Microstructural Changes in the Spinothalamic Tract of CPSS Patients: Preliminary Results from a Single-Center Diffusion-Weighted Magnetic Resonance Imaging Study
Abstract
:1. Introduction
2. Methods
2.1. Ethical Approval
2.2. Study Population
2.3. MRI Protocol
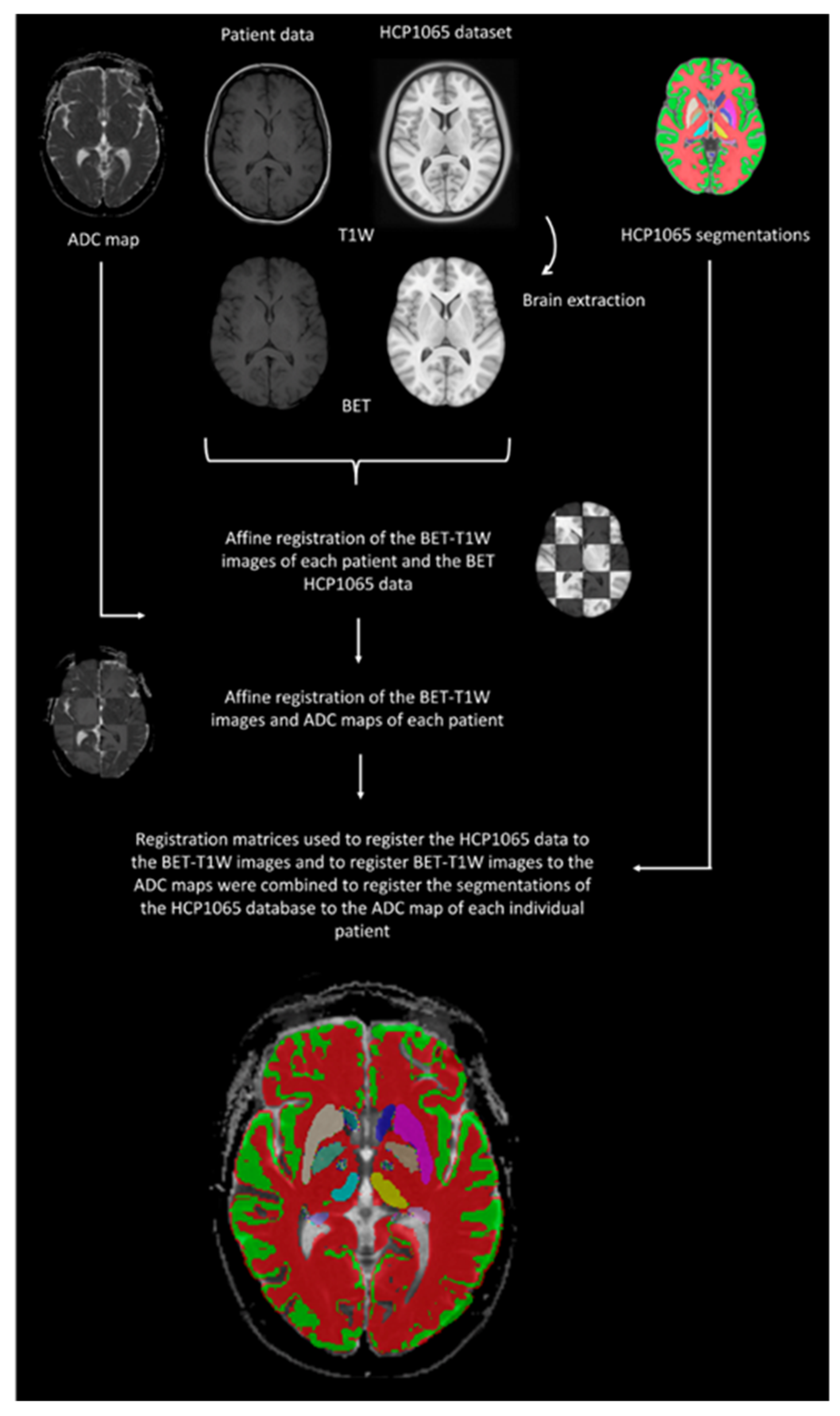
2.4. Data Processing
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schug, S.A.; Lavand’Homme, P.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.-D.; IASP Taskforce for the Classification of Chronic Pain. The IASP classification of chronic pain for ICD-11: Chronic postsurgical or posttraumatic pain. Pain 2019, 160, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Thomson, S. Failed back surgery syndrome—definition, epidemiology and demographics. Br. J. Pain 2013, 7, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Christelis, N.; Simpson, B.; Russo, M.; Stanton-Hicks, M.; Barolat, G.; Thomson, S.; Schug, S.; Baron, R.; Buchser, E.; Carr, D.B.; et al. Persistent Spinal Pain Syndrome: A Proposal for Failed Back Surgery Syndrome and ICD-11. Pain Med. 2021, 22, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Manca, A.; Eldabe, S.; Buchser, E.; Kumar, K.; Taylor, R.S. Relationship between health-related quality of life, pain, and functional disability in neuropathic pain patients with failed back surgery syndrome. Value Health 2010, 13, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Thomson, S.; Jacques, L. Demographic characteristics of patients with severe neuropathic pain secondary to failed back surgery syndrome. Pain Pract. 2009, 9, 206–215. [Google Scholar] [CrossRef]
- Baber, Z.; Erdek, M.A. Failed back surgery syndrome: Current perspectives. J. Pain Res. 2016, 9, 979–987. [Google Scholar] [CrossRef]
- Chan, C.W.; Peng, P. Failed back surgery syndrome. Pain Med. 2011, 12, 577–606. [Google Scholar] [CrossRef]
- Amirdelfan, K.; Webster, L.; Poree, L.; Sukul, V.; McRoberts, P. Treatment Options for Failed Back Surgery Syndrome Patients with Refractory Chronic Pain: An Evidence Based Approach. Spine 2017, 42, S41–S52. [Google Scholar] [CrossRef]
- Grider, J.S.; Manchikanti, L.; Carayannopoulos, A.; Sharma, M.L.; Balog, C.C.; E Harned, M.; Grami, V.; Justiz, R.; Nouri, K.H.; Hayek, S.M.; et al. Effectiveness of Spinal Cord Stimulation in Chronic Spinal Pain: A Systematic Review. Pain Physician 2016, 19, E33–E54. [Google Scholar] [CrossRef]
- Beaulieu, C. The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed. 2002, 15, 435–455. [Google Scholar] [CrossRef]
- Sener, R.N. Sener Diffusion MRI: Apparent diffusion coefficient (ADC) values in the normal brain and a classification of brain disorders based on ADC values. Comput. Med Imaging Graph. 2001, 25, 299–326. [Google Scholar] [CrossRef] [PubMed]
- Le Bihan, D. Apparent diffusion coefficient and beyond: What diffusion MR imaging can tell us about tissue structure. Radiology 2013, 268, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Goyal, M.; Al Azri, F.; Lum, C.; Higaki, T.; Nakamura, Y.; Tatsugami, F.; Kaichi, Y.; Akagi, M.; Akiyama, Y.; et al. State-of-the-art imaging of acute stroke. Radiographics 2006, 26 (Suppl. 1), S75–S95. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.M.; Caylor, J.; Strigo, I.; Lerman, I.; Henry, B.; Lopez, E.; Wallace, M.S.; Ellis, R.J.; Simmons, A.N.; Keltner, J.R. Toward Composite Pain Biomarkers of Neuropathic Pain-Focus on Peripheral Neuropathic Pain. Front. Pain Res. 2022, 3, 869215. [Google Scholar] [CrossRef]
- Tracey, I.; Woolf, C.J.; Andrews, N.A. Composite Pain Biomarker Signatures for Objective Assessment and Effective Treatment. Neuron 2019, 101, 783–800. [Google Scholar] [CrossRef]
- Sisignano, M.; Lötsch, J.; Parnham, M.J.; Geisslinger, G. Potential biomarkers for persistent and neuropathic pain therapy. Pharmacol. Ther. 2019, 199, 16–29. [Google Scholar] [CrossRef]
- Mackey, S.; Greely, H.T.; Martucci, K.T. Neuroimaging-based pain biomarkers: Definitions, clinical and research applications, and evaluation frameworks to achieve personalized pain medicine. Pain Rep. 2019, 4, e762. [Google Scholar] [CrossRef]
- van der Miesen, M.M.; Lindquist, M.A.; Wager, T.D. Neuroimaging-based biomarkers for pain: State of the field and current directions. Pain Rep. 2019, 4, e751. [Google Scholar] [CrossRef]
- Wager, T.D.; Atlas, L.Y.; Lindquist, M.A.; Roy, M.; Woo, C.W.; Kross, E. An fMRI-based neurologic signature of physical pain. N. Engl. J. Med. 2013, 368, 1388–1397. [Google Scholar] [CrossRef]
- Maarrawi, J.; Peyron, R.; Mertens, P.; Costes, N.; Magnin, M.; Sindou, M.; Laurent, B.; Garcia-Larrea, L. Motor cortex stimulation for pain control induces changes in the endogenous opioid system. Neurology 2007, 69, 827–834. [Google Scholar] [CrossRef]
- Smith, S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002, 17, 143–155. [Google Scholar] [CrossRef]
- Mazziotta, J.; Toga, A.; Evans, A.; Fox, P.; Lancaster, J.; Zilles, K.; Woods, R.; Paus, T.; Simpson, G.; Pike, B.; et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2001, 356, 1293–1322. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, J.C.; Toga, A.W.; Evans, A.; Fox, P.; Lancaster, J. A probabilistic atlas of the human brain: Theory and rationale for its development. The International Consortium for Brain Mapping (ICBM). NeuroImage 1995, 2, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.L.; Lee, J.E.; Lazar, M.; Field, A.S. Diffusion tensor imaging of the brain. Neurotherapeutics 2007, 4, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Kwon, S.; Chang, M.C. The Usefulness of Diffusion Tensor Tractography in Diagnosing Neuropathic Pain: A Narrative Review. Front. Neurosci. 2021, 15, 591018. [Google Scholar] [CrossRef]
- Yeo, S.S.; Jang, S.H.; Lee, J. Central post-stroke pain due to injury of the spinothalamic tract in patients with cerebral infarction: A diffusion tensor tractography imaging study. Neural Regen. Res. 2017, 12, 2021–2024. [Google Scholar] [CrossRef]
- Jang, S.H.; Seo, J.P.; Lee, S.J. Diffusion Tensor Tractography Studies of Central Post-stroke Pain Due to the Spinothalamic Tract Injury: A Mini-Review. Front. Neurol. 2019, 10, 787. [Google Scholar] [CrossRef]
- Hong, J.H.; Bai, D.S.; Jeong, J.Y.; Choi, B.Y.; Chang, C.H.; Kim, S.H.; Ahn, S.H.; Jang, S.H. Injury of the Spino-Thalamo-Cortical Pathway Is Necessary for Central Post-Stroke Pain. Eur. Neurol. 2010, 64, 163–168. [Google Scholar] [CrossRef]
- Yoon, E.J.; Kim, Y.K.; Shin, H.I.; Lee, Y.; Kim, S.E. Cortical and white matter alterations in patients with neuropathic pain after spinal cord injury. Brain Res. 2013, 1540, 64–73. [Google Scholar] [CrossRef]
- Edgar, J.M.; Griffiths, I.R. White Matter Structure: A Microscopist’s View. In Diffusion Mri: From Quantitative Measurement to In Vivo Neuroanatomy; Academic Press: Cambridge, MA, USA, 2009; pp. 75–103. [Google Scholar]
- Gustin, S.M.; Wrigley, P.J.; Siddall, P.J.; Henderson, L.A. Brain anatomy changes associated with persistent neuropathic pain following spinal cord injury. Cereb. Cortex 2010, 20, 1409–1419. [Google Scholar] [CrossRef]
- Keenan, K.E.; Peskin, A.P.; Wilmes, L.J.; Aliu, S.O.; Jones, E.F.; Li, W.; Kornak, J.; Newitt, D.C.; Hylton, N.M. Variability and bias assessment in breast ADC measurement across multiple systems. J. Magn. Reson. Imaging 2016, 44, 846–855. [Google Scholar] [CrossRef] [PubMed]
- deSouza, N.M.; Winfield, J.M.; Waterton, J.C.; Weller, A.; Papoutsaki, M.-V.; Doran, S.J.; Collins, D.J.; Fournier, L.; Sullivan, D.; Chenevert, T.; et al. Implementing diffusion-weighted MRI for body imaging in prospective multicentre trials: Current considerations and future perspectives. Eur. Radiol. 2018, 28, 1118–1131. [Google Scholar] [CrossRef] [PubMed]
| CPSS Group (Mean ± SD) | Healthy Controls (Mean ± SD) | p-Value | |
|---|---|---|---|
| White matter | 966.2 ± 71.7 | 1005.6 ± 105.8 | 0.227 |
| Gray matter (cortical) | 1090 ± 87.4 | 1163.4 ± 123.3 | 0.061 |
| Cerebellum | 874.6 ± 84 | 862.4 ± 76.4 | 0.671 |
| Thalamus | 983 ± 218.3 | 896.4 ± 139.1 | 0.191 |
| Putamen | 815.1 ± 45.3 | 835.3 ± 101.5 | 0.474 |
| Globus pallidus externa | 949.9 ± 94.3 | 997.2 ± 163.9 | 0.325 |
| Globus pallidus interna | 1071.9 ± 183.1 | 1138.2 ± 242.7 | 0.390 |
| Caudate nucleus | 1262.6 ± 422.4 | 1320.5 ± 500.8 | 0.726 |
| Amygdala | 950.4 ± 94.9 | 952.4 ± 169.4 | 0.969 |
| Hippocampus | 969.9 ± 88.4 | 952.7 ± 135.3 | 0.674 |
| Precentral gyrus | 1109.8 ± 62.7 | 1145.5 ± 119.2 | 0.301 |
| Postcentral gyrus | 1127 ± 71.3 | 1155.2 ± 128.4 | 0.449 |
| Insula | 1035.1 ± 110.6 | 1040 ± 139.8 | 0.912 |
| Corticothalamic tract | 901.7 ± 95.6 | 893.3 ± 110.9 | 0.820 |
| Spinothalamic tract | 922.8 ± 130.9 | 826.6 ± 52.2 | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witkam, R.L.; Burmeister, L.S.; Van Goethem, J.W.M.; van der Kolk, A.G.; Vissers, K.C.P.; Henssen, D.J.H.A. Microstructural Changes in the Spinothalamic Tract of CPSS Patients: Preliminary Results from a Single-Center Diffusion-Weighted Magnetic Resonance Imaging Study. Brain Sci. 2023, 13, 1370. https://doi.org/10.3390/brainsci13101370
Witkam RL, Burmeister LS, Van Goethem JWM, van der Kolk AG, Vissers KCP, Henssen DJHA. Microstructural Changes in the Spinothalamic Tract of CPSS Patients: Preliminary Results from a Single-Center Diffusion-Weighted Magnetic Resonance Imaging Study. Brain Sciences. 2023; 13(10):1370. https://doi.org/10.3390/brainsci13101370
Chicago/Turabian StyleWitkam, Richard L., Lara S. Burmeister, Johan W. M. Van Goethem, Anja G. van der Kolk, Kris C. P. Vissers, and Dylan J. H. A. Henssen. 2023. "Microstructural Changes in the Spinothalamic Tract of CPSS Patients: Preliminary Results from a Single-Center Diffusion-Weighted Magnetic Resonance Imaging Study" Brain Sciences 13, no. 10: 1370. https://doi.org/10.3390/brainsci13101370
APA StyleWitkam, R. L., Burmeister, L. S., Van Goethem, J. W. M., van der Kolk, A. G., Vissers, K. C. P., & Henssen, D. J. H. A. (2023). Microstructural Changes in the Spinothalamic Tract of CPSS Patients: Preliminary Results from a Single-Center Diffusion-Weighted Magnetic Resonance Imaging Study. Brain Sciences, 13(10), 1370. https://doi.org/10.3390/brainsci13101370






