Peripheral Blood and Cerebrospinal Fluid Levels of YKL-40 in Alzheimer’s Disease: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Eligibility Criteria
2.2.1. Inclusion Criteria
- Human participants.
- Samples from peripheral blood or CSF.
- The study reports detailed groupings of AD and HC, along with the corresponding YKL-40 concentration values for each group.
- The study describes the specific measurements of peripheral blood and CSF levels of YKL-40 samples.
- Case-control studies or cross-sectional studies with complete and available data.
- Written or published in English.
2.2.2. Exclusion Criteria
- Reviews, guidelines, letters, conference abstracts, commentaries, and case reports.
- Medical history includes neurological, psychiatric, or other systemic disorders that may have an impact on cognitive function (e.g., depression, stroke, VaD, and mild cognitive impairment).
- Lack of quantitative data on YKL-40 concentration and research with incomplete or unavailable data.
- Failure to provide study data for mean and SD or SE or CI of YKL-40.
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
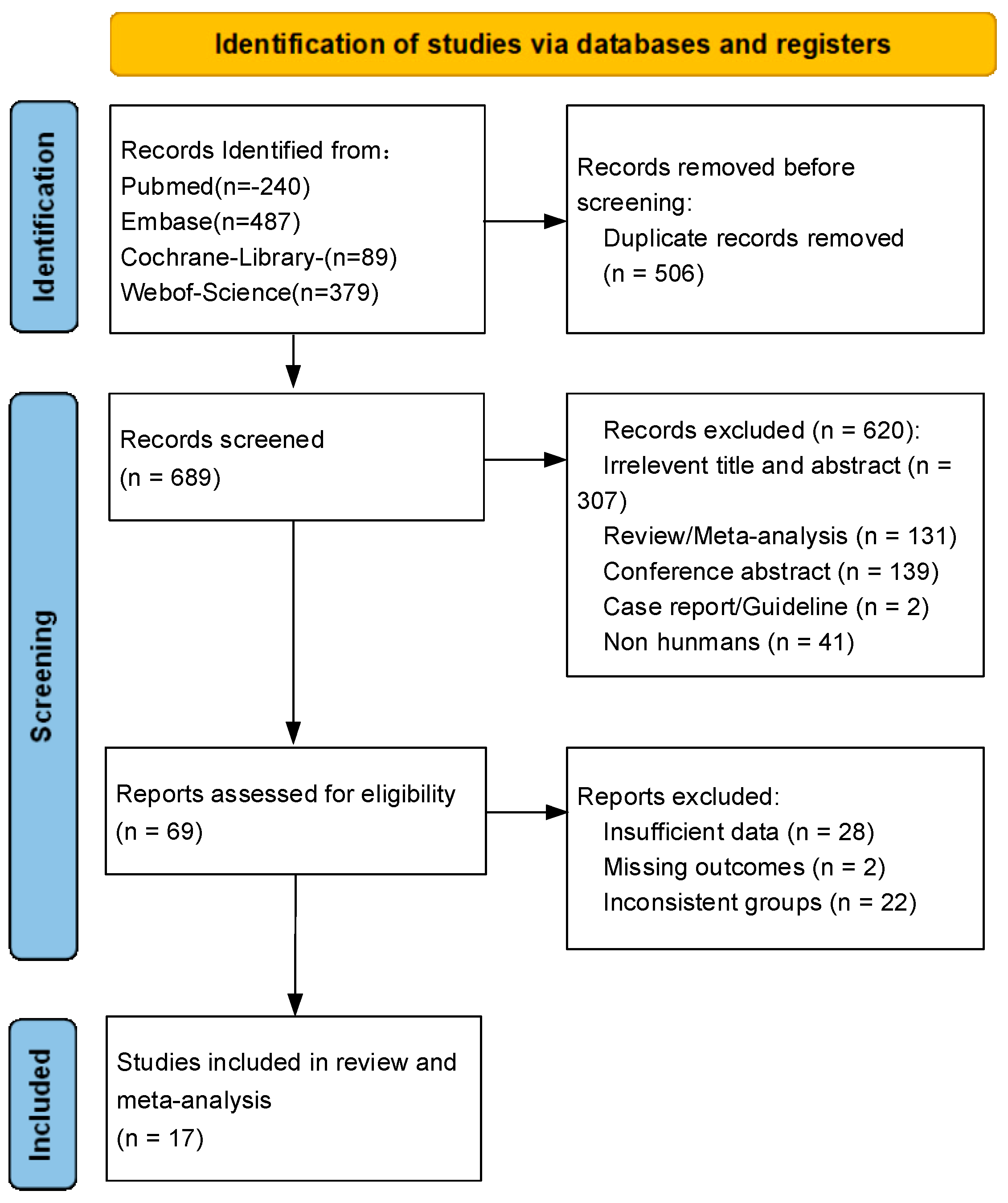
3.1. Literature Search and Study Characteristics
3.2. Meta-Analysis of Peripheral Blood Levels of YKL-40 between AD and HCs
3.3. Meta-Analysis of CSF Levels of YKL-40
3.4. Meta-Analysis of Overall Levels of YKL-40
3.5. Results of Sensitivity Analysis and Publication Bias Analysis
3.6. Meta-Analysis of CSF Levels of Aβ42
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- d’Abramo, C.; D’Adamio, L.; Giliberto, L. Significance of Blood and Cerebrospinal Fluid Biomarkers for Alzheimer’s Disease: Sensitivity, Specificity and Potential for Clinical Use. J. Pers. Med. 2020, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- McGrowder, D.A.; Miller, F.; Vaz, K.; Nwokocha, C.; Wilson-Clarke, C.; Anderson-Cross, M.; Brown, J.; Anderson-Jackson, L.; Williams, L.; Latore, L.; et al. Cerebrospinal Fluid Biomarkers of Alzheimer’s Disease: Current Evidence and Future Perspectives. Brain Sci. 2021, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Mavroudis, I.A.; Petridis, F.; Chatzikonstantinou, S.; Karantali, E.; Kazis, D. A meta-analysis on the levels of VILIP-1 in the CSF of Alzheimer’s disease compared to normal controls and other neurodegenerative conditions. Aging Clin. Exp. Res. 2021, 33, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Bridel, C.; van Wieringen, W.N.; Zetterberg, H.; Tijms, B.M.; Teunissen, C.E.; Alvarez-Cermeño, J.C.; Andreasson, U.; Axelsson, M.; Bäckström, D.C.; Bartos, A.; et al. Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology: A Systematic Review and Meta-analysis. JAMA Neurol. 2019, 76, 1035–1048. [Google Scholar] [CrossRef]
- Mavroudis, I.A.; Petridis, F.; Chatzikonstantinou, S.; Kazis, D. A meta-analysis on CSF neurogranin levels for the diagnosis of Alzheimer’s disease and mild cognitive impairment. Aging Clin. Exp. Res. 2020, 32, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Roveta, F.; Cermelli, A.; Boschi, S.; Ferrandes, F.; Grassini, A.; Marcinnò, A.; Spina, M.; Rubino, E.; Borsello, T.; Vercelli, A.; et al. Synaptic Proteins as Fluid Biomarkers in Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. JAD 2022, 90, 1381–1393. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhu, M.; Kong, C.; Pang, Y.; Zhang, H.; Qiu, Q.; Wei, C.; Tang, Y.; Wang, Q.; Li, Y.; et al. Blood neuro-exosomal synaptic proteins predict Alzheimer’s disease at the asymptomatic stage. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2021, 17, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Chang, X.; Lang, M. Iron Homeostasis Disorder and Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 12442. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Collins, A.E.; Hickey, J.P.; Pfeifer, J.A.; Kalisch, B.E. Sex Differences in the Level of Homocysteine in Alzheimer’s Disease and Parkinson’s Disease Patients: A Meta-Analysis. Brain Sci. 2023, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Martens, Y.A.; Zhao, N.; Liu, C.C.; Kanekiyo, T.; Yang, A.J.; Goate, A.M.; Holtzman, D.M.; Bu, G. ApoE Cascade Hypothesis in the pathogenesis of Alzheimer’s disease and related dementias. Neuron 2022, 110, 1304–1317. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, W.; Xing, Y.; Jia, J.; Tang, Y. B vitamins and prevention of cognitive decline and incident dementia: A systematic review and meta-analysis. Nutr. Rev. 2022, 80, 931–949. [Google Scholar] [CrossRef]
- Michetti, F.; Clementi, M.E.; Di Liddo, R.; Valeriani, F.; Ria, F.; Rende, M.; Di Sante, G.; Romano Spica, V. The S100B Protein: A Multifaceted Pathogenic Factor More Than a Biomarker. Int. J. Mol. Sci. 2023, 24, 9605. [Google Scholar] [CrossRef]
- Wang, Q.; Duan, L.; Li, X.; Wang, Y.; Guo, W.; Guan, F.; Ma, S. Glucose Metabolism, Neural Cell Senescence and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 4351. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, C.G.; Assogna, M.; Di Donna, M.G.; Bernocchi, F.; De Lucia, V.; Nuccetelli, M.; Fiorelli, D.; Loizzo, S.; Mercuri, N.B.; Koch, G.; et al. Cerebrospinal Fluid sTREM-2, GFAP, and β-S100 in Symptomatic Sporadic Alzheimer’s Disease: Microglial, Astrocytic, and APOE Contributions Along the Alzheimer’s Disease Continuum. J. Alzheimer’s Dis. JAD 2023, 92, 1385–1397. [Google Scholar] [CrossRef] [PubMed]
- Oeckl, P.; Anderl-Straub, S.; Von Arnim, C.A.F.; Baldeiras, I.; Diehl-Schmid, J.; Grimmer, T.; Halbgebauer, S.; Kort, A.M.; Lima, M.; Marques, T.M.; et al. Serum GFAP differentiates Alzheimer’s disease from frontotemporal dementia and predicts MCI-to-dementia conversion. J. Neurol. Neurosurg. Psychiatry 2022, 93, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, Y.K. Inflammatory Biomarkers in AD: Implications for Diagnosis. Curr. Alzheimer Res. 2020, 17, 962–971. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, M.; Liu, X. C-reactive protein and risk of Alzheimer’s disease. Neurobiol. Aging 2022, 109, 259–263. [Google Scholar] [CrossRef]
- Custodero, C.; Ciavarella, A.; Panza, F.; Gnocchi, D.; Lenato, G.M.; Lee, J.; Mazzocca, A.; Sabbà, C.; Solfrizzi, V. Role of inflammatory markers in the diagnosis of vascular contributions to cognitive impairment and dementia: A systematic review and meta-analysis. Geroscience 2022, 44, 1373–1392. [Google Scholar] [CrossRef]
- Mavroudis, I.; Chowdhury, R.; Petridis, F.; Karantali, E.; Chatzikonstantinou, S.; Balmus, I.M.; Luca, I.S.; Ciobica, A.; Kazis, D. YKL-40 as a Potential Biomarker for the Differential Diagnosis of Alzheimer’s Disease. Medicina 2021, 58, 60. [Google Scholar] [CrossRef]
- Yasuno, F.; Watanabe, A.; Kimura, Y.; Yamauchi, Y.; Ogata, A.; Ikenuma, H.; Abe, J.; Minami, H.; Nihashi, T.; Yokoi, K.; et al. Estimation of blood-based biomarkers of glial activation related to neuroinflammation. Brain Behav. Immun.-Health 2022, 26, 100549. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener 2020, 9, 42. [Google Scholar] [CrossRef]
- Blanco-Palmero, V.A.; Rubio-Fernández, M.; Antequera, D.; Villarejo-Galende, A.; Molina, J.A.; Ferrer, I.; Bartolome, F.; Carro, E. Increased YKL-40 but Not C-Reactive Protein Levels in Patients with Alzheimer’s Disease. Biomedicines 2021, 9, 1094. [Google Scholar] [CrossRef]
- Al-Ghraiybah, N.F.; Wang, J.; Alkhalifa, A.E.; Roberts, A.B.; Raj, R.; Yang, E.; Kaddoumi, A. Glial Cell-Mediated Neuroinflammation in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 10572. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Han, S.M.; Kim, C.E. New fluid biomarkers tracking non-amyloid-β and non-tau pathology in Alzheimer’s disease. Exp. Mol. Med. 2020, 52, 556–568. [Google Scholar] [CrossRef]
- Lananna, B.V.; McKee, C.A.; King, M.W.; Del-Aguila, J.L.; Dimitry, J.M.; Farias, F.H.G.; Nadarajah, C.J.; Xiong, D.D.; Guo, C.; Cammack, A.J.; et al. Chi3l1/YKL-40 is controlled by the astrocyte circadian clock and regulates neuroinflammation and Alzheimer’s disease pathogenesis. Sci. Transl. Med. 2020, 12, eaax3519. [Google Scholar] [CrossRef]
- Tizaoui, K.; Yang, J.W.; Lee, K.H.; Kim, J.H.; Kim, M.; Yoon, S.; Jung, Y.; Park, J.B.; An, K.; Choi, H.; et al. The role of YKL-40 in the pathogenesis of autoimmune diseases: A comprehensive review. Int. J. Biol. Sci. 2022, 18, 3731–3746. [Google Scholar] [CrossRef]
- Hok, A.H.Y.S.; Hoozemans, J.J.M.; Hu, W.T.; Wouters, D.; Howell, J.C.; Rábano, A.; van der Flier, W.M.; Pijnenburg, Y.A.L.; Teunissen, C.E.; Del Campo, M. YKL-40 changes are not detected in post-mortem brain of patients with Alzheimer’s disease and frontotemporal lobar degeneration. Alzheimers Res. Ther. 2022, 14, 100. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, Y.; Li, Z.; Yu, J.; Chen, Y.; Wan, H.; Wang, B. Changes and significance of serum BK and YKL40 levels in elderly patients with Alzheimer’s disease and mild cognitive impairment. Acta Medica Mediterr. 2019, 35, 2335–2339. [Google Scholar] [CrossRef]
- Villar-Piqué, A.; Schmitz, M.; Hermann, P.; Goebel, S.; Bunck, T.; Varges, D.; Ferrer, I.; Riggert, J.; Llorens, F.; Zerr, I. Plasma YKL-40 in the spectrum of neurodegenerative dementia. J. Neuroinflamm. 2019, 16, 145. [Google Scholar] [CrossRef] [PubMed]
- Ko, P.W.; Lee, H.W.; Lee, M.; Youn, Y.C.; Kim, S.; Kim, J.H.; Kang, K.; Suk, K. Increased plasma levels of chitinase 3-like 1 (CHI3L1) protein in patients with idiopathic normal-pressure hydrocephalus. J. Neurol. Sci. 2021, 423, 117353. [Google Scholar] [CrossRef]
- Schulz, I.; Kruse, N.; Gera, R.G.; Kremer, T.; Cedarbaum, J.; Barbour, R.; Zago, W.; Schade, S.; Otte, B.; Bartl, M.; et al. Systematic Assessment of 10 Biomarker Candidates Focusing on α-Synuclein-Related Disorders. Mov. Disord. 2021, 36, 2874–2887. [Google Scholar] [CrossRef] [PubMed]
- Manniche, C.; Simonsen, A.H.; Hasselbalch, S.G.; Andreasson, U.; Zetterberg, H.; Blennow, K.; Høgh, P.; Juhler, M.; Hejl, A.M. Cerebrospinal Fluid Biomarkers to Differentiate Idiopathic Normal Pressure Hydrocephalus from Subcortical Ischemic Vascular Disease. J. Alzheimer’s Dis. 2020, 75, 937–947. [Google Scholar] [CrossRef]
- Toschi, N.; Lista, S.; Baldacci, F.; Cavedo, E.; Zetterberg, H.; Blennow, K.; Kilimann, I.; Teipel, S.J.; Melo dos Santos, A.; Epelbaum, S.; et al. Biomarker-guided clustering of Alzheimer’s disease clinical syndromes. Neurobiol. Aging 2019, 83, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Nordengen, K.; Kirsebom, B.E.; Henjum, K.; Selnes, P.; Gísladóttir, B.; Wettergreen, M.; Torsetnes, S.B.; Grøntvedt, G.R.; Waterloo, K.K.; Aarsland, D.; et al. Glial activation and inflammation along the Alzheimer’s disease continuum. J. Neuroinflamm. 2019, 16, 46. [Google Scholar] [CrossRef] [PubMed]
- Morenas-Rodríguez, E.; Alcolea, D.; Suárez-Calvet, M.; Muñoz-Llahuna, L.; Vilaplana, E.; Sala, I.; Subirana, A.; Querol-Vilaseca, M.; Carmona-Iragui, M.; Illán-Gala, I.; et al. Different pattern of CSF glial markers between dementia with Lewy bodies and Alzheimer’s disease. Sci. Rep. 2019, 9, 7803. [Google Scholar] [CrossRef]
- Lleó, A.; Alcolea, D.; Martínez-Lage, P.; Scheltens, P.; Parnetti, L.; Poirier, J.; Simonsen, A.H.; Verbeek, M.M.; Rosa-Neto, P.; Slot, R.E.R.; et al. Longitudinal cerebrospinal fluid biomarker trajectories along the Alzheimer’s disease continuum in the BIOMARKAPD study. Alzheimer’s Dement. 2019, 15, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Llorens, F.; Schmitz, M.; Knipper, T.; Schmidt, C.; Lange, P.; Fischer, A.; Hermann, P.; Zerr, I. Cerebrospinal fluid biomarkers of Alzheimer’s disease show different but partially overlapping profile compared to vascular dementia. Front. Aging Neurosci. 2017, 9, 289. [Google Scholar] [CrossRef]
- Alcolea, D.; Vilaplana, E.; Suárez-Calvet, M.; Illán-Gala, I.; Blesa, R.; Clarimón, J.; Lladó, A.; Sánchez-Valle, R.; Molinuevo, J.L.; García-Ribas, G.; et al. CSF sAPPβ, YKL-40, and neurofilament light in frontotemporal lobar degeneration. Neurology 2017, 89, 178–188. [Google Scholar] [CrossRef]
- Janelidze, S.; Hertze, J.; Zetterberg, H.; Landqvist Waldö, M.; Santillo, A.; Blennow, K.; Hansson, O. Cerebrospinal fluid neurogranin and YKL-40 as biomarkers of Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2016, 3, 12–20. [Google Scholar] [CrossRef]
- Olsson, B.; Hertze, J.; Lautner, R.; Zetterberg, H.; Nägga, K.; Höglund, K.; Basun, H.; Annas, P.; Lannfelt, L.; Andreasen, N.; et al. Microglial markers are elevated in the prodromal phase of Alzheimer’s disease and vascular dementia. J. Alzheimer’s Dis. 2013, 33, 45–53. [Google Scholar] [CrossRef]
- Mattsson, N.; Tabatabaei, S.; Johansson, P.; Hansson, O.; Andreasson, U.; Månsson, J.E.; Johansson, J.O.; Olsson, B.; Wallin, A.; Svensson, J.; et al. Cerebrospinal fluid microglial markers in Alzheimer’s disease: Elevated chitotriosidase activity but lack of diagnostic utility. NeuroMol. Med. 2011, 13, 151–159. [Google Scholar] [CrossRef]
- Zhang, H.; Ng, K.P.; Therriault, J.; Kang, M.S.; Pascoal, T.A.; Rosa-Neto, P.; Gauthier, S. Cerebrospinal fluid phosphorylated tau, visinin-like protein-1, and chitinase-3-like protein 1 in mild cognitive impairment and Alzheimer’s disease 11 Medical and Health Sciences 1109 Neurosciences. Transl. Neurodegener. 2018, 7, 23. [Google Scholar] [CrossRef]
- Kester, M.I.; Teunissen, C.E.; Sutphen, C.; Herries, E.M.; Ladenson, J.H.; Xiong, C.; Scheltens, P.; Van Der Flier, W.M.; Morris, J.C.; Holtzman, D.M.; et al. Cerebrospinal fluid VILIP-1 and YKL-40, candidate biomarkers to diagnose, predict and monitor Alzheimer’s disease in a memory clinic cohort. Alzheimer’s Res. Ther. 2015, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Kosyreva, A.M.; Sentyabreva, A.V.; Tsvetkov, I.S.; Makarova, O.V. Alzheimer’s Disease and Inflammaging. Brain Sci. 2022, 12, 1237. [Google Scholar] [CrossRef]
- Shen, X.N.; Niu, L.D.; Wang, Y.J.; Cao, X.P.; Liu, Q.; Tan, L.; Zhang, C.; Yu, J.T. Inflammatory markers in Alzheimer’s disease and mild cognitive impairment: A meta-analysis and systematic review of 170 studies. J. Neurol. Neurosurg. Psychiatry 2019, 90, 590–598. [Google Scholar] [CrossRef]
- Baldacci, F.; Lista, S.; Palermo, G.; Giorgi, F.S.; Vergallo, A.; Hampel, H. The neuroinflammatory biomarker YKL-40 for neurodegenerative diseases: Advances in development. Expert Rev. Proteom. 2019, 16, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Prins, S.; de Kam, M.L.; Teunissen, C.E.; Groeneveld, G.J. Inflammatory plasma biomarkers in subjects with preclinical Alzheimer’s disease. Alzheimers Res. Ther. 2022, 14, 106. [Google Scholar] [CrossRef]
- Thordardottir, S.; Almkvist, O.; Johansson, C.; Zetterberg, H.; Blennow, K.; Graff, C. Cerebrospinal Fluid YKL-40 and Neurogranin in Familial Alzheimer’s Disease: A Pilot Study. J. Alzheimer’s Dis. JAD 2020, 76, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Baldacci, F.; Lista, S.; Cavedo, E.; Bonuccelli, U.; Hampel, H. Diagnostic function of the neuroinflammatory biomarker YKL-40 in Alzheimer’s disease and other neurodegenerative diseases. Expert Rev. Proteom. 2017, 14, 285–299. [Google Scholar] [CrossRef]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef]
- Ferrari-Souza, J.P.; Ferreira, P.C.L.; Bellaver, B.; Tissot, C.; Wang, Y.T.; Leffa, D.T.; Brum, W.S.; Benedet, A.L.; Ashton, N.J.; De Bastiani, M.A.; et al. Astrocyte biomarker signatures of amyloid-β and tau pathologies in Alzheimer’s disease. Mol. Psychiatry 2022, 27, 4781–4789. [Google Scholar] [CrossRef] [PubMed]
- Jiwaji, Z.; Tiwari, S.S.; Avilés-Reyes, R.X.; Hooley, M.; Hampton, D.; Torvell, M.; Johnson, D.A.; McQueen, J.; Baxter, P.; Sabari-Sankar, K.; et al. Reactive astrocytes acquire neuroprotective as well as deleterious signatures in response to Tau and Aß pathology. Nat. Commun. 2022, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- De Bastiani, M.A.; Bellaver, B.; Brum, W.S.; Souza, D.G.; Ferreira, P.C.L.; Rocha, A.S.; Povala, G.; Ferrari-Souza, J.P.; Benedet, A.L.; Ashton, N.J.; et al. Hippocampal GFAP-positive astrocyte responses to amyloid and tau pathologies. Brain Behav. Immun. 2023, 110, 175–184. [Google Scholar] [CrossRef]
- Craig-Schapiro, R.; Perrin, R.J.; Roe, C.M.; Xiong, C.; Carter, D.; Cairns, N.J.; Mintun, M.A.; Peskind, E.R.; Li, G.; Galasko, D.R.; et al. YKL-40: A novel prognostic fluid biomarker for preclinical Alzheimer’s disease. Biol. Psychiatry 2010, 68, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Bonneh-Barkay, D.; Wang, G.; Starkey, A.; Hamilton, R.L.; Wiley, C.A. In vivo CHI3L1 (YKL-40) expression in astrocytes in acute and chronic neurological diseases. J. Neuroinflamm. 2010, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Bonneh-Barkay, D.; Bissel, S.J.; Kofler, J.; Starkey, A.; Wang, G.; Wiley, C.A. Astrocyte and macrophage regulation of YKL-40 expression and cellular response in neuroinflammation. Brain Pathol. 2012, 22, 530–546. [Google Scholar] [CrossRef] [PubMed]
- Vergallo, A.; Lista, S.; Lemercier, P.; Chiesa, P.A.; Zetterberg, H.; Blennow, K.; Potier, M.C.; Habert, M.O.; Baldacci, F.; Cavedo, E.; et al. Association of plasma YKL-40 with brain amyloid-β levels, memory performance, and sex in subjective memory complainers. Neurobiol. Aging 2020, 96, 22–32. [Google Scholar] [CrossRef]





| Study | Sample Source | Ethnicity | AD Criteria | Mean Age (y) | Method | Male/Female | Mean MMSE | Biomaterial |
|---|---|---|---|---|---|---|---|---|
| Lu 2019 [28] | Serum | China (A) | NR | AD = 79.77 ± 12.97, NC = 74.50 ± 6.45 | ELISA | AD = 18/27, NC = 23/17 | AD = 15.10 ± 5.78, NC = 29.06 ± 1.07 | Fasting blood glucose, Triglyceride, Total cholesterol lipoprotein |
| Yasuno 2022 [20] | Serum | Japan (A) | NIA-AA | AD = 78.1 ± 3.9, NC = 78.9 ± 5.2 | ELISA | AD = 7/8, NC = 4/6 | AD = 20.7 ± 2.4, NC = 26.2 ± 2.2 | ApoE4, BMI, PET |
| Villar-Piqué 2019 [29] | Plasma | Germany (C) | NIA-AA | AD = 69 ± 10, NC = 66 ± 5 | ELISA | AD = 25/25, NC = 48/22 | NR | NR |
| Ko 2021 [30] | Plasma | Korea (A) | NIA-AA | AD = 65.2 ± 9.9, NC = 64.7 ± 9.9 | ELISA | AD = 51/70, NC = 36/47 | AD = 17.3 ± 6.3, NC = 28.1 ± 1.9 | CDR, Aβ42, t-tau, p-tau |
| Schulz 2021 [31] | Serum/CSF | Germany (C) | NR | AD = 74.27 ± 4.64, NC = 68.75 ± 6.38 | ELISA | AD = 6/5, NC = 14/6 | AD = 17.89 ± 7.72, NC = 28.20 ± 1.47 | α-Synuclein, NfL, tTau, UCHL-1, GFAP, S100B, sTREM-2 |
| Manniche 2020 [32] | CSF | Denmark (C) | NIA-AA | AD = 70.28 ± 8.0, NC = 64.52 ± 7.6 | ELISA | AD = 30/27, NC = 19/14 | AD = 23.44 ± 4.6, NC = 28.96 ± 1.3 | NFL, NG, Aβ42, t-tau, p-tau |
| Toschi 2019 [33] | CSF | France/Germany/Sweden (C) | NINCDS-ADRDA | AD = 70.4 ± 7.7, NC = 60.7 ± 10.3 | ELISA | AD = 18/19, NC = 7/13 | AD = 21.7 ± 5.0, NC = 29.4 ± 0.8 | p-tau181, t-tau, Aβ1-42, NFL |
| Nordengen 2019 [34] | CSF | Norway (C) | NIA-AA | AD = 67.6 ± 5.2, NC = 61.1 ± 9.2 | MSD | AD = 14/13, NC = 17/19 | AD = 19.0 ± 5.8, NC = 29.4 ± 0.7 | ApoE4, Aβ42, t-tau, p-tau |
| Morenas-Rodríguez 2019 [35] | CSF | Spain (C) | NIA-AA | AD = 74.6 ± 5.6, NC = 67.4 ± 5.1 | ELISA | AD = 19/31, NC = 19/25 | AD = 22.5 ± 3.4, NC = 28.9 ± 1.2 | Aβ1-42, t-tau, p-tau, sTREM2, PGRN |
| Lleó 2019 [36] | CSF | Spain (C) | NIA-AA | AD = 68.5 ± 8.5, NC = 58.2 ± 7.2 | ELISA | AD = 63/47, NC = 68/86 | AD = 22.6 ± 4.1, NC = 28.7 ± 1.2 | APOE ε4, t-tau, p-tau, Aβ1-38, Aβ1-40, Aβ1-42, Aβ1-42/Aβ1-40, Aβ1-42/t-tau, NFL |
| Llorens 2017 [37] | CSF | Germany (C) | NINCDS-ADRDA | AD = 67 ± 11, NC = 70 ± 6 | ELISA | AD = 22/43, NC = 23/27 | NR | tau, p-tau, Aβ42, S100B, NSE, 14-3-3 |
| Alcolea 2017 [38] | CSF | Spain (C) | NIA-AA | AD = 70.8 ± 7.8, NC = 60.2 ± 8.3 | ELISA | AD = 28/44, NC = 31/45 | AD = 21.6 ± 4.6, NC = 29.0 ± 1.1 | NR |
| Janelidze 2016 [39] | CSF | Sweden (C) | DSM-III-R combine NINCDS-ADRDA | AD = 76.4 ± 7.4, NC = 75.3 ± 6.4 | ELISA | AD = 24/50, NC = 16/37 | AD = 19.4 ± 3.3, NC = 28.6 ± 1.8 | Neurogranin, Aβ40, Aβ42, Tau |
| Olsson 2013 [40] | CSF | Sweden (C) | DSM-III-R | AD = 76.2 ± 7.4, NC = 74.7 ± 7.5 | ELISA | AD = 34/62, NC = 17/48 | AD = 19.0 ± 3.8, NC = 28.7 ± 1.6 | Aβ1-42, t-tau, p-tau, sCD14 |
| Mattsson 2011 [41] | CSF | Sweden (C) | DSM-III-R | AD = 74 ± 4, NC = 74 ± 5 | ELISA | AD = 11/14, NC = 9/10 | NR | CCL2, IL6, IL8 |
| Zhang 2018 [42] | CSF | USA (C) | NINCDS/ADRDA | AD = 74.3 ± 6.8, NC = 76 ± 5.7 | ELISA | AD = 7/11, NC = 19/13 | AD = 24.2 ± 2.1, NC = 29.2 ± 1.1 | Aβ42, t-tau, p-tau181, VILIP-1 |
| Kester 2015 [43] | CSF | Netherlands (C) | NINCDS-ADRDA | AD = 65 ± 8.1, NC = 64 ± 12.2 | ELISA | AD = 36/29, NC = 23/14 | AD = 22 ± 5.6, NC = 28 ± 1.8 | Aβ42, tau, p-tau181, VILIP-1 |
| Subgroups | n of Studies | SMD (95%CI) | I2 | p-Value |
|---|---|---|---|---|
| All studies | 5 | |||
| Sample types Source | ||||
| Serum | 3 | −0.638 (−2.636, 1.361) | 95.9% | 0.532 |
| Plasma | 2 | 0.527 (0.302, 0.752) | 0.0% | 0.000 |
| Ethnicity | ||||
| Asian | 3 | −0.605 (−2.598, 1.388) | 97.7% | 0.552 |
| Caucasian | 2 | 0.507 (0.176, 0.838) | 0.0% | 0.003 |
| AD Criteria | ||||
| NIA-AA | 3 | 0.498 (0.281, 0.715) | 0.0% | 0.000 |
| NR | 2 | −1.009 (−3.917, 1.899) | 97.4% | 0.497 |
| Mean Age Range | ||||
| 70–79 y | 3 | −0.638 (−2.636, 1.361) | 95.9% | 0.532 |
| 60–69 y | 2 | 0.527 (0.302, 0.752) | 0.0% | 0.000 |
| Subgroups | n of Studies | SMD (95%CI) | I2 | p-Value |
|---|---|---|---|---|
| All studies | 13 | |||
| AD Criteria | ||||
| NIA-AA | 5 | 1.180 (0.825, 1.535) | 74.9% | 0.000 |
| NINCDS-ADRDA | 4 | 0.763 (0.530, 0.996) | 0.4% | 0.000 |
| DSM-III-R | 2 | 0.487 (0.205, 0.769) | 0.0% | 0.001 |
| NR | 1 | 1.002 (0.222, 1.781) | - | 0.012 |
| DSM-IIIR combine NINCDS-ADRDA | 1 | 0.710 (0.347, 1.074) | - | 0.000 |
| Mean Age Range | ||||
| 70–79 y | 9 | 0.823 (0.526, 1.121) | 73.1% | 0.000 |
| 60–69 y | 4 | 1.034 (0.689, 1.378) | 67.9% | 0.000 |
| Subgroups | n of Studies | SMD (95%CI) | I2 | p-Value |
|---|---|---|---|---|
| All studies | 18 | |||
| Sample types of Sources | ||||
| Serum | 3 | −0.638 (−2.636, 1.361) | 95.9% | 0.532 |
| Plasma | 2 | 0.527 (0.302, 0.752) | 0.0% | 0.000 |
| CSF | 13 | 0.893 (0.665, 1.121) | 72.2% | 0.000 |
| Ethnicity | ||||
| Asian | 3 | −0.605 (−2.598, 1.388) | 97.7% | 0.552 |
| Caucasian | 15 | 0.846 (0.634, 1.058) | 71.3% | 0.000 |
| AD Criteria | ||||
| NIA-AA | 8 | 0.908 (0.560, 1.256) | 83.8% | 0.000 |
| NINCDS-ADRDA | 4 | 0.763 (0.530, 0.996) | 0.4% | 0.000 |
| DSM-III-R | 2 | 0.487 (0.205, 0.769) | 0.0% | 0.001 |
| NR | 3 | −0.344 (−2.635, 1.947) | 96.9% | 0.769 |
| DSM-IIIR combine NINCDS-ADRDA | 1 | 0.710 (0.347, 1.074) | - | 0.000 |
| Mean Age Range | ||||
| 70–79 y | 12 | 0.465 (−0.068, 0.998) | 92.8% | 0.087 |
| 60–69 y | 6 | 0.852 (0.536, 1.169) | 78.3% | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Tian, J.; Ni, J.; Wei, M.; Li, T.; Shi, J. Peripheral Blood and Cerebrospinal Fluid Levels of YKL-40 in Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Brain Sci. 2023, 13, 1364. https://doi.org/10.3390/brainsci13101364
Zhang Y, Tian J, Ni J, Wei M, Li T, Shi J. Peripheral Blood and Cerebrospinal Fluid Levels of YKL-40 in Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Brain Sciences. 2023; 13(10):1364. https://doi.org/10.3390/brainsci13101364
Chicago/Turabian StyleZhang, Yuchen, Jinzhou Tian, Jingnian Ni, Mingqing Wei, Ting Li, and Jing Shi. 2023. "Peripheral Blood and Cerebrospinal Fluid Levels of YKL-40 in Alzheimer’s Disease: A Systematic Review and Meta-Analysis" Brain Sciences 13, no. 10: 1364. https://doi.org/10.3390/brainsci13101364
APA StyleZhang, Y., Tian, J., Ni, J., Wei, M., Li, T., & Shi, J. (2023). Peripheral Blood and Cerebrospinal Fluid Levels of YKL-40 in Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Brain Sciences, 13(10), 1364. https://doi.org/10.3390/brainsci13101364








