten, M.; Dell’Acqua, F.; Weintraub, S.; et al. A Novel Frontal Pathway Underlies Verbal Fluency in Primary Progressive Aphasia. Brain 2013, 136, 2619–2628. [Google Scholar] [CrossRef]
- Mars, R.B.; Piekema, C.; Coles, M.G.H.; Hulstijn, W.; Toni, I. On the Programming and Reprogramming of Actions. Cereb. Cortex 2007, 17, 2972–2979. [Google Scholar] [CrossRef] [PubMed]
- Shriberg, L.D.; Aram, D.M.; Kwiatkowski, J. Developmental Apraxia of Speech: II. Toward a Diagnostic Marker. J. Speech Lang. Hear. Res. 1997, 40, 286–312. [Google Scholar] [CrossRef] [PubMed]


| Speech Assessment Protocol | CAS Group | Reference Data |
|---|---|---|
| Phonetic inventory | M = 12.7 (SD = 4.09) | 40 TD children, mean age = 4.7 years (SD = 0.47 years) Mean number of phonemes: 19.2 (SD = 0.9) |
| Word Inaccuracy | 61% | 40 TD children, mean age = 4.7 years (SD = 0.47 years) Mean percentage of inaccurate productions: 8.8% (SD = 10.7) |
| Inconsistent errors on consonants and vowels | 24% | 40 TD children mean age = 4.7 (SD = 0.47 years) Mean percentage of inconsistent errors: 0.4% (SD = 1.3) |
| DDK rate (maximum performance task) | M = 15.13 (SD = 4.01) | 40 TD children (mean age = 4.7 years (SD = 0.9 years) Mean number of repetitions: 23.18 (SD = 4.5) |
| Intelligibility | M = 2.32 (SD = 0.87). | Qualitative rating scale ranging from 5 to 1 (5 = always, 4 = usually, 3 = sometimes, 2 = rarely, 1 = never intelligible) |
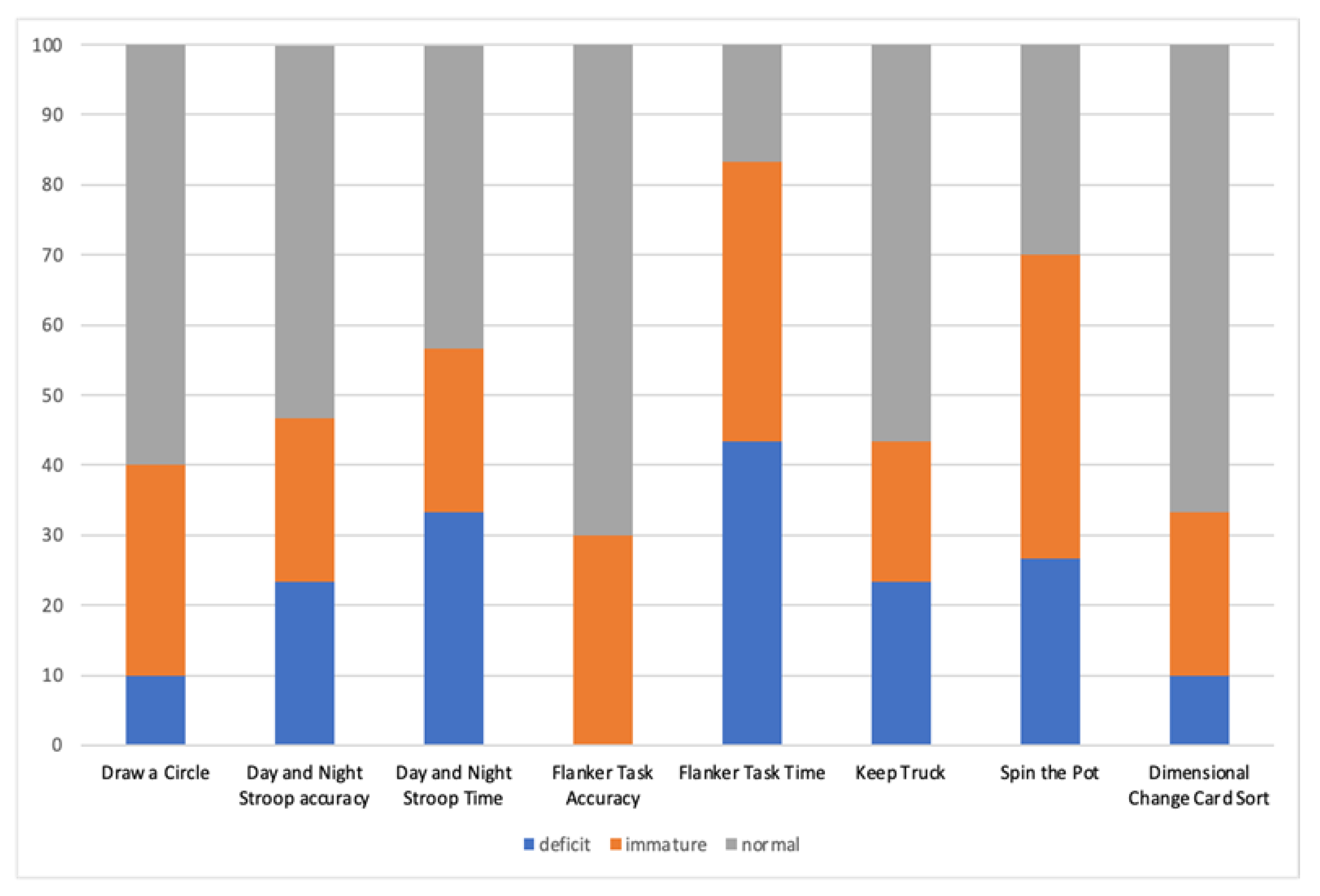
| EF Assessment Protocol of CAS Group | % <5° Percentile | % <10° Percentile | % >25° Percentile |
|---|---|---|---|
| Motor Response Inhibition (Draw a Circle) | 10% | 30% | 60% |
| Visual-Verbal Response Inhibition (Day and Night Stroop, accuracy) | 23.4% | 23.3% | 53.3% |
| Visual-Verbal Response Inhibition (Day and Night Stroop, time) | 33.4% | 23.3% | 43.3% |
| Visual-Spatial Control Interference (Flanker Task, accuracy) | 0% | 30% | 70% |
| Visual-Spatial Control Interference (Flanker Task, time) | 43.3% | 40% | 16.7% |
| Visual-Verbal Updating (Keep Truck) | 23.3% | 20% | 56.7% |
| Visual-Spatial Updating (Spin the Pot) | 26.7% | 43.3% | 30% |
| Visual Cognitive Flexibility (Dimensional Change Card Sort) | 10% | 23.3% | 66.7% |
| CAS Mean (SD) | TD Mean (SD) | F | p | |
|---|---|---|---|---|
| FA-left preSMA FAT | 0.36 (0.02) | 0.39 (0.02) | 5.93 | 0.02 * |
| FA-right preSMA FAT | 0.37 (0.02) | 0.37 (0.03) | 0.01 | 0.90 |
| FA-left SMA FAT | 0.37 (0.02) | 0.39 (0.02) | 2.79 | 0.10 |
| FA-right SMA FAT | 0.37 (0.02) | 0.37 (0.03) | 1.71 | 0.19 |
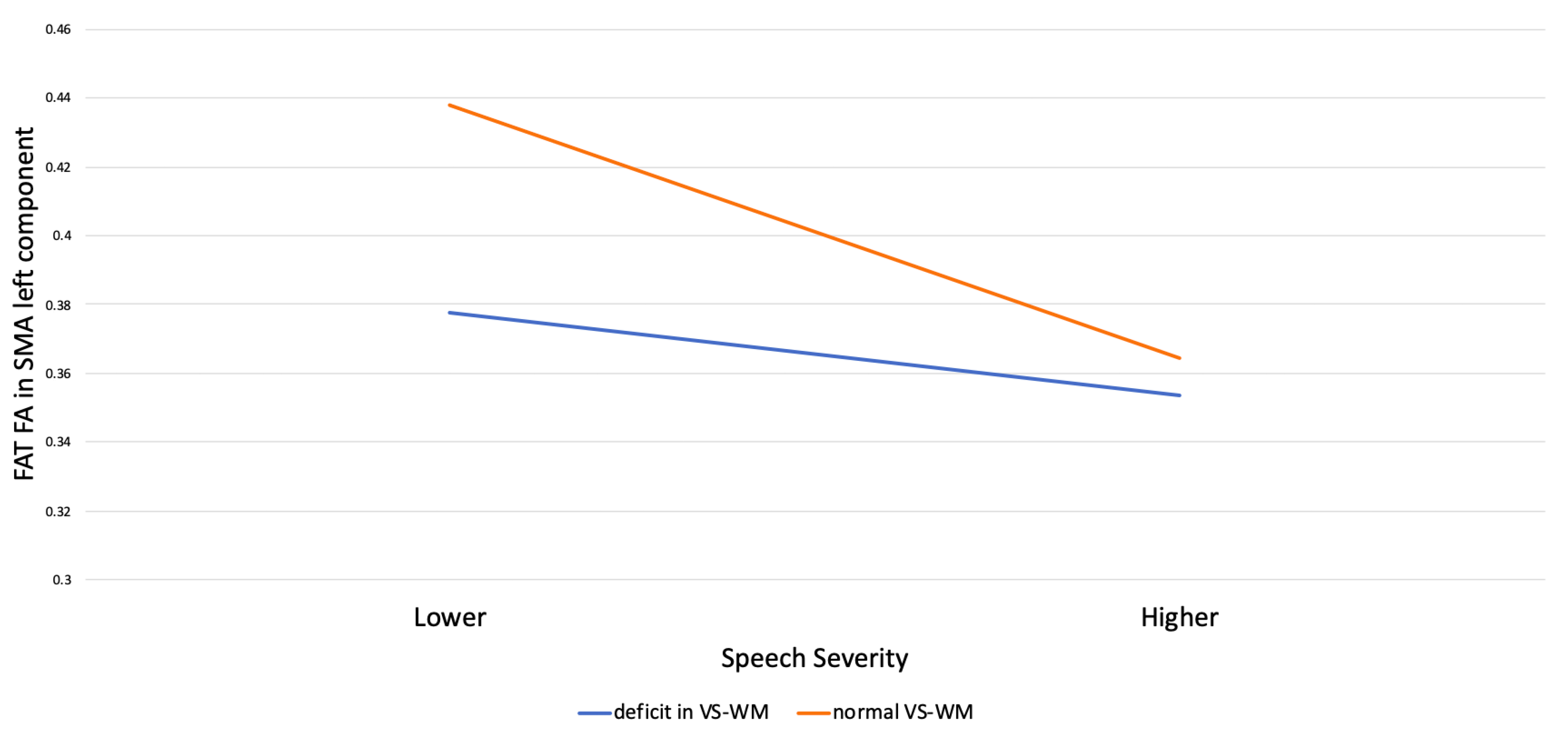
| B | S.E. | t | p | 95% CI | |
|---|---|---|---|---|---|
| Speech severity (SS) | −0.025 | −0.03 | −0.89 | 0.38 | −0.015–0.064 |
| Visual-spatial working memory (VS-WM) | −0.039 | 0.03 | −1.33 | 0.02 | 0.076–0.142 |
| SS × VS-WM | 0.05 | 0.02 | 2.11 | <0.05 | −0.072–−0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bombonato, C.; Cipriano, E.; Pecini, C.; Casalini, C.; Bosco, P.; Podda, I.; Tosetti, M.; Biagi, L.; Chilosi, A.M. Relationship among Connectivity of the Frontal Aslant Tract, Executive Functions, and Speech and Language Impairment in Children with Childhood Apraxia of Speech. Brain Sci. 2023, 13, 78. https://doi.org/10.3390/brainsci13010078
Bombonato C, Cipriano E, Pecini C, Casalini C, Bosco P, Podda I, Tosetti M, Biagi L, Chilosi AM. Relationship among Connectivity of the Frontal Aslant Tract, Executive Functions, and Speech and Language Impairment in Children with Childhood Apraxia of Speech. Brain Sciences. 2023; 13(1):78. https://doi.org/10.3390/brainsci13010078
Chicago/Turabian StyleBombonato, Clara, Emilio Cipriano, Chiara Pecini, Claudia Casalini, Paolo Bosco, Irina Podda, Michela Tosetti, Laura Biagi, and Anna Maria Chilosi. 2023. "Relationship among Connectivity of the Frontal Aslant Tract, Executive Functions, and Speech and Language Impairment in Children with Childhood Apraxia of Speech" Brain Sciences 13, no. 1: 78. https://doi.org/10.3390/brainsci13010078
APA StyleBombonato, C., Cipriano, E., Pecini, C., Casalini, C., Bosco, P., Podda, I., Tosetti, M., Biagi, L., & Chilosi, A. M. (2023). Relationship among Connectivity of the Frontal Aslant Tract, Executive Functions, and Speech and Language Impairment in Children with Childhood Apraxia of Speech. Brain Sciences, 13(1), 78. https://doi.org/10.3390/brainsci13010078






