Behavioral Effects of Repetitive Transcranial Magnetic Stimulation in Disorders of Consciousness: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
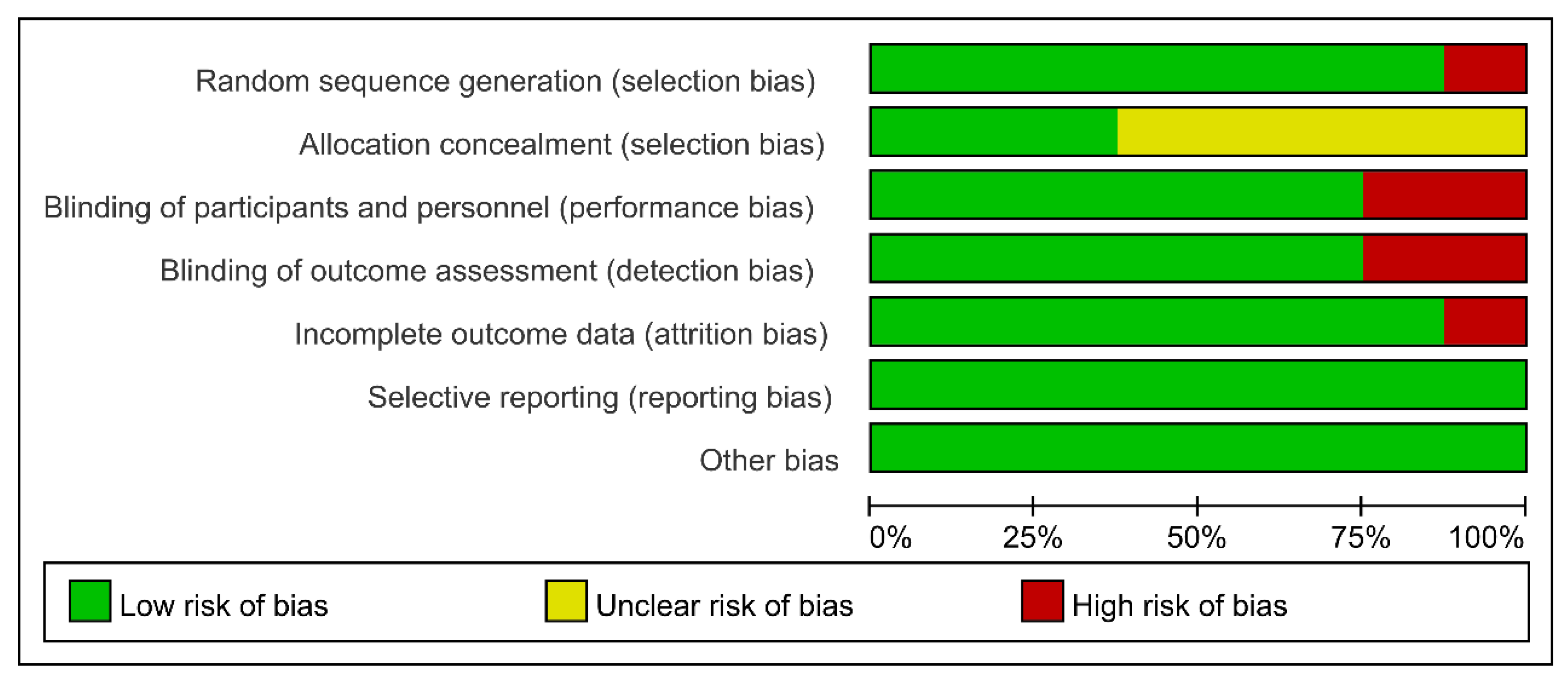
2.3. Quality Assessment
2.4. Data Extraction
2.5. Data Analysis
3. Results
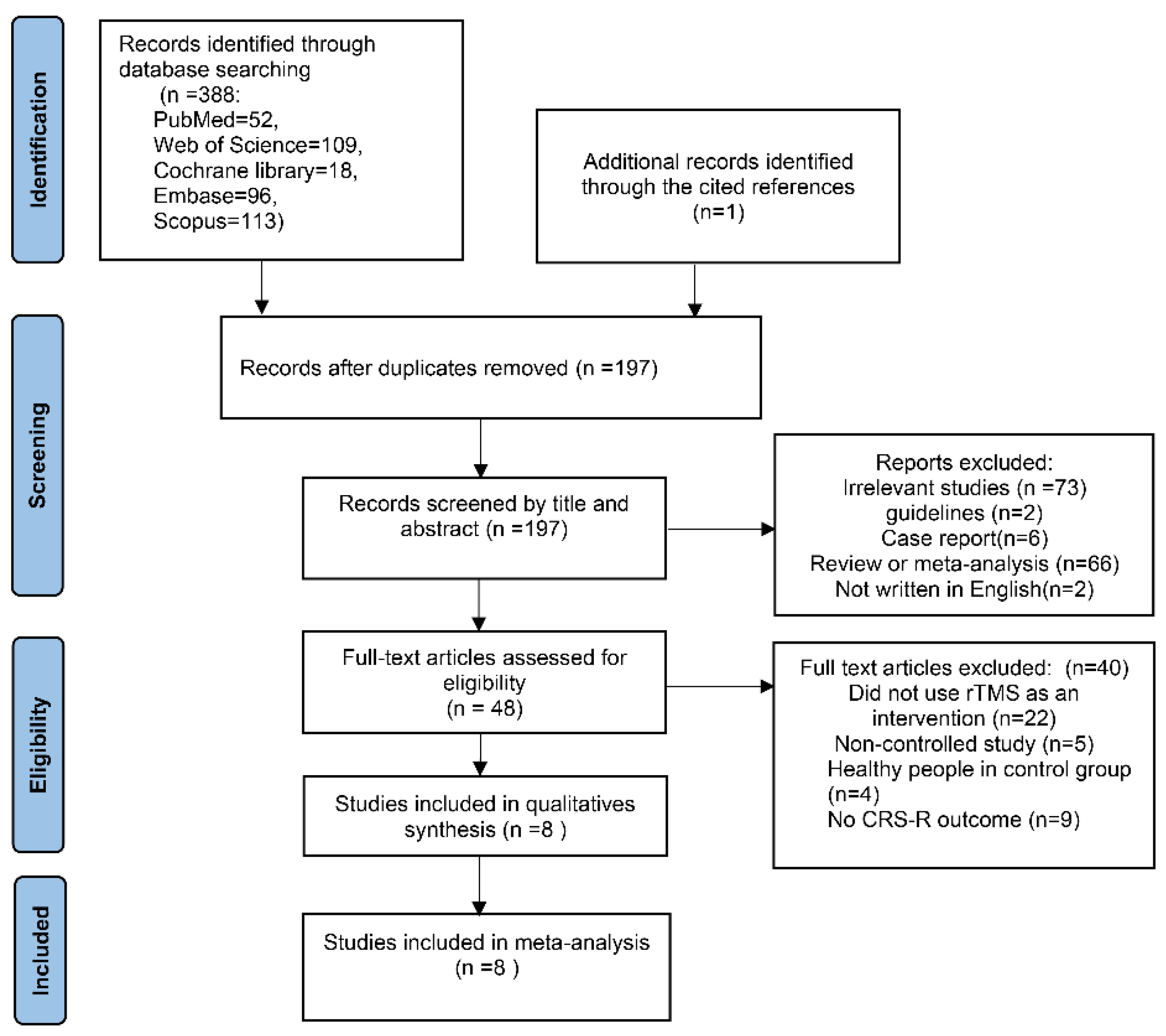
3.1. Search Results
3.2. Characteristics of Included Studies
3.3. Heterogeneity Analysis
3.4. Meta-Analysis in All Protocols
3.5. Subgroup Analysis: Target Region
3.6. Subgroup Analysis: Stimulation Frequency
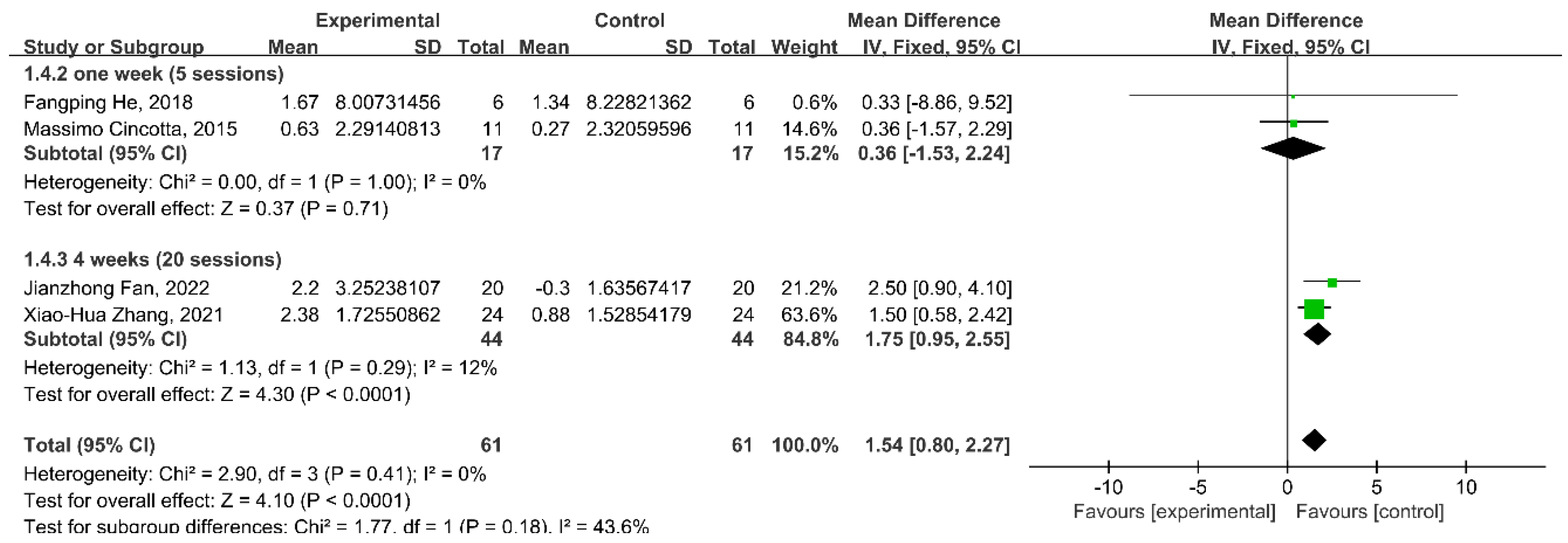
3.7. Subgroup Analysis: Stimulation Duration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edlow, B.L.; Claassen, J.; Schiff, N.D.; Greer, D.M. Recovery from disorders of consciousness: Mechanisms, prognosis and emerging therapies. Nat. Rev. Neurol. 2021, 17, 135–156. [Google Scholar] [CrossRef] [PubMed]
- Laureys, S.; Celesia, G.G.; Cohadon, F.; Lavrijsen, J.; León-Carrión, J.; Sannita, W.G.; Sazbon, L.; Schmutzhard, E.; von Wild, K.R.; Zeman, A.; et al. Unresponsive wakefulness syndrome: A new name for the vegetative state or apallic syndrome. BMC Med. 2010, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Kondziella, D.; Bender, A.; Diserens, K.; van Erp, W.; Estraneo, A.; Formisano, R.; Laureys, S.; Naccache, L.; Ozturk, S.; Rohaut, B.; et al. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur. J. Neurol. 2020, 27, 741–756. [Google Scholar] [CrossRef] [PubMed]
- Giacino, J.T.; Ashwal, S.; Childs, N.; Cranford, R.; Jennett, B.; Katz, D.I.; Kelly, J.P.; Rosenberg, J.H.; Whyte, J.; Zafonte, R.D.; et al. The minimally conscious state: Definition and diagnostic criteria. Neurology 2002, 58, 349–353. [Google Scholar] [CrossRef]
- Seel, R.T.; Sherer, M.; Whyte, J.; Katz, D.I.; Giacino, J.T.; Rosenbaum, A.M.; Hammond, F.M.; Kalmar, K.; Pape, T.L.; Zafonte, R.; et al. Assessment scales for disorders of consciousness: Evidence-based recommendations for clinical practice and research. Arch. Phys. Med. Rehabil. 2010, 91, 1795–1813. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Yan, F.; Zhang, H.; Zhang, E.; Wang, X.; Wei, M.; Pei, Y.; Yang, Z.; Li, Y.; et al. Investigating the mechanism and prognosis of patients with disorders of consciousness on the basis of brain networks between the thalamus and whole-brain. Front. Neurol. 2022, 13, 990686. [Google Scholar] [CrossRef]
- Fischer, D.B.; Boes, A.D.; Demertzi, A.; Evrard, H.C.; Laureys, S.; Edlow, B.L.; Liu, H.; Saper, C.B.; Pascual-Leone, A.; Fox, M.D.; et al. A human brain network derived from coma-causing brainstem lesions. Neurology 2016, 87, 2427–2434. [Google Scholar] [CrossRef]
- Timofeev, I.; Grenier, F.; Bazhenov, M.; Sejnowski, T.J.; Steriade, M. Origin of slow cortical oscillations in deafferented cortical slabs. Cereb. Cortex 2000, 10, 1185–1199. [Google Scholar] [CrossRef]
- Hammond, F.M.; Giacino, J.T.; Nakase Richardson, R.; Sherer, M.; Zafonte, R.D.; Whyte, J.; Arciniegas, D.B.; Tang, X. Disorders of Consciousness due to Traumatic Brain Injury: Functional Status Ten Years Post-Injury. J. Neurotrauma 2019, 36, 1136–1146. [Google Scholar] [CrossRef]
- Kowalski, R.G.; Hammond, F.M.; Weintraub, A.H.; Nakase-Richardson, R.; Zafonte, R.D.; Whyte, J.; Giacino, J.T. Recovery of Consciousness and Functional Outcome in Moderate and Severe Traumatic Brain Injury. JAMA Neurol. 2021, 78, 548–557. [Google Scholar] [CrossRef]
- Katz, D.I.; Polyak, M.; Coughlan, D.; Nichols, M.; Roche, A. Natural history of recovery from brain injury after prolonged disorders of consciousness: Outcome of patients admitted to inpatient rehabilitation with 1–4 year follow-up. Prog. Brain Res. 2009, 177, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Whyte, J.; Nakase-Richardson, R.; Hammond, F.M.; McNamee, S.; Giacino, J.T.; Kalmar, K.; Greenwald, B.D.; Yablon, S.A.; Horn, L.J. Functional outcomes in traumatic disorders of consciousness: 5-year outcomes from the National Institute on Disability and Rehabilitation Research Traumatic Brain Injury Model Systems. Arch. Phys. Med. Rehabil. 2013, 94, 1855–1860. [Google Scholar] [CrossRef] [PubMed]
- Klomjai, W.; Katz, R.; Lackmy-Vallée, A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann. Phys. Rehabil. Med. 2015, 58, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Graff-Radford, J. Executive Dysfunction and the Prefrontal Cortex. Continuum 2021, 27, 1586–1601. [Google Scholar] [CrossRef]
- Fitzsimmons, S.; van der Werf, Y.D.; van Campen, A.D.; Arns, M.; Sack, A.T.; Hoogendoorn, A.W.; van den Heuvel, O.A. Repetitive transcranial magnetic stimulation for obsessive-compulsive disorder: A systematic review and pairwise/network meta-analysis. J. Affect. Disord. 2022, 302, 302–312. [Google Scholar] [CrossRef]
- Bodien, Y.G.; Chatelle, C.; Edlow, B.L. Functional Networks in Disorders of Consciousness. Semin. Neurol. 2017, 37, 485–502. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, E.; Yuan, C.; Zhang, H.; Wang, X.; Yan, F.; Pei, Y.; Li, Y.; Wei, M.; Yang, Z.; et al. Abnormal default mode network could be a potential prognostic marker in patients with disorders of consciousness. Clin. Neurol. Neurosurg. 2022, 218, 107294. [Google Scholar] [CrossRef]
- McDonnell, M.N.; Stinear, C.M. TMS measures of motor cortex function after stroke: A meta-analysis. Brain Stimul. 2017, 10, 721–734. [Google Scholar] [CrossRef]
- Grefkes, C.; Ward, N.S. Cortical reorganization after stroke: How much and how functional? Neurosci. A Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2014, 20, 56–70. [Google Scholar] [CrossRef]
- Corti, M.; Patten, C.; Triggs, W. Repetitive transcranial magnetic stimulation of motor cortex after stroke: A focused review. Am. J. Phys. Med. Rehabil. 2012, 91, 254–270. [Google Scholar] [CrossRef]
- Du, J.; Yang, F.; Hu, J.; Hu, J.; Xu, Q.; Cong, N.; Zhang, Q.; Liu, L.; Mantini, D.; Zhang, Z.; et al. Effects of high- and low-frequency repetitive transcranial magnetic stimulation on motor recovery in early stroke patients: Evidence from a randomized controlled trial with clinical, neurophysiological and functional imaging assessments. NeuroImage. Clin. 2019, 21, 101620. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Zhang, J.; Fong, K.N.K. Effects of transcranial magnetic stimulation in modulating cortical excitability in patients with stroke: A systematic review and meta-analysis. J. Neuroeng. Rehabil. 2022, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Kashyap, R.; Abualait, T.; Annabel Chen, S.H.; Yoo, W.K.; Bashir, S. The Role of Primary Motor Cortex: More Than Movement Execution. J. Mot. Behav. 2021, 53, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Cincotta, M.; Giovannelli, F.; Chiaramonti, R.; Bianco, G.; Godone, M.; Battista, D.; Cardinali, C.; Borgheresi, A.; Sighinolfi, A.; D’Avanzo, A.M.; et al. No effects of 20 Hz-rTMS of the primary motor cortex in vegetative state: A randomised, sham-controlled study. Cortex A J. Devoted Study Nerv. Syst. Behav. 2015, 71, 368–376. [Google Scholar] [CrossRef]
- He, F.; Wu, M.; Meng, F.; Hu, Y.; Gao, J.; Chen, Z.; Bao, W.; Liu, K.; Luo, B.; Pan, G. Effects of 20 Hz Repetitive Transcranial Magnetic Stimulation on Disorders of Consciousness: A Resting-State Electroencephalography Study. Neural Plast. 2018, 2018, 5036184. [Google Scholar] [CrossRef]
- Zhang, X.H.; Han, P.; Zeng, Y.Y.; Wang, Y.L.; Lv, H.L. The Clinical Effect of Repetitive Transcranial Magnetic Stimulation on the Disturbance of Consciousness in Patients in a Vegetative State. Front. Neurosci. 2021, 15, 647517. [Google Scholar] [CrossRef]
- Xu, C.; Wu, W.; Zheng, X.; Liang, Q.; Huang, X.; Zhong, H.; Xiao, Q.; Lan, Y.; Bai, Y.; Xie, Q. Repetitive transcranial magnetic stimulation over the posterior parietal cortex improves functional recovery in nonresponsive patients: A crossover, randomized, double-blind, sham-controlled study. Front. Neurol. 2023, 14, 1059789. [Google Scholar] [CrossRef]
- Fan, J.; Zhong, Y.; Wang, H.; Aierken, N.; He, R. Repetitive transcranial magnetic stimulation improves consciousness in some patients with disorders of consciousness. Clin. Rehabil. 2022, 36, 916–925. [Google Scholar] [CrossRef]
- Shen, L.; Huang, Y.; Liao, Y.; Yin, X.; Huang, Y.; Ou, J.; Ouyang, H.; Chen, Z.; Long, J. Effect of high-frequency repetitive transcranial magnetic stimulation over M1 for consciousness recovery after traumatic brain injury. Brain Behav. 2023, 13, e2971. [Google Scholar] [CrossRef]
- Ge, X.; Zhang, Y.; Xin, T.; Luan, X. Effects of 10 Hz repetitive transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in the vegetative state. Exp. Ther. Med. 2021, 21, 206. [Google Scholar] [CrossRef]
- Chen, J.M.; Chen, Q.F.; Wang, Z.Y.; Chen, Y.J.; Zhang, N.N.; Xu, J.W.; Ni, J. Influence of High-Frequency Repetitive Transcranial Magnetic Stimulation on Neurobehavioral and Electrophysiology in Patients with Disorders of Consciousness. Neural Plast. 2022, 2022, 7195699. [Google Scholar] [CrossRef] [PubMed]
- Baek, A.; Park, E.J.; Kim, S.Y.; Nam, B.G.; Kim, J.H.; Jun, S.W.; Kim, S.H.; Cho, S.R. High-Frequency Repetitive Magnetic Stimulation Enhances the Expression of Brain-Derived Neurotrophic Factor Through Activation of Ca(2+)-Calmodulin-Dependent Protein Kinase II-cAMP-Response Element-Binding Protein Pathway. Front. Neurol. 2018, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, M.; Yee, K.M.; Steward, O. Non-invasive High Frequency Repetitive Transcranial Magnetic Stimulation (hfrTMS) Robustly Activates Molecular Pathways Implicated in Neuronal Growth and Synaptic Plasticity in Select Populations of Neurons. Front. Neurosci. 2020, 14, 558. [Google Scholar] [CrossRef]
- Brown, J.C.; Higgins, E.S.; George, M.S. Synaptic Plasticity 101: The Story of the AMPA Receptor for the Brain Stimulation Practitioner. Neuromodulation J. Int. Neuromodulation Soc. 2022, 25, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, J.; Zhou, Y.; Bai, Z.; Yin, Y. Noninvasive brain stimulation for patients with a disorder of consciousness: A systematic review and meta-analysis. Rev. Neurosci. 2020. epub ahead of print. [Google Scholar] [CrossRef]
- Eapen, B.C.; Georgekutty, J.; Subbarao, B.; Bavishi, S.; Cifu, D.X. Disorders of Consciousness. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 245–258. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Fan, J.; Wang, H.; Zhong, Y.; Ma, J. Differentiating Responders and Non-responders to rTMS Treatment for Disorder of Consciousness Using EEG After-Effects. Front. Neurol. 2020, 11, 583268. [Google Scholar] [CrossRef]
- Liu, X.; Meng, F.; Gao, J.; Zhang, L.; Zhou, Z.; Pan, G.; Luo, B. Behavioral and Resting State Functional Connectivity Effects of High Frequency rTMS on Disorders of Consciousness: A Sham-Controlled Study. Front. Neurol. 2018, 9, 982. [Google Scholar] [CrossRef]
- Bestmann, S.; Baudewig, J.; Siebner, H.R.; Rothwell, J.C.; Frahm, J. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur. J. Neurosci. 2004, 19, 1950–1962. [Google Scholar] [CrossRef]
- Ragazzoni, A.; Pirulli, C.; Veniero, D.; Feurra, M.; Cincotta, M.; Giovannelli, F.; Chiaramonti, R.; Lino, M.; Rossi, S.; Miniussi, C. Vegetative versus minimally conscious states: A study using TMS-EEG, sensory and event-related potentials. PLoS ONE 2013, 8, e57069. [Google Scholar] [CrossRef]
- Seth, A.K.; Bayne, T. Theories of consciousness. Nat. Rev. Neurosci. 2022, 23, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Fingelkurts, A.A.; Fingelkurts, A.A.; Bagnato, S.; Boccagni, C.; Galardi, G. DMN Operational Synchrony Relates to Self-Consciousness: Evidence from Patients in Vegetative and Minimally Conscious States. Open Neuroimaging J. 2012, 6, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Marino, S.; Bonanno, L.; Giorgio, A. Functional connectivity in disorders of consciousness: Methodological aspects and clinical relevance. Brain Imaging Behav. 2016, 10, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Zis, P.; Shafique, F.; Hadjivassiliou, M.; Blackburn, D.; Venneri, A.; Iliodromiti, S.; Mitsikostas, D.D.; Sarrigiannis, P.G. Safety, Tolerability, and Nocebo Phenomena during Transcranial Magnetic Stimulation: A Systematic Review and Meta-Analysis of Placebo-Controlled Clinical Trials. Neuromodulation J. Int. Neuromodulation Soc. 2020, 23, 291–300. [Google Scholar] [CrossRef]
- Wang, W.L.; Wang, S.Y.; Hung, H.Y.; Chen, M.H.; Juan, C.H.; Li, C.T. Safety of transcranial magnetic stimulation in unipolar depression: A systematic review and meta-analysis of randomized-controlled trials. J. Affect. Disord. 2022, 301, 400–425. [Google Scholar] [CrossRef]
- Wu, L.N.; Zheng, H.Y.; Xue, S.A.; Chen, K.Y.; Li, R.Y. The Efficacy and Safety of Different Noninvasive Therapies in the Treatment of Central Poststroke Pain (CPSP): A Network Meta-Analysis and Systematic Review. J. Integr. Neurosci. 2023, 22, 102. [Google Scholar] [CrossRef]
- Rossi, S.; Antal, A.; Bestmann, S.; Bikson, M.; Brewer, C.; Brockmöller, J.; Carpenter, L.L.; Cincotta, M.; Chen, R.; Daskalakis, J.D.; et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2021, 132, 269–306. [Google Scholar] [CrossRef]
- Kim, W.S.; Paik, N.J. Safety Review for Clinical Application of Repetitive Transcranial Magnetic Stimulation. Brain NeuroRehabilitation 2021, 14, e6. [Google Scholar] [CrossRef]


| Authors | Study Design | Participants | Time after Injury | Intervention | Duration | Brain Target | Control | Behavioral Outcome |
|---|---|---|---|---|---|---|---|---|
| Massimo Cincotta et al., 2015 [24] | Cross-over | 11 VS | 9–80 months | 20 Hz rTMS | 10 min per session; 5 sessions | LM1 | The utilization of a sham coil in rTMS | CRS-R |
| Fangping He et al., 2018 [25] | Cross-over | 3 VS/UWS, 3 MCS | 1–3 months | 20 Hz rTMS | 10 min per session; 5 sessions | LM1 | Sham rTMS by positioning the coil away from the head | CRS-R |
| Xin Ge et al., 2021 [31] | Parallel | 32 VS | <1 month | 10 Hz rTMS | 20 min per session; 1 session | RDLPFC | The utilization of a sham coil in rTMS | CRS-R |
| Xiao-Hua Zhang et al., 2021 [26] | Parallel | 48 PVS | >3 months | 5 Hz rTMS | 40 sessions (5 times a week over 8 consecutive weeks) | LM1 | Sham rTMS using a sham coil | CRS-R |
| Jian-Min Chen et al., 2022 [30] | Parallel | 50 PVS | 1–3 months | 10 Hz rTMS | 20 min per session; 1 session | LDLPFC (F3) | The utilization of a sham coil in rTMS | CRS-R |
| Jianzhong Fan et al., 2022 [28] | Parallel | 40 PVS | 1–3 months | 20 Hz rTMS | 20 sessions (5 times a week over 4 consecutive weeks) | LDLPFC (F3) | The utilization of a sham coil in rTMS | CRS-R |
| Chengwei Xu et al., 2023 [27] | Cross-over | 20 UWS | <12 months and >28 days | 10 Hz rTMS | 20 min per session; 10 sessions | LPPC | The utilization of a sham coil in rTMS | CRS-R |
| Longbin Shen et al., 2023 [29] | Parallel | 99 VS | 1–3 months | 20 Hz rTMS | 10 min per session; 5 sessions | LDLPFC (F3) | The utilization of a sham coil in rTMS | CRS-R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Yue, T.; Zschorlich, V.R.; Li, D.; Wang, D.; Qi, F. Behavioral Effects of Repetitive Transcranial Magnetic Stimulation in Disorders of Consciousness: A Systematic Review and Meta-Analysis. Brain Sci. 2023, 13, 1362. https://doi.org/10.3390/brainsci13101362
Yang Z, Yue T, Zschorlich VR, Li D, Wang D, Qi F. Behavioral Effects of Repetitive Transcranial Magnetic Stimulation in Disorders of Consciousness: A Systematic Review and Meta-Analysis. Brain Sciences. 2023; 13(10):1362. https://doi.org/10.3390/brainsci13101362
Chicago/Turabian StyleYang, Zihan, Tian Yue, Volker R. Zschorlich, Dai Li, Duanwei Wang, and Fengxue Qi. 2023. "Behavioral Effects of Repetitive Transcranial Magnetic Stimulation in Disorders of Consciousness: A Systematic Review and Meta-Analysis" Brain Sciences 13, no. 10: 1362. https://doi.org/10.3390/brainsci13101362
APA StyleYang, Z., Yue, T., Zschorlich, V. R., Li, D., Wang, D., & Qi, F. (2023). Behavioral Effects of Repetitive Transcranial Magnetic Stimulation in Disorders of Consciousness: A Systematic Review and Meta-Analysis. Brain Sciences, 13(10), 1362. https://doi.org/10.3390/brainsci13101362








