Impact of Cranioplasty on Rehabilitation Course of Patients with Traumatic or Hemorrhagic Brain Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Variables, Data Sources, and Measurements
2.3. Statistical Analysis
3. Results
3.1. Clinical and Functional Characteristics
3.2. Post-DC Cranioplasty and Functional Outcome
3.3. Post-DC Cranioplasty and Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bullock, M.R.; Chesnut, R.; Ghajar, J.; Gordon, D.; Hartl, R.; Newell, D.W.; Servadei, F.; Walters, B.C.; Wilberger, J. Surgical Management of Traumatic Brain Injury Author Group. Surgical management of traumatic parenchymal lesions. Neurosurgery 2006, 58, S25–S46. [Google Scholar] [PubMed]
- Marinkovic, I.; Strbian, D.; Pedrono, E.; Vekovischeva, O.Y.; Shekhar, S.; Durukan, A.; Korpi, E.R.; Abo-Ramadan, U.; Tatlisumak, T. Decompressive craniectomy for intracerebral hemorrhage. Neurosurgery 2009, 65, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Hofmeijer, J.; Kappelle, L.J.; Algra, A.; Amelink, G.J.; van Gijn, J.; van der Worp, H.B.; HAMLET investigators. Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): A multicentre, open, randomised trial. Lancet Neurol. 2009, 8, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, P.J.; Kolias, A.G.; Tajsic, T.; Adeleye, A.; Aklilu, A.T.; Apriawan, T.; Bajamal, A.H.; Barthélemy, E.J.; Devi, B.I.; Bhat, D.; et al. Consensus statement from the International Consensus Meeting on the Role of Decompressive Craniectomy in the Management of Traumatic Brain Injury: Consensus statement. Acta Neurochir. 2019, 161, 1261–1274. [Google Scholar] [CrossRef]
- Cooper, D.J.; Rosenfeld, J.V.; Murray, L.; Arabi, Y.M.; Davies, A.R.; D’Urso, P.; Kossmann, T.; Ponsford, J.; Seppelt, I.; Reilly, P.; et al. Decompressive craniectomy in diffuse traumatic brain injury. N. Engl. J. Med. 2011, 364, 1493–1502. [Google Scholar] [CrossRef]
- Jaeger, M.; Soehle, M.; Meixensberger, J. Effects of decompressive craniectomy on brain tissue oxygen in patients with intracranial hypertension. J. Neurol. Neurosurg. Psychiatry 2003, 74, 513–515. [Google Scholar] [CrossRef]
- Hutchinson, P.J.; Kolias, A.G.; Timofeev, I.S.; Corteen, E.A.; Czosnyka, M.; Timothy, J.; Anderson, I.; Bulters, D.O.; Belli, A.; Eynon, C.A.; et al. Trial of Decompressive Craniectomy for Traumatic Intracranial Hypertension. N. Engl. J. Med. 2016, 375, 1119–1130. [Google Scholar] [CrossRef]
- Dujovny, M.; Fernandez, P.; Alperin, N.; Betz, W.; Misra, M.; Mafee, M. Post-cranioplasty cerebrospinal fluid hydrodynamic changes: Magnetic resonance imaging quantitative analysis. Neurol. Res. 1997, 19, 311–316. [Google Scholar] [CrossRef]
- Winkler, P.A.; Stummer, W.; Linke, R.; Krishnan, K.G.; Tatsch, K. Influence of cranioplasty on postural blood flow regulation, cerebrovascular reserve capacity, and cerebral glucose metabolism. J. Neurosurg. 2000, 93, 53–61. [Google Scholar] [CrossRef]
- Jasey, N.; Ward, I.; Lequerica, A.; Chiaravalloti, N.D. The therapeutic value of cranioplasty in individuals with brain injury. Brain Inj. 2018, 32, 318–324. [Google Scholar] [CrossRef]
- Feroze, A.H.; Walmsley, G.G.; Choudhri, O.; Lorenz, H.P.; Grant, G.A.; Edwards, M.S. Evolution of cranioplasty techniques in neurosurgery: Historical review, pediatric considerations, and current trends. J. Neurosurg. 2015, 123, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, C.; Kolias, A.G.; Roumy, L.G.; Fountas, K.; Adeleye, A.O. Cranioplasty Following Decompressive Craniectomy. Front. Neurol. 2020, 10, 1357. [Google Scholar] [CrossRef] [PubMed]
- Piazza, M.; Grady, M.S. Cranioplasty. Neurosurg. Clin. N. Am. 2017, 28, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Yadla, S.; Campbell, P.G.; Chitale, R.; Maltenfort, M.G.; Jabbour, P.; Sharan, A.D. Effect of early surgery, material, and method of flap preservation on cranioplasty infections: A systematic review. Neurosurgery 2011, 68, 1124–1129. [Google Scholar] [CrossRef]
- Beauchamp, K.M.; Kashuk, J.; Moore, E.E.; Bolles, G.; Rabb, C.; Seinfeld, J.; Szentirmai, O.; Sauaia, A. Cranioplasty after postinjury decompressive craniectomy: Is timing of the essence? J. Trauma 2010, 69, 270–274. [Google Scholar] [CrossRef]
- Liang, W.; Xiaofeng, Y.; Weiguo, L.; Gang, S.; Xuesheng, Z.; Fei, C.; Gu, L. Cranioplasty of large cranial defect at an early stage after decompressive craniectomy performed for severe head trauma. J. Craniofac. Surg. 2007, 18, 526–532. [Google Scholar] [CrossRef]
- Shahid, A.H.; Mohanty, M.; Singla, N.; Mittal, B.R.; Gupta, S.K. The effect of cranioplasty following decompressive craniectomy on cerebral blood perfusion, neurological, and cognitive outcome. J. Neurosurg. 2018, 128, 229–235. [Google Scholar] [CrossRef]
- Nasi, D.; Dobran, M. Can early cranioplasty reduce the incidence of hydrocephalus after decompressive craniectomy? A meta-analysis. Surg. Neurol. Int. 2020, 11, 94. [Google Scholar] [CrossRef]
- Corallo, F.; Marra, A.; Bramanti, P.; Calabrò, R.S. Effect of cranioplasty on functional and neuro-psychological recovery after severe acquired brain injury: Fact or fake? Considerations on a single case. Funct. Neurol. 2014, 29, 273–275. [Google Scholar] [CrossRef]
- Paredes, I.; Castaño, A.M.; Cepeda, S.; Alén, J.A.; Salvador, E.; Millán, J.M.; Lagares, A. The Effect of Cranioplasty on Cerebral Hemodynamics as Measured by Perfusion Computed Tomography and Doppler Ultrasonography. J. Neurotrauma 2016, 33, 1586–1597. [Google Scholar] [CrossRef]
- Bender, A.; Heulin, S.; Röhrer, S.; Mehrkens, J.H.; Heidecke, V.; Straube, A.; Pfefferkorn, T. Early cranioplasty may improve outcome in neurological patients with decompressive craniectomy. Brain Inj. 2013, 27, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Ozoner, B. Cranioplasty Following Severe Traumatic Brain Injury: Role in Neurorecovery. Curr. Neurol. Neurosci. Rep. 2021, 21, 62. [Google Scholar] [CrossRef]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Linacre, J.M.; Heinemann, J.W.; Wright, B.D.; Granger, C.V.; Hamilton, B.B. The structure and stability of the functional independence measure. Arch. Phys. Med. Rehabil. 1994, 75, 127–132. [Google Scholar] [CrossRef]
- Mackintosh, S. Functional independence measure. Aust. J. Physiother. 2009, 55, 65. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, A.W.; Linacre, J.M.; Wright, B.D.; Hamilton, B.B.; Granger, C. Relationships between impairment and physical disability as measured by the functional independence measure. Arch. Phys. Med. Rehabil. 1993, 74, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Dinet, V.; Petry, K.G.; Badaut, J. Brain-Immune Interactions and Neuroinflammation After Traumatic Brain Injury. Front. Neurosci. 2019, 13, 1178. [Google Scholar] [CrossRef]
- Mele, C.; Pingue, V.; Caputo, M.; Zavattaro, M.; Pagano, L.; Prodam, F.; Nardone, A.; Aimaretti, G.; Marzullo, P. Neuroinflammation and Hypothalamo-Pituitary Dysfunction: Focus of Traumatic Brain Injury. Int. J. Mol. Sci. 2021, 22, 2686. [Google Scholar] [CrossRef]
- Dujovny, M.; Aviles, A.; Agner, C.; Fernandez, P.; Charbel, F.T. Cranioplasty: Cosmetic or therapeutic? Surg. Neurol. 1997, 47, 238–241. [Google Scholar] [CrossRef]
- Ashayeri, K.; Jackson, E.M.; Huang, J.; Brem, H.; Gordon, C.R. Syndrome of the Trephined: A Systematic Review. Neurosurgery 2016, 79, 525–534. [Google Scholar] [CrossRef]
- Sedney, C.L.; Dillen, W.; Julien, T. Clinical spectrum and radiographic features of the syndrome of the trephined. J. Neurosci. Rural. Pract. 2015, 6, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishnan, A.; Alperin, N.; Surapaneni, S.; Lichtor, T. Evaluating the effect of decompression surgery on cerebrospinal fluid flow and intracranial compliance in patients with chiari malformation with magnetic resonance imaging flow studies. Neurosurgery 2004, 55, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, J.; Gur, R.; Nisim, U.; Pollak, L. Symptomatic patients after craniectomy. Surg. Neurol. 1997, 47, 231–237. [Google Scholar] [CrossRef]
- Schirmer, C.M.; Ackil, A.A., Jr.; Malek, A.M. Decompressive Craniectomy. Neurocrit. Care 2008, 8, 456–470. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.R.; Wang, C.C.; Chio, C.C.; Cheng, T.J. Neurological improvement after cranioplasty-analysis by transcranial doppler ultrasonography. J. Clin. Neurosci. 2004, 11, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Jelcic, N.; De Pellegrin, S.; Cecchin, D.; Della Puppa, A.; Cagnin, A. Cognitive improvement after cranioplasty: A possible volume transmission-related effect. Acta Neurochir. 2013, 155, 1597–1599. [Google Scholar] [CrossRef]
- Coelho, F.; Oliveira, A.M.; Paiva, W.S.; Freire, F.R.; Calado, V.T.; Amorim, R.L.; Neville, I.S.; de Andrade, A.F.; Bor-Seng-Shu, E.; Anghinah, R.; et al. Comprehensive cognitive and cerebral hemodynamic evaluation after cranioplasty. Neuropsychiatr. Dis. Treat. 2014, 10, 695–701. [Google Scholar]
- Rynkowski, C.B.; Robba, C.; Loreto, M.; Theisen, A.C.W.; Kolias, A.G.; Finger, G.; Czosnyka, M.; Bianchin, M.M. Effects of Cranioplasty After Decompressive Craniectomy on Neurological Function and Cerebral Hemodynamics in Traumatic Versus Nontraumatic Brain Injury. Acta Neurochir. Suppl. 2021, 131, 79–82. [Google Scholar]
- Honeybul, S.; Janzen, C.; Kruger, K.; Ho, K.M. The impact of cranioplasty on neurological function. Br. J. Neurosurg. 2013, 27, 636–641. [Google Scholar] [CrossRef]
- Mee, H.; Castano Leon, A.; Timofeev, I.; Adeleye, A.; Devi, I.; Marklund, N.; Muehlschlegel, S.; Bond, K.; Clement, C.; Grieve, K.; et al. Study Protocol: Core Outcome Set for cranioplasty following stroke or TBI ‘COAST Study’. JMIR Res. Protoc. 2022; Epub Ahead of Print. [Google Scholar]
- Kim, B.W.; Kim, T.U.; Hyun, J.K. Effects of Early Cranioplasty on the Restoration of Cognitive and Functional Impairments. Ann. Rehabil. Med. 2017, 41, 354–361. [Google Scholar] [CrossRef]
- Shao, Z.; Tu, S.; Shao, A. Pathophysiological Mechanisms and Potential Therapeutic Targets in Intracerebral Hemorrhage. Front. Pharmacol. 2019, 10, 1079. [Google Scholar] [CrossRef] [PubMed]
- Mee, H.; Clement, C.; Anwar, F.; Whiting, G.; Timofeev, I.; Helmy, A.; Hutchinson, P.J.; Kolias, A.G. Exploring the experiences and challenges for patients undergoing cranioplasty: A mixed-methods study protocol. BMJ Open 2022, 12, e048072. [Google Scholar] [CrossRef] [PubMed]
- Management of Concussion/mTBI Working Group. VA/DoD Clinical Practice Guideline for Management of Concussion/Mild Traumatic Brain Injury. J. Rehabil. Res. Dev. 2009, 46, CP1–CP68. [Google Scholar] [CrossRef]
- Malcolm, J.G.; Rindler, R.S.; Chu, J.K.; Chokshi, F.; Grossberg, J.A.; Pradilla, G.; Ahmad, F.U. Early Cranioplasty is Associated with Greater Neurological Improvement: A Systematic Review and Meta-Analysis. Neurosurgery 2018, 82, 278–288. [Google Scholar] [CrossRef] [PubMed]
- von Steinbüchel, N.; Meeuwsen, M.; Zeldovich, M.; Vester, J.C.; Maas, A.; Koskinen, S.; Covic, A. Differences in Health-Related Quality of Life after Traumatic Brain Injury between Varying Patient Groups: Sensitivity of a Disease-Specific (QOLIBRI) and a Generic (SF-36) Instrument. J. Neurotrauma 2020, 37, 1242–1254. [Google Scholar] [CrossRef] [PubMed]
- von Steinbuechel, N.; Covic, A.; Polinder, S.; Kohlmann, T.; Cepulyte, U.; Poinstingl, H.; Backhaus, J.; Bakx, W.; Bullinger, M.; Christensen, A.L.; et al. Assessment of Health-Related Quality of Life after TBI: Comparison of a Disease-Specific (QOLIBRI) with a Generic (SF-36) Instrument. Behav. Neurol. 2016, 2016, 7928014. [Google Scholar] [CrossRef][Green Version]
| Variables | Whole Population n = 253 n (%) | Cranioplasty | p-Value | ||
|---|---|---|---|---|---|
| No n (%) 145 (57.3) | Yes n (%) 108 (42.7) | ||||
| Sex | M | 153 (60.5) | 98 (67.6) | 55 (50.9) | 0.007 |
| F | 100 (39.5) | 47 (32.4) | 53 (49.1) | ||
| Age | ≤65 | 131 (51.8) | 70 (48.3) | 47 (43.5) | 0.45 |
| >65 | 122 (48.2) | 75 (51.7) | 61 (56.5) | ||
| Type of lesion | HS | 145 (57.3) | 70 (48.3) | 75 (69.4) | 0.0008 |
| TBI | 108 (42.7) | 75 (51.7) | 33 (30.6) | ||
| Classification of lesion | Cerebral edema | 69 (27.3) | 47 (32.4) | 22 (20.4) | 0.03 |
| ICH | 83 (32.8) | 55 (37.9) | 28 (25.9) | 0.04 | |
| SAH | 86 (34.0) | 38 (26.2) | 48 (44.4) | 0.002 | |
| ICH + SAH | 15 (5.9) | 5 (3.5) | 10 (9.3) | 0.05 | |
| Site of lesion | Frontal | 30 (11.9) | 17 (11.7) | 13 (12.0) | 0.94 |
| Temporal | 20 (7.9) | 8 (5.5) | 12 (11.1) | 0.10 | |
| Parietal | 4 (1.6) | 3 (2.1) | 1 (0.9) | 0.47 | |
| Occipital | 4 (1.6) | 3 (2.1) | 1 (0.9) | 0.47 | |
| Deep brain structures | 51 (20.1) | 37 (25.5) | 14 (13.0) | 0.01 | |
| Multiple sites | 144 (56.9) | 77 (53.1) | 67 (62.1) | 0.16 | |
| GCS T0 | Mild | 51 (20.2) | 32 (22.1) | 19 (17.6) | 0.38 |
| Moderate | 103 (40.7) | 55 (37.9) | 48 (44.4) | 0.30 | |
| Severe | 99 (39.1) | 58 (40.0) | 41 (38.9) | 0.74 | |
| GCS T1 Available for 212 patients | Mild | 115 (54.2) | 61 (53.0) | 54 (55.7) | 0.70 |
| Moderate | 50 (23.6) | 29 (25.2) | 21 (21.6) | 0.54 | |
| Severe | 47 (22.2) | 25 (21.8) | 22 (22.7) | 0.87 | |
| FIM T0 [median (IQR)] | 18 (18–29) | 18 (18–25) | 18 (18–32) | 0.67 | |
| FIM T1 [median (IQR)] | 37 (18–84) | 34 (18–83) | 44 (18–87) | 0.20 | |
| ΔFIM [median (IQR)] | 13 (0–36.3) | 8 (0–34) | 18 (0–39) | 0.09 | |
| Death | 41 (16.2) | 30 (20.7) | 11 (10.2) | 0.02 | |
| Variables | HS 145 Patients | TBI 108 Patients | |||||
|---|---|---|---|---|---|---|---|
| No Cranioplasty 70 Patients | Cranioplasty 75 Patients | p-Value | No Cranioplasty 75 Patients | Cranioplasty 33 Patients | p-Value | ||
| Sex | M | 42 (60.0) | 33 (44.0) | 0.06 | 56 (74.7) | 22 (66.7) | 0.39 |
| F | 28 (40.0) | 42 (56.0) | 19 (25.3) | 11 (33.3) | |||
| Age | ≤65 | 33 (47.1) | 38 (50.7) | 0.67 | 37 (49.3) | 23 (69.7) | 0.04 |
| >65 | 37 (52.9) | 37 (49.3) | 38 (50.7) | 10 (30.3) | |||
| Classification of lesion | Cerebral edema | 0 (0.0) | 0 (0.0) | - | 48 (64.0) | 21 (63.6) | 0.11 |
| ICH | 55 (78.6) | 28 (37.3) | <0.0001 | 0 (0.0) | 0 (0.0) | - | |
| SAH | 11 (15.7) | 38 (50.7) | <0.0001 | 26 (34.7) | 11 (33.3) | 0.89 | |
| ICH + SAH | 4 (5.7) | 9 (12.0) | 0.18 | 1 (1.3) | 1 (3.0) | 0.55 | |
| Site of lesion | Frontal | 9 (12.9) | 12 (16.0) | 0.59 | 8 (10.7) | 1 (3.0) | 0.18 |
| Temporal | 4 (5.8) | 9 (12.0) | 0.18 | 4 (5.4) | 3 (9.1) | 0.46 | |
| Parietal | 2 (2.8) | 1 (1.3) | 0.52 | 1 (1.3) | 0 (0.0) | 0.50 | |
| Occipital | 2 (2.8) | 0 (0.0) | 0.14 | 1 (1.3) | 1 (3.0) | 0.55 | |
| Deep brain structures | 25 (35.7) | 7 (9.4) | 0.0001 | 12 (16.0) | 7 (21.2) | 0.51 | |
| Multiple sites | 28 (40.0) | 46 (61.3) | 0.01 | 49 (65.3) | 21 (63.7) | 0.86 | |
| GCS T0 | Mild | 18 (25.7) | 11 (14.7) | 0.10 | 14 (18.7) | 8 (24.3) | 0.51 |
| Moderate | 27 (38.6) | 34 (45.3) | 0.41 | 28 (37.3) | 14 (42.4) | 0.62 | |
| Severe | 25 (35.7) | 30 (40.0) | 0.59 | 33 (44.0) | 11 (33.3) | 0.30 | |
| GCS T1 | Mild | 34 (56.6) | 36 (52.2) | 0.61 | 27 (49.1) | 18 (64.3) | 0.19 |
| Moderate | 13 (21.7) | 17 (24.6) | 0.69 | 16 (29.1) | 4 (14.3) | 0.13 | |
| Severe | 13 (21.7) | 16 (23.2) | 0.84 | 12 (21.8) | 6 (21.4) | 0.97 | |
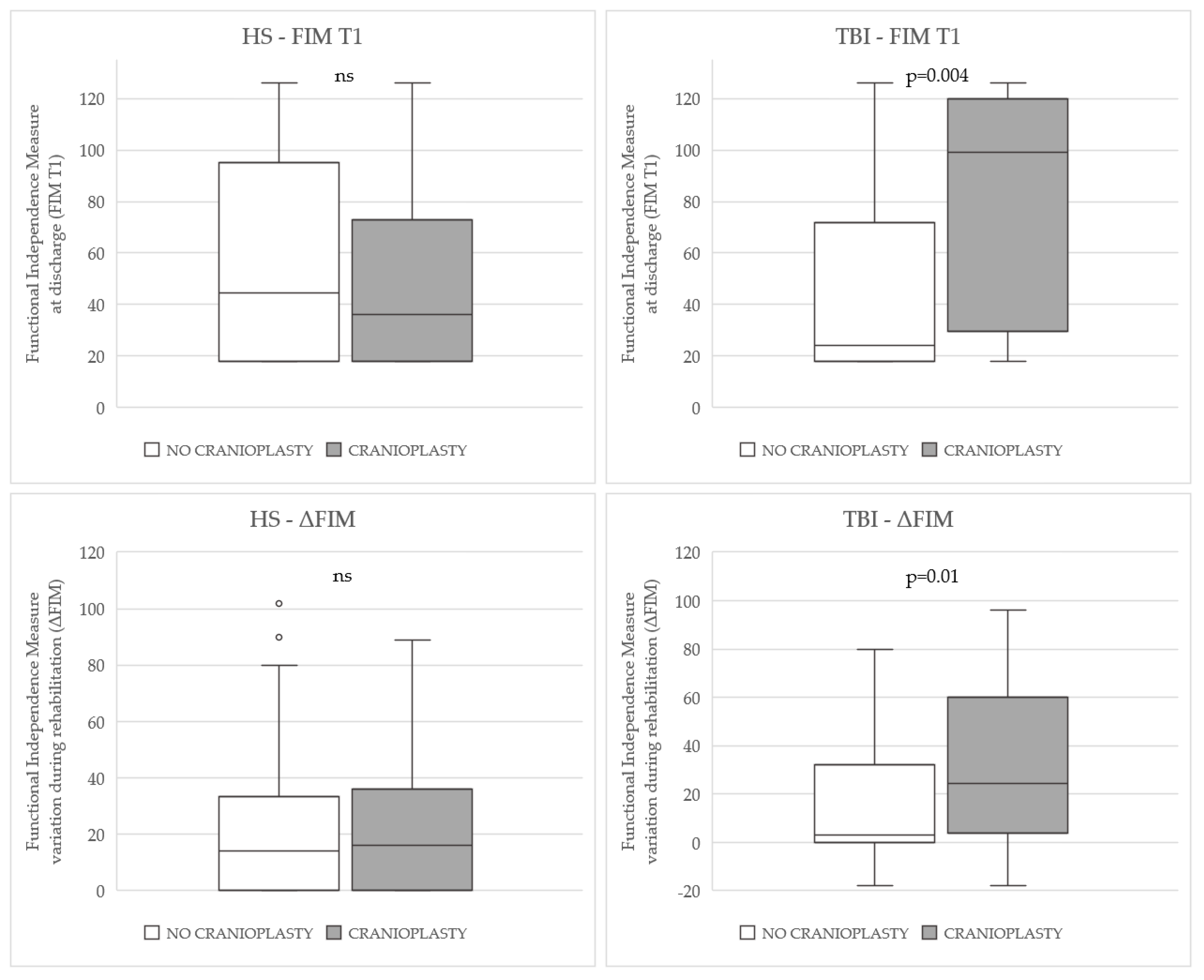
| FIM T0 [median (IQR)] | 18 (18–30) | 18 (18–25) | 0.20 | 18 (18–20) | 21 (18–49) | 0.01 | |
| FIM T1 [median (IQR)] | 45 (18–95) | 36 (18–74) | 0.55 | 24 (18–72) | 99 (23–120) | 0.004 | |
| Δ FIM [median (IQR)] | 14 (0–34) | 16 (0–37) | 0.94 | 3 (0–35) | 25 (1–60) | 0.01 | |
| Death | 10 (14.3) | 6 (8.0) | 0.23 | 20 (26.7) | 5 (15.2) | 0.19 | |
| Dependent Variable FIMT1 Total Score | Dependent Variable ΔFIM Total Score | |||||
|---|---|---|---|---|---|---|
| HS | Regression Models | Beta | p-Value | Beta | p-Value | |
| Model 1 R2 FIMT1 = 0.52 R2 ΔFIM = 0.21 | Sex | −0.182 | 0.004 | −0.197 | 0.02 | |
| GCS T0 severity | −0.679 | <0.0001 | −0.395 | <0.0001 | ||
| Cranioplasty | −0.019 | 0.76 | 0.022 | 0.78 | ||
| Model 2 R2 FIMT1 = 0.51 R2 ΔFIM = 0.20 | Age | −0.135 | 0.03 | −0.171 | 0.04 | |
| GCS T0 severity | −0.695 | <0.0001 | −0.412 | <0.0001 | ||
| Cranioplasty | −0.040 | 0.52 | 0.011 | 0.90 | ||
| Model 3 R2 FIMT1 = 0.49 R2 ΔFIM = 0.17 | Site of lesion | −0.053 | 0.42 | 0.015 | 0.86 | |
| GCS T0 severity | −0.688 | <0.0001 | −0.415 | <0.0001 | ||
| Cranioplasty | −0.035 | 0.59 | −0.004 | 0.97 | ||
| Model 4 R2 FIMT1 = 0.50 R2 ΔFIM = 0.18 | Classification of lesion | 0.083 | 0.22 | 0.088 | 0.31 | |
| GCS T0 severity | −0.704 | <0.0001 | −0.421 | <0.0001 | ||
| Cranioplasty | −0.066 | 0.32 | −0.028 | 0.74 | ||
| Model 5 R2 FIMT1 = 0.63 R2 ΔFIM = 0.22 | GCS T0 severity | −0.407 | <0.0001 | −0.590 | <0.0001 | |
| FIM T0 total score | 0.473 | <0.0001 | −0.291 | 0.004 | ||
| Cranioplasty | −0.013 | 0.81 | −0.019 | 0.81 | ||
| TBI | Model 1 R2 FIMT1 = 0.63 R2 ΔFIM = 0.31 | Sex | 0.067 | 0.33 | 0.099 | 0.29 |
| GCS T0 severity | −0.711 | <0.0001 | −0.453 | <0.0001 | ||
| Cranioplasty | 0.278 | <0.0001 | 0.277 | 0.004 | ||
| Model 2 R2 FIMT1 = 0.64 R2 ΔFIM = 0.30 | Age | −0.130 | 0.07 | −0.059 | 0.54 | |
| GCS T0 severity | −0.706 | <0.0001 | −0.453 | <0.0001 | ||
| Cranioplasty | 0.248 | 0.001 | 0.263 | 0.008 | ||
| Model 3 R2 FIMT1 = 0.62 R2 ΔFIM = 0.31 | Site of lesion | 0.029 | 0.68 | 0.092 | 0.34 | |
| GCS T0 severity | −0.711 | <0.0001 | −0.455 | <0.0001 | ||
| Cranioplasty | 0.283 | <0.0001 | 0.291 | 0.003 | ||
| Model 4 R2 FIMT1 = 0.62 R2 ΔFIM = 0.32 | Classification of lesion | −0.025 | 0.73 | −0.111 | 0.24 | |
| GCS T0 severity | −0.709 | <0.0001 | −0.450 | <0.0001 | ||
| Cranioplasty | 0.279 | <0.0001 | 0.279 | 0.004 | ||
| Model 5 R2 FIMT1 = 0.73 R2 ΔFIM = 0.35 | FIM T0 total score | 0.444 | <0.0001 | −0.641 | <0.0001 | |
| GCS T0 severity | −0.422 | <0.0001 | −0.282 | 0.03 | ||
| Cranioplasty | 0.217 | 0.001 | 0.315 | 0.001 | ||
| Covariates | Death during Rehabilitation (Dependent Variable) (No = 0, Yes = 1) | |||
|---|---|---|---|---|
| OR | CI 95% | p-Value | ||
| HS | Sex | 0.347 | 0.101–1.196 | 0.35 |
| Age | 2.459 | 0.701–8.628 | 0.16 | |
| Classification of lesion | 1.072 | 0.391–2.936 | 0.89 | |
| Site of lesion | 0.188 | 0.021–1.724 | 0.14 | |
| Cranioplasty | 0.428 | 0.122–1.500 | 0.18 | |
| GCS T0 severity | 1.424 | 0.459–4.420 | 0.54 | |
| FIM T0 | 0.754 | 0.478–1.188 | 0.22 | |
| TBI | Sex | 0.474 | 0.132–1.708 | 0.25 |
| Age | 4.926 | 1.568–15.470 | 0.006 | |
| Classification of lesion | 1.917 | 0.520–7.072 | 0.33 | |
| Site of lesion | 0.766 | 0.425–1.381 | 0.38 | |
| Cranioplasty | 1.005 | 0.275–3.671 | 0.99 | |
| GCS T0 severity | 1.279 | 0.467–3.502 | 0.63 | |
| FIM T0 | 0.799 | 0.577–1.106 | 0.17 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mele, C.; Bassetto, A.; Boetto, V.; Nardone, A.; Pingue, V. Impact of Cranioplasty on Rehabilitation Course of Patients with Traumatic or Hemorrhagic Brain Injury. Brain Sci. 2023, 13, 80. https://doi.org/10.3390/brainsci13010080
Mele C, Bassetto A, Boetto V, Nardone A, Pingue V. Impact of Cranioplasty on Rehabilitation Course of Patients with Traumatic or Hemorrhagic Brain Injury. Brain Sciences. 2023; 13(1):80. https://doi.org/10.3390/brainsci13010080
Chicago/Turabian StyleMele, Chiara, Anna Bassetto, Valentina Boetto, Antonio Nardone, and Valeria Pingue. 2023. "Impact of Cranioplasty on Rehabilitation Course of Patients with Traumatic or Hemorrhagic Brain Injury" Brain Sciences 13, no. 1: 80. https://doi.org/10.3390/brainsci13010080
APA StyleMele, C., Bassetto, A., Boetto, V., Nardone, A., & Pingue, V. (2023). Impact of Cranioplasty on Rehabilitation Course of Patients with Traumatic or Hemorrhagic Brain Injury. Brain Sciences, 13(1), 80. https://doi.org/10.3390/brainsci13010080







