Effects of Physiological Signal Removal on Resting-State Functional MRI Metrics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. MRI Acquisition
2.3. Physiological Signals Acquisition
2.4. Data Processing
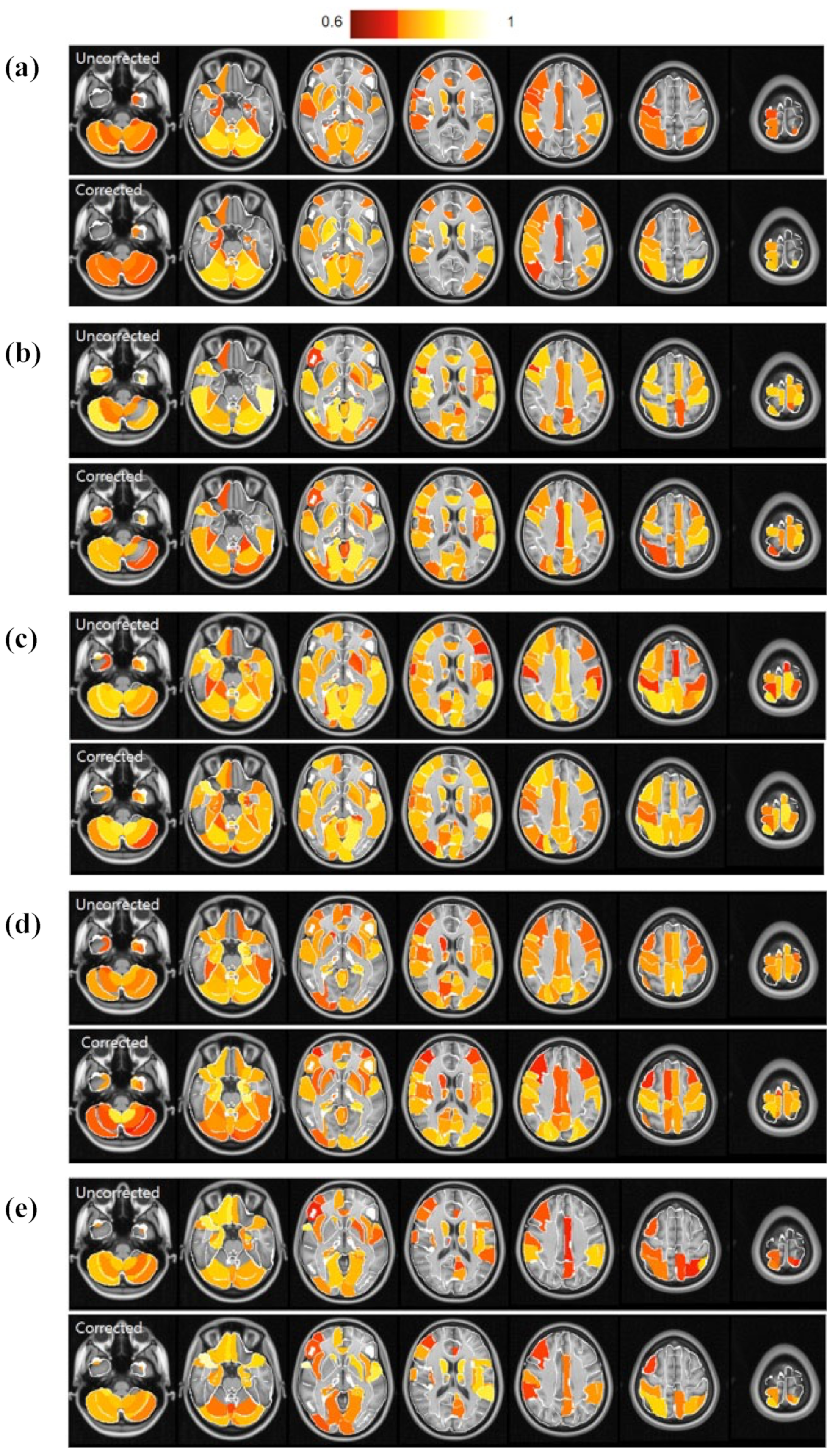
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biswal, B.; Yetkin, F.Z.; Haughton, V.M.; Hyde, J.S. Functional Connectivity in the Motor Cortex of Resting Human Brain Using Echo-Planar MRI. Magn. Reson. Med. 1995, 34, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Fransson, P. Spontaneous Low-Frequency BOLD Signal Fluctuations: An FMRI Investigation of the Resting-State Default Mode of Brain Function Hypothesis. Hum. Brain Mapp. 2005, 26, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.D.; Raichle, M.E. Spontaneous Fluctuations in Brain Activity Observed with Functional Magnetic Resonance Imaging. Nat. Rev. Neurosci. 2007, 8, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Craig, M.M.; Manktelow, A.E.; Sahakian, B.J.; Menon, D.K.; Stamatakis, E.A. Spectral Diversity in Default Mode Network Connectivity Reflects Behavioral State. J. Cogn. Neurosci. 2018, 30, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Chen, H.; He, C.; Long, Z.; Guo, X.; Zhou, Y.; Uddin, L.Q.; Chen, H. Resting-State Functional under-Connectivity within and between Large-Scale Cortical Networks across Three Low-Frequency Bands in Adolescents with Autism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 79, 434–441. [Google Scholar] [CrossRef]
- Finn, E.S.; Shen, X.; Scheinost, D.; Rosenberg, M.D.; Huang, J.; Chun, M.M.; Papademetris, X.; Constable, R.T. Functional Connectome Fingerprinting: Identifying Individuals Using Patterns of Brain Connectivity. Nat. Neurosci. 2015, 18, 1664–1671. [Google Scholar] [CrossRef]
- Göttler, J.; Preibisch, C.; Riederer, I.; Pasquini, L.; Alexopoulos, P.; Bohn, K.P.; Yakushev, I.; Beller, E.; Kaczmarz, S.; Zimmer, C.; et al. Reduced Blood Oxygenation Level Dependent Connectivity Is Related to Hypoperfusion in Alzheimer’s Disease. J. Cereb. Blood Flow Metab. 2019, 39, 1314–1325. [Google Scholar] [CrossRef]
- Hegarty, J.P.; Ferguson, B.J.; Zamzow, R.M.; Rohowetz, L.J.; Johnson, J.D.; Christ, S.E.; Beversdorf, D.Q. Beta-Adrenergic Antagonism Modulates Functional Connectivity in the Default Mode Network of Individuals with and without Autism Spectrum Disorder. Brain Imaging Behav. 2017, 11, 1278–1289. [Google Scholar] [CrossRef]
- Jack, A. Neuroimaging in Neurodevelopmental Disorders: Focus on Resting-State FMRI Analysis of Intrinsic Functional Brain Connectivity. Curr. Opin. Neurol. 2018, 31, 140–148. [Google Scholar] [CrossRef]
- Lei, X.; Zhong, M.; Liu, Y.; Jin, X.; Zhou, Q.; Xi, C.; Tan, C.; Zhu, X.; Yao, S.; Yi, J. A Resting-State FMRI Study in Borderline Personality Disorder Combining Amplitude of Low Frequency Fluctuation, Regional Homogeneity and Seed Based Functional Connectivity. J. Affect. Disord. 2017, 218, 299–305. [Google Scholar] [CrossRef]
- Meskaldji, D.-E.; Preti, M.G.; Bolton, T.A.; Montandon, M.-L.; Rodriguez, C.; Morgenthaler, S.; Giannakopoulos, P.; Haller, S.; Van De Ville, D. Prediction of Long-Term Memory Scores in MCI Based on Resting-State FMRI. NeuroImage Clin. 2016, 12, 785–795. [Google Scholar] [CrossRef]
- Song, X.; Qian, S.; Liu, K.; Zhou, S.; Zhu, H.; Zou, Q.; Liu, Y.; Sun, G.; Gao, J.-H. Resting-State BOLD Oscillation Frequency Predicts Vigilance Task Performance at Both Normal and High Environmental Temperatures. Brain Struct. Funct. 2017, 222, 4065–4077. [Google Scholar] [CrossRef]
- Sung, Y.-W.; Kawachi, Y.; Choi, U.-S.; Kang, D.; Abe, C.; Otomo, Y.; Ogawa, S. A Set of Functional Brain Networks for the Comprehensive Evaluation of Human Characteristics. Front. Neurosci. 2018, 12, 149. [Google Scholar] [CrossRef]
- Tavor, I.; Jones, O.P.; Mars, R.B.; Smith, S.M.; Behrens, T.E.; Jbabdi, S. Task-Free MRI Predicts Individual Differences in Brain Activity during Task Performance. Science 2016, 352, 216–220. [Google Scholar] [CrossRef]
- Wang, B.; Niu, Y.; Miao, L.; Cao, R.; Yan, P.; Guo, H.; Li, D.; Guo, Y.; Yan, T.; Wu, J.; et al. Decreased Complexity in Alzheimer’s Disease: Resting-State FMRI Evidence of Brain Entropy Mapping. Front. Aging Neurosci. 2017, 9, 378. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Guo, Z.; Liu, X.; Jia, X.; Li, J.; Li, Y.; Lv, D.; Chen, W. Abnormal Functional Connectivity of the Posterior Cingulate Cortex Is Associated with Depressive Symptoms in Patients with Alzheimer’s Disease. Neuropsychiatr. Dis. Treat. 2017, 13, 2589–2598. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.L.; Kahn, I.; Snyder, A.Z.; Raichle, M.E.; Buckner, R.L. Evidence for a Frontoparietal Control System Revealed by Intrinsic Functional Connectivity. J. Neurophysiol. 2008, 100, 3328–3342. [Google Scholar] [CrossRef] [Green Version]
- Zou, Q.-H.; Zhu, C.-Z.; Yang, Y.; Zuo, X.-N.; Long, X.-Y.; Cao, Q.-J.; Wang, Y.-F.; Zang, Y.-F. An Improved Approach to Detection of Amplitude of Low-Frequency Fluctuation (ALFF) for Resting-State FMRI: Fractional ALFF. J. Neurosci. Methods 2008, 172, 137–141. [Google Scholar] [CrossRef] [Green Version]
- Zang, Y.; Jiang, T.; Lu, Y.; He, Y.; Tian, L. Regional Homogeneity Approach to FMRI Data Analysis. NeuroImage 2004, 22, 394–400. [Google Scholar] [CrossRef]
- Zuo, X.-N.; Kelly, C.; Martino, A.D.; Mennes, M.; Margulies, D.S.; Bangaru, S.; Grzadzinski, R.; Evans, A.C.; Zang, Y.-F.; Castellanos, F.X.; et al. Growing Together and Growing Apart: Regional and Sex Differences in the Lifespan Developmental Trajectories of Functional Homotopy. J. Neurosci. 2010, 30, 15034–15043. [Google Scholar] [CrossRef] [Green Version]
- Buckner, R.L.; Sepulcre, J.; Talukdar, T.; Krienen, F.M.; Liu, H.; Hedden, T.; Andrews-Hanna, J.R.; Sperling, R.A.; Johnson, K.A. Cortical Hubs Revealed by Intrinsic Functional Connectivity: Mapping, Assessment of Stability, and Relation to Alzheimer’s Disease. J. Neurosci. 2009, 29, 1860–1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, X.-N.; Ehmke, R.; Mennes, M.; Imperati, D.; Castellanos, F.X.; Sporns, O.; Milham, M.P. Network Centrality in the Human Functional Connectome. Cereb. Cortex 2012, 22, 1862–1875. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L.; DiNicola, L.M. The Brain’s Default Network: Updated Anatomy, Physiology and Evolving Insights. Nat. Rev. Neurosci. 2019, 20, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Greicius, M.D.; Srivastava, G.; Reiss, A.L.; Menon, V. Default-Mode Network Activity Distinguishes Alzheimer’s Disease from Healthy Aging: Evidence from Functional MRI. Proc. Natl. Acad. Sci. USA 2004, 101, 4637–4642. [Google Scholar] [CrossRef] [Green Version]
- Mason, M.F.; Norton, M.I.; Horn, J.D.V.; Wegner, D.M.; Grafton, S.T.; Macrae, C.N. Wandering Minds: The Default Network and Stimulus-Independent Thought. Science 2007, 315, 393–395. [Google Scholar] [CrossRef] [Green Version]
- Birn, R.M.; Cornejo, M.D.; Molloy, E.K.; Patriat, R.; Meier, T.B.; Kirk, G.R.; Nair, V.A.; Meyerand, M.E.; Prabhakaran, V. The Influence of Physiological Noise Correction on Test–Retest Reliability of Resting-State Functional Connectivity. Brain Connect. 2014, 4, 511–522. [Google Scholar] [CrossRef] [Green Version]
- Kassinopoulos, M.; Mitsis, G.D. Physiological Noise Modeling in FMRI Based on the Pulsatile Component of Photoplethysmograph. NeuroImage 2021, 242, 118467. [Google Scholar] [CrossRef]
- Chang, C.; Glover, G.H. Effects of Model-Based Physiological Noise Correction on Default Mode Network Anti-Correlations and Correlations. NeuroImage 2009, 47, 1448–1459. [Google Scholar] [CrossRef] [Green Version]
- Murphy, K.; Birn, R.M.; Bandettini, P.A. Resting-State FMRI Confounds and Cleanup. NeuroImage 2013, 80, 349–359. [Google Scholar] [CrossRef] [Green Version]
- Birn, R.M.; Diamond, J.B.; Smith, M.A.; Bandettini, P.A. Separating Respiratory-Variation-Related Fluctuations from Neuronal-Activity-Related Fluctuations in FMRI. NeuroImage 2006, 31, 1536–1548. [Google Scholar] [CrossRef]
- Glasser, M.F.; Coalson, T.S.; Bijsterbosch, J.D.; Harrison, S.J.; Harms, M.P.; Anticevic, A.; Van Essen, D.C.; Smith, S.M. Using Temporal ICA to Selectively Remove Global Noise While Preserving Global Signal in Functional MRI Data. NeuroImage 2018, 181, 692–717. [Google Scholar] [CrossRef]
- Shin, W.; Koenig, K.A.; Lowe, M.J. A Comprehensive Investigation of Physiologic Noise Modeling in Resting State FMRI; time Shifted Cardiac Noise in EPI and Its Removal without External Physiologic Signal Measures. NeuroImage 2022, 254, 119136. [Google Scholar] [CrossRef]
- Cole, D.; Smith, S.; Beckmann, C. Advances and Pitfalls in the Analysis and Interpretation of Resting-State FMRI Data. Front. Syst. Neurosci. 2010, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Bright, M.G.; Tench, C.R.; Murphy, K. Potential Pitfalls When Denoising Resting State FMRI Data Using Nuisance Regression. NeuroImage 2017, 154, 159–168. [Google Scholar] [CrossRef]
- Jarrahi, B.; Mackey, S. Measuring the Influence of Physiological Noise Corrections on ICA Derived Intrinsic Connectivity Brain Networks in Rest and Task FMRI. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), IEEE, Honolulu, HI, USA, 18–21 July 2018; pp. 1046–1049. [Google Scholar]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.J.; Woolrich, M.W.; Smith, S.M. FSL. NeuroImage 2012, 62, 782–790. [Google Scholar] [CrossRef] [Green Version]
- Kasper, L.; Bollmann, S.; Diaconescu, A.O.; Hutton, C.; Heinzle, J.; Iglesias, S.; Hauser, T.U.; Sebold, M.; Manjaly, Z.-M.; Pruessmann, K.P.; et al. The PhysIO Toolbox for Modeling Physiological Noise in FMRI Data. J. Neurosci. Methods 2017, 276, 56–72. [Google Scholar] [CrossRef] [Green Version]
- Cox, R.W. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Comput. Biomed. Res. 1996, 29, 162–173. [Google Scholar] [CrossRef]
- Brooks, J.C.W.; Beckmann, C.F.; Miller, K.L.; Wise, R.G.; Porro, C.A.; Tracey, I.; Jenkinson, M. Physiological Noise Modelling for Spinal Functional Magnetic Resonance Imaging Studies. NeuroImage 2008, 39, 680–692. [Google Scholar] [CrossRef]
- Yan, C.-G.; Wang, X.-D.; Zuo, X.-N.; Zang, Y.-F. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics 2016, 14, 339–351. [Google Scholar]
- Rolls, E.T.; Huang, C.-C.; Lin, C.-P.; Feng, J.; Joliot, M. Automated Anatomical Labelling Atlas 3. NeuroImage 2020, 206, 116189. [Google Scholar] [CrossRef]
- McGraw, K.O.; Wong, S.P. Forming Inferences about Some Intraclass Correlation Coefficients. Psychol. Methods 1996, 1, 30–46. [Google Scholar] [CrossRef]
- Liljequist, D.; Elfving, B.; Roaldsen, K.S. Intraclass Correlation—A Discussion and Demonstration of Basic Features. PLoS ONE 2019, 14, e0219854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birn, R.M. The Role of Physiological Noise in Resting-State Functional Connectivity. NeuroImage 2012, 62, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Cauzzo, S.; Callara, A.L.; Sole Morelli, M.; Hartwig, V.; Montanaro, D.; Passino, C.; Emdin, M.; Giannoni, A.; Vanello, N. On the Use of Linear-Modelling-Based Algorithms for Physiological Noise Correction in FMRI Studies of the Central Breathing Control. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), IEEE, Berlin, Germany, 23–27 July 2019; pp. 808–811. [Google Scholar]
- Cao, A.A.; Noll, D.C. A Retrospective Physiological Noise Correction Method for Oscillating Steady-State Imaging. Magn. Reson. Med. 2021, 85, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Glover, G.H.; Li, T.Q.; Ress, D. Image-Based Method for Retrospective Correction of Physiological Motion Effects in FMRI: RETROICOR. Magn. Reson. Med. 2000, 44, 162–167. [Google Scholar] [CrossRef]
- Marchitelli, R.; Minati, L.; Marizzoni, M.; Bosch, B.; Bartrés-Faz, D.; Müller, B.W.; Wiltfang, J.; Fiedler, U.; Roccatagliata, L.; Picco, A.; et al. Test-Retest Reliability of the Default Mode Network in a Multi-Centric FMRI Study of Healthy Elderly: Effects of Data-Driven Physiological Noise Correction Techniques. Hum. Brain Mapp. 2016, 37, 2114–2132. [Google Scholar] [CrossRef]
- Krishnamurthy, V.; Krishnamurthy, L.C.; Schwam, D.M.; Ealey, A.; Shin, J.; Greenberg, D.; Morris, R.D. Retrospective Correction of Physiological Noise: Impact on Sensitivity, Specificity, and Reproducibility of Resting-State Functional Connectivity in a Reading Network Model. Brain Connect. 2018, 8, 94–105. [Google Scholar] [CrossRef]
- Van der Zwaag, W.; Jorge, J.; Butticaz, D.; Gruetter, R. Physiological Noise in Human Cerebellar FMRI. Magn. Reson. Mater. Phys. Biol. Med. 2015, 28, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Golestani, A.M.; Kwinta, J.B.; Khatamian, Y.B.; Chen, J.J. The Effect of Low-Frequency Physiological Correction on the Reproducibility and Specificity of Resting-State FMRI Metrics: Functional Connectivity, ALFF, and ReHo. Front. Neurosci. 2017, 11, 546. [Google Scholar] [CrossRef] [Green Version]
- Brooks, J.C.W.; Faull, O.K.; Pattinson, K.T.S.; Jenkinson, M. Physiological Noise in Brainstem FMRI. Front. Hum. Neurosci. 2013, 7, 623. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, A.; Masaoka, Y.; Yoshida, M.; Koiwa, N.; Honma, M.; Watanabe, K.; Kubota, S.; Natsuko, I.; Ida, M.; Izumizaki, M. Heart Rate and Respiration Affect the Functional Connectivity of Default Mode Network in Resting-State Functional Magnetic Resonance Imaging. Front. Neurosci. 2020, 14, 631. [Google Scholar] [CrossRef]
- Kamba, M.; Sung, Y.-W.; Ogawa, S. A Dynamic System Model-Based Technique for Functional MRI Data Analysis. NeuroImage 2004, 22, 179–187. [Google Scholar] [CrossRef]

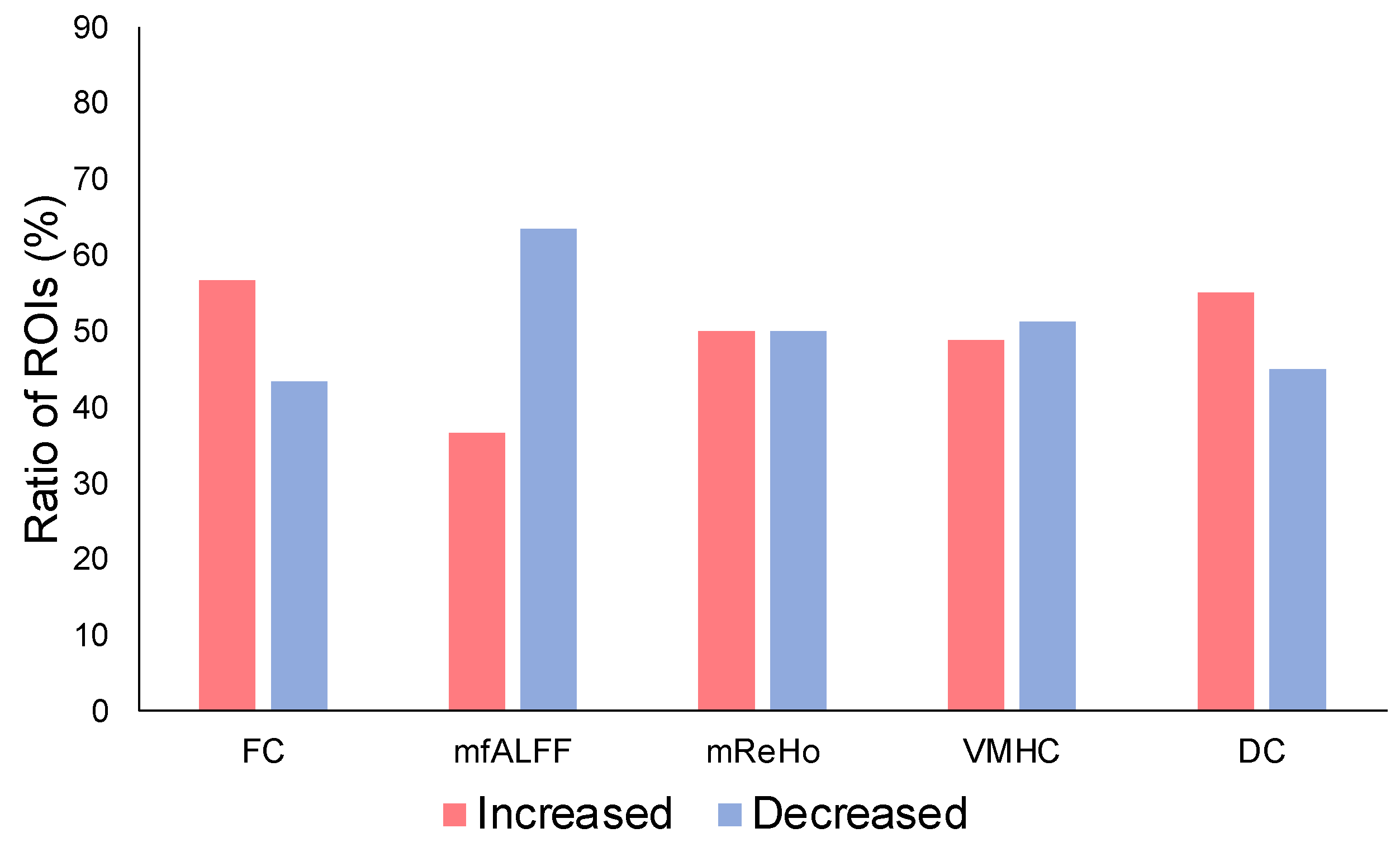

| Measure | Effect | AAL3 Brain Region |
|---|---|---|
| FC | Increased | Precentral_R |
| Frontal_Inf_Oper_R | ||
| Occipital_Inf_R | ||
| Postcentral_R | ||
| Parietal_Sup_L | ||
| Parietal_Sup_R | ||
| Caudate_L | ||
| Caudate_R | ||
| Putamen_L | ||
| Putamen_R | ||
| Pallidum_L | ||
| Temporal_Sup_L | ||
| Temporal_Sup_R | ||
| Thal_AV_L | ||
| Thal_AV_R | ||
| Thal_LP_R | ||
| Thal_VPL_L | ||
| Thal_PuL_R | ||
| SN_pr_L | ||
| Decreased | Parietal_Inf_R | |
| Cerebellum_3_R | ||
| Cerebellum_7b_R | ||
| Red_N_L | ||
| Red_N_R | ||
| LC_L | ||
| Raphe_D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, U.-S.; Sung, Y.-W.; Ogawa, S. Effects of Physiological Signal Removal on Resting-State Functional MRI Metrics. Brain Sci. 2023, 13, 8. https://doi.org/10.3390/brainsci13010008
Choi U-S, Sung Y-W, Ogawa S. Effects of Physiological Signal Removal on Resting-State Functional MRI Metrics. Brain Sciences. 2023; 13(1):8. https://doi.org/10.3390/brainsci13010008
Chicago/Turabian StyleChoi, Uk-Su, Yul-Wan Sung, and Seiji Ogawa. 2023. "Effects of Physiological Signal Removal on Resting-State Functional MRI Metrics" Brain Sciences 13, no. 1: 8. https://doi.org/10.3390/brainsci13010008





