Modified Vertical Parasagittal Sub-Insular Hemispherotomy—Case Series and Technical Note
Abstract
:1. Introduction
1.1. Historical Context
1.2. Technical Evolution
1.3. Goal of this Study
2. Materials and Methods
2.1. Population and Data Collection
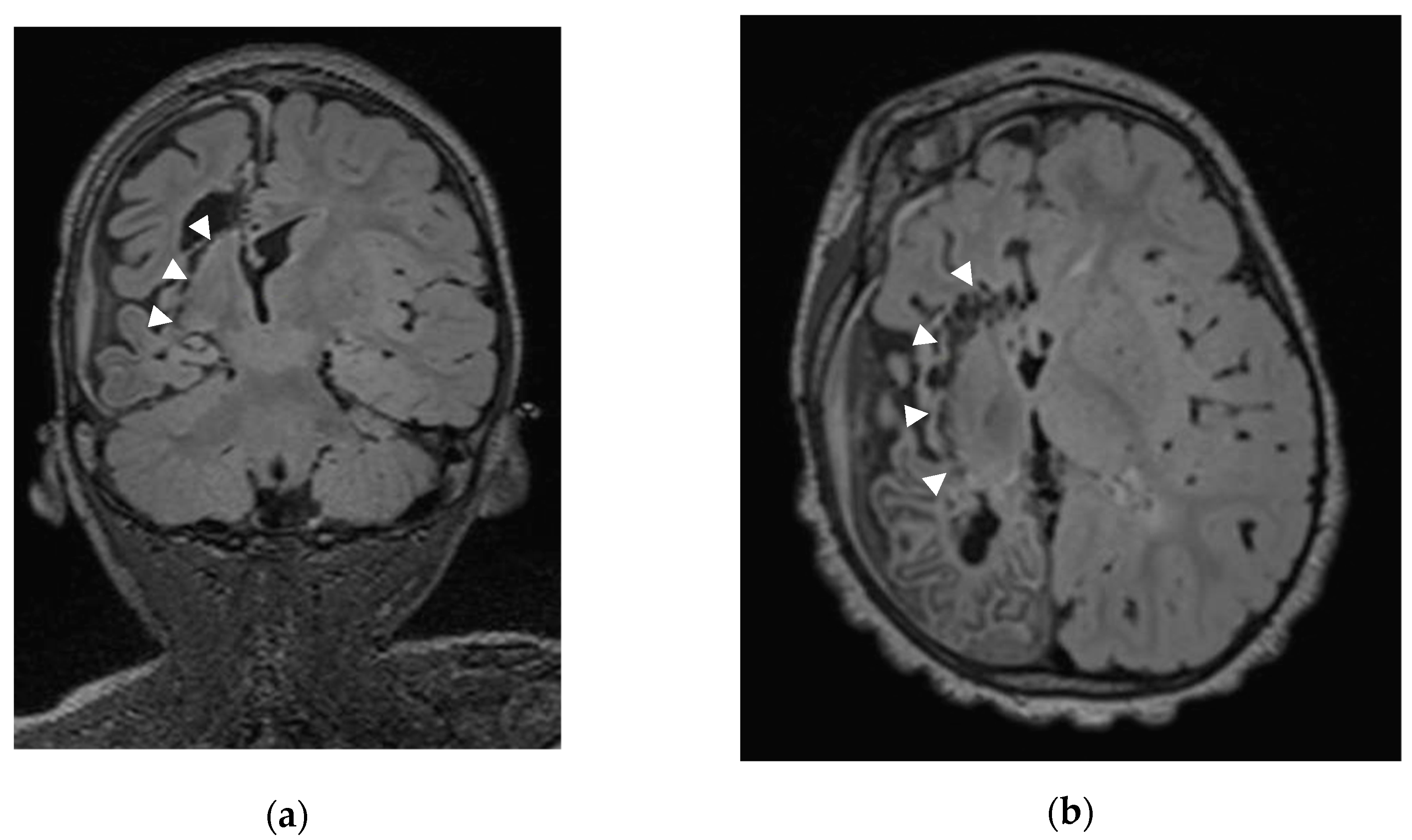
2.2. Sub-Insular VPH Method Description (Figure 1)

2.3. Outcome
2.4. Statistical Analysis
2.5. Ethics
3. Results
3.1. Demographic Data and Clinical Findings
Medical History
3.2. Surgical Procedure and Postoperative Course (Table 3)
| Case No | Sex | Age at (y) | Side | Etiology | Complications | 2nd Surgery | FUp (y) | mRS | Sz Outcome (Engel) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Onset | Surgery | Cong | Ac | Prog | BTF (mL) | HCP | APOS | AF | |||||||
| 1 | F | 6.6 | 6.8 | R | + | 0 | - | + | 14.91 | 1 | I | ||||
| 2 | M | 7.4 | 8.9 | R | + | 0 | - | + | - | 13.59 | 1 | I | |||
| 3 | F | 3.2 | 5.7 | R | + | 100 | - | + | + | + | 11.77 | 5 | IV | ||
| 4 | M | 0 | 6.8 | L | + | 0 | - | - | 9.72 | 2 | I | ||||
| 5 | M | 9.6 | 12.7 | R | + | 0 | + | + | 9.96 | 3 | II | ||||
| 6 | M | 3.2 | 5.5 | R | + | 0 | - | - | 7.32 | 3 | I | ||||
| 7 | M | 0.1 | 12.8 | R | + | 0 | - | - | 10.04 | 3 | I | ||||
| 8 | M | 1 | 22.1 | L | + | 0 | - | + | - | 9.71 | 3 | I | |||
| 9 | M | 1.8 | 10 | R | + | 0 | - | + | 7.74 | 3 | I | ||||
| 10 | F | 3 | 15.8 | L | + | 0 | - | + | 4.49 | NA | II | ||||
| 11 | F | 0.3 | 9.7 | R | + | 0 | - | - | 7.6 | 3 | I | ||||
| 12 | F | 1.5 | 4.9 | L | + | 180 | - | + | 7.02 | 2 | I | ||||
| 13 | M | 3 | 5.5 | L | + | 0 | - | + | 6.86 | 3 | I | ||||
| 14 | F | 3.7 | 4.2 | R | + | 0 | + | - | + | 6.2 | 2 | I | |||
| 15 | M | 1.3 | 6.9 | R | + | 0 | - | - | 4.31 | 3 | I | ||||
| 16 | F | 6 | 7.4 | R | + | 0 | - | - | + | 5.15 | 2 | I | |||
| 17 | F | 4.6 | 5.5 | R | + | 0 | - | + | 4.6 | 3 | I | ||||
| 18 | M | 0.3 | 1.5 | L | + | 90 | - | - | 4.95 | 3 | I | ||||
| 19 | M | 0 | 1.6 | R | + | 0 | - | + | + | 4.93 | 4 | I | |||
| 20 | F | 3 | 4.8 | L | + | + | - | + | 4.82 | 4 | I | ||||
| 21 | F | 0.17 | 2.38 | R | + | 0 | - | - | 3.95 | 2 | I | ||||
| 22 | M | 0.75 | 7.19 | R | + | 0 | - | - | 3.33 | 3 | I | ||||
| 23 | F | 5.98 | 10.51 | L | + | 0 | - | + | 0.32 | 3 | I | ||||
| 24 | M | 0 | 0.16 | R | + | 100 | - | + | - | + | 0.16 | NA | III | ||
| 25 | M | 0.5 | 3.28 | R | + | 0 | - | + | - | 0.08 | 4 | I | |||
| M/F | 1.27 | ||||||||||||||
| Mean | 2.68 | 7.3 | 6.5 | 2.8 | |||||||||||
| Range | 9.6–0 | 22.1–0.16 | 14.9–0.08 | ||||||||||||
3.2.1. Acute Postoperative Seizures
3.2.2. Hydrocephalus and Shunting
3.3. Seizure Outcome
- Patient 3, with Rasmussen encephalitis, was seizure-free for 10 months after the first VPH before recurrence of catastrophic status epilepticus. Postoperative MRI showed suspected persistence of a callosal connection. We performed a second surgery for completion of the hemispherotomy. Unfortunately, the patient’s seizures did not improve despite radiological confirmation of complete disconnection.
- Patient 5 developed epilepsy after severe head trauma that required decompressive craniectomy. He was seizure-free for two years after VPH and then presented recurrent spasms despite complete disconnection on MRI.
- Patient 10 still suffered from morpheic seizures after surgery, but her last video-EEG showed a bilateralization of the epileptic foci.
| Author, yr | N | Etiologies (%) | Mean Age at | Complications | Mean FUp (yr) | Sz Outcome (Engel at Last FUp) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cong | Acq | Prog | Onset (yr) | Surgery (yr) | Mty | HCP | BTF | APOS | Other | I | II | III | IV | ||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | ||||||||
| Delalande, 2007 ¹ [17] | 83 | 40 (48) | 18 (21) | 25 (30) | 2.1 | 8.0 | 3 | 3.6 | 12 | 14.5 | 6 | 7.2 | NA | NA | 2 | 2.4 | 4.4 | 60 | 72.3 | 10 | 12.0 | 9 | 10.8 | 2 | 2.4 |
| Honda, 2013 [23] | 12 | 12 (100) 2 | 0 | 0 | 0.05 | 0.36 | 0 | 0.0 | 1 | 8.3 | 12 | 100.0 | NA | NA | 0 | 0.0 | 6.5 | 8 | 66.7 | 0 | 0.0 | 1 | 8.3 | 3 | 25.0 |
| Dorfer, 2013 3 [24] | 37 | 13 (32) | 26 (65) | 1 (2) | 1.2 | 5.5 | 1 | 2.7 | 1 ⁴ | 2.7 | 2 | 5.4 | NA | NA | 0 | 0.0 | 37 | 34 | 91.9 | 0 | 0.0 | 0 | 0.0 | 3 | 8.1 |
| Kawai, 2014 [25] | 7 | 4 (57) | 3 (43) | 0 | 2.1 | 14.8 | 0 | 0.0 | 0 | 0.0 | NA | NA | 0 | 0.0 | 0 | 0.0 | 3.1 | 6 | 85.7 | 0 | 0.0 | 0 | 0.0 | 1 | 14.3 |
| Panigrahi, 2016 [26] | 16 | 1 (6) | 10 (62) | 5 (31) | 2.9 | 6.5 | 0 | 0.0 | 1 | 6.3 | NA | NA | 4 | 25.0 | NA | NA | 2.2 | 15 | 93.8 | NA | NA | NA | NA | NA | NA |
| Fohlen, 2019 [27] | 18 | 18 (100) | 0 | 0 | 2 | 7.2 | 0 | 0.0 | 2 | 11.1 | NA | NA | NA | NA | 1 | 5.6 | 12.8 | 16 | 88.9 | 2 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Saint-Luc, 2023 | 23 | 5 | 13 | 4 | 2.9 | 7.8 | 0 | 0.0 | 2 | 8.7 | 4 | 17.4 | 4 | 17.4 | 4 ⁵ | 17 | 6.81 | 20 | 86.9 | 2 | 8.7 | 0 | 0.0 | 1 | 4.3 |
3.4. Cognitive Outcome
4. Discussion
4.1. Elegance of the Vertical Parasagittal Hemispherotomy (VPH)
4.2. Gelfoam Plug into the Foramen of Monro
4.3. Sub-Insular Disconnection with LN Preservation
4.4. Acute Postoperative Seizures (APOSs)
4.5. VPH: Literature Review and Comparison with Our Series (Table 4)
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alotaibi, F.; Albaradie, R.; Almubarak, S.; Baeesa, S.; Steven, D.A.; Girvin, J.P. Hemispherotomy for Epilepsy: The Procedure Evolution and Outcome. Can. J. Neurol. Sci./J. Can. Des Sci. Neurol. 2021, 48, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Dorfer, C.; Khalaveh, F.; Dorfmüller, G.; Czech, T. Surgical Anatomy of Vertical Perithalamic Hemispherotomy. Oper. Neurosurg. 2020, 18, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Dandy, W.E. Dandy Removal of Right Cerebral Hemisphere for Certain Tumors with Hemiplegia: Preliminary Report. JAMA 1928, 90, 823–825. [Google Scholar] [CrossRef]
- Krynauw, R.A. Infantile hemiplegia treated by removing one cerebral hemisphere. J. Neurol. Neurosurg. Psychiatry 1950, 13, 243–267. [Google Scholar] [CrossRef]
- Rasmussen, T. Hemispherectomy for Seizures Revisited. Can. J. Neurol. Sci./J. Can. Des Sci. Neurol. 1983, 10, 71–78. [Google Scholar] [CrossRef]
- Laine, E.; Pruvot, P.; Osson, D. Ultimate Results of Hemispherectomy in Cases on Infantile Cerebral Hemiatrophy Productive of Epilepsy. Neurochirurgie 1964, 10, 507–522. [Google Scholar]
- Noetzel Mi, V.H. Diffusion Yon Blutfarbstoff in Der Inneren Rantlzone Unr Iiul~eren 0berfliiehe Des Zentralnervensystems Bei Subarachnoidaler Blutung. Arch. f Psychiatr. 1940, 111, 129–138. [Google Scholar] [CrossRef]
- Delalande, O.P. Hemispherotomy: A New Procedure for Central Disconnection. Epilepsia 1992, 33, 99–100. [Google Scholar]
- Morino, M.; Shimizu, H.; Ohata, K.; Tanaka, K.; Hara, M. Anatomical Analysis of Different Hemispherotomy Procedures Based on Dissection of Cadaveric Brains. J. Neurosurg. 2002, 97, 423–431. [Google Scholar] [CrossRef]
- Nieuwenhuys, R.; Voogd, J.; Huijzen, C. The Human Central Nervous System; Steinkopff: Heidelberg, Germany, 2008. [Google Scholar]
- Türe, U.; Yaşargil, D.C.H.; Al-Mefty, O.; Yaşargil, M.G. Topographic Anatomy of the Insular Region. J. Neurosurg. 1999, 90, 720–733. [Google Scholar] [CrossRef]
- Blanchet, B.; Roland, J.; Braun, M.; Anxionnat, R.; Moret, C.; Picard, L. The Anatomy and the MRI Anatomy of the Interhemispheric Cerebral Commissures. J. Neuroradiol. 1995, 22, 237–251. [Google Scholar] [PubMed]
- Schramm, J.; Behrens, E.; Entzian, W. Hemispherical Deafferentation. Neurosurgery 1995, 36, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Villemure, J.-G.; Mascott, C.R. Peri-Insular Hemispherotomy. Neurosurgery 1995, 37, 975–980. [Google Scholar] [CrossRef]
- Shimizu, H.; Maehara, T. Modification of Peri-Insular Hemispherotomy and Surgical Results. Neurosurgery 2000, 47, 367–373. [Google Scholar] [CrossRef]
- Hmaimess, G.; Raftopoulos, C.; Kadhim, H.; Nassogne, M.-C.; Ghariani, S.; de Tourtchaninoff, M.; van Rijckevorsel, K. Impact of Early Hemispherotomy in a Case of Ohtahara Syndrome with Left Parieto-Occipital Megalencephaly. Seizure 2005, 14, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Delalande, O.; Bulteau, C.; Dellatolas, G.; Fohlen, M.; Jalin, C.; Buret, V.; Viguier, D.; Dorfmüller, G.; Jambaqué, I. Vertical parasagittal hemispherotomy. Oper. Neurosurg. 2007, 60, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Delalande, O.; Dorfmüller, G. Hémisphérotomie Verticale Parasagittale: Technique Opératoire. Neurochirurgie 2008, 54, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Cossu, M.; Nichelatti, M.; De Benedictis, A.; Rizzi, M. Lateral versus Vertical Hemispheric Disconnection for Epilepsy: A Systematic Review and Meta-Analysis. J. Neurosurg. 2022, 136, 1627–1637. [Google Scholar] [CrossRef]
- Ramantani, G.; Bulteau, C.; Cserpan, D.; Otte, W.M.; Dorfmüller, G.; Cross, J.H.; Zentner, J.; Tisdall, M.; Braun, K.P.J. Not Surgical Technique, but Etiology, Contralateral MRI, Prior Surgery, and Side of Surgery Determine Seizure Outcome after Pediatric Hemispherotomy. Epilepsia 2023, 64, 1214–1224. [Google Scholar] [CrossRef]
- Engel, J.V.N.P.; Rasmussen, T.B.; Ojemann, L.M. Outcome with Respect to Epileptic Seizures, 2nd ed.; Raven Press: New York, NY, USA, 1993. [Google Scholar]
- Baltus, C.; El M’Kaddem, B.; Ferrao Santos, S.; Ribeiro Vaz, J.G.; Raftopoulos, C. Second Surgery after Vertical Paramedian Hemispherotomy for Epilepsy Recurrence. Heliyon 2023, 9, e14326. [Google Scholar] [CrossRef]
- Honda, R.; Kaido, T.; Sugai, K.; Takahashi, A.; Kaneko, Y.; Nakagwa, E.; Sasaki, M.; Otsuki, T. Long-Term Developmental Outcome after Early Hemispherotomy for Hemimegalencephaly in Infants with Epileptic Encephalopathy. Epilepsy Behav. 2013, 29, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Dorfer, C.; Czech, T.; Dressler, A.; Gröppel, G.; Mühlebner-Fahrngruber, A.; Novak, K.; Reinprecht, A.; Reiter-Fink, E.; Traub-Weidinger, T.; Feucht, M. Vertical Perithalamic Hemispherotomy: A Single-Center Experience in 40 Pediatric Patients with Epilepsy. Epilepsia 2013, 54, 1905–1912. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Morino, M.; Iwasaki, M. Modification of Vertical Hemispherotomy for Refractory Epilepsy. Brain Dev. 2014, 36, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, M.; Krishnan, S.S.; Vooturi, S.; Vadapalli, R.; Somayajula, S.; Jayalakshmi, S. An Observational Study on Outcome of Hemispherotomy in Children with Refractory Epilepsy. Int. J. Surg. 2016, 36, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Fohlen, M.; Dorfmüller, G.; Ferrand-Sorbets, S.; Dorison, N.; Chipaux, M.; Taussig, D. Parasagittal Hemispherotomy in Hemispheric Polymicrogyria with Electrical Status Epilepticus during Slow Sleep: Indications, Results and Follow-Up. Seizure 2019, 71, 190–200. [Google Scholar] [CrossRef]
- Bulteau, C.; Otsuki, T.; Delalande, O. Epilepsy Surgery for Hemispheric Syndromes in Infants: Hemimegalencepahly and Hemispheric Cortical Dysplasia. Brain Dev. 2013, 35, 742–747. [Google Scholar] [CrossRef] [PubMed]
- de Palma, L.; Pietrafusa, N.; Gozzo, F.; Barba, C.; Carfi-Pavia, G.; Cossu, M.; De Benedictis, A.; Genitori, L.; Giordano, F.; Lo Russo, G.; et al. Outcome after Hemispherotomy in Patients with Intractable Epilepsy: Comparison of Techniques in the Italian Experience. Epilepsy Behav. 2019, 93, 22–28. [Google Scholar] [CrossRef]
- Lew, S.M.; Koop, J.I.; Mueller, W.M.; Matthews, A.E.; Mallonee, J.C. Fifty Consecutive Hemispherectomies. Neurosurgery 2014, 74, 182–195. [Google Scholar] [CrossRef]
- Strowitzki, M.; Kiefer, M.; Steudel, W.I. Acute Hydrocephalus as a Late Complication of Hemispherectomy. Acta Neurochir 1994, 131, 253–259. [Google Scholar] [CrossRef]
- Greenberg, M.S. Greenberg’s Handbook of Neurosurgery, 10th ed.; Thieme: New York, NY, USA, 2023. [Google Scholar]
- Massicotte, E.M.; Del Bigio, M.R. Human Arachnoid Villi Response to Subarachnoid Hemorrhage: Possible Relationship to Chronic Hydrocephalus. J. Neurosurg. 1999, 91, 80–84. [Google Scholar] [CrossRef]
- Durieux, P.F.; Schiffmann, S.N.; d’Exaerde, A.d.K. Targeting Neuronal Populations of the Striatum. Front. Neuroanat. 2011, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Hikosaka, O. The Globus Pallidus Sends Reward-Related Signals to the Lateral Habenula. Neuron 2008, 60, 720–729. [Google Scholar] [CrossRef] [PubMed]
- van Schouwenburg, M.; Aarts, E.; Cools, R. Dopaminergic Modulation of Cognitive Control: Distinct Roles for the Prefrontal Cortex and the Basal Ganglia. Curr. Pharm. Des. 2010, 16, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Rahayel, S.; Postuma, R.B.; Montplaisir, J.; Génier Marchand, D.; Escudier, F.; Gaubert, M.; Bourgouin, P.-A.; Carrier, J.; Monchi, O.; Joubert, S.; et al. Cortical and Subcortical Gray Matter Bases of Cognitive Deficits in REM Sleep Behavior Disorder. Neurology 2018, 90, e1759–e1770. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Zhong, S.; Liao, X.; Wang, Y.; Huang, J.; Zhang, S.; Sun, Y.; Zhao, H.; Jia, Y. Biochemical Abnormalities in Basal Ganglia and Executive Dysfunction in Acute- and Euthymic-Episode Patients with Bipolar Disorder: A Proton Magnetic Resonance Spectroscopy Study. J. Affect. Disord. 2018, 225, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Delvecchio, G.; Pigoni, A.; Perlini, C.; Barillari, M.; Versace, A.; Ruggeri, M.; Altamura, A.C.; Bellani, M.; Brambilla, P. A Diffusion Weighted Imaging Study of Basal Ganglia in Schizophrenia. Int. J. Psychiatry Clin. Pract. 2018, 22, 6–12. [Google Scholar] [CrossRef]
- Falconer, M.A.; Serafetinides, E.A. A Follow-up Study of Surgery in Temporal Lobe Epilepsy. J. Neurol. Neurosurg. Psychiatry 1963, 26, 154–165. [Google Scholar] [CrossRef]
- Garcia, P.A.; Barbaro, N.M.; Laxer, K.D. The Prognostic Value of Postoperative Seizures Following Epilepsy Surgery. Neurology 1991, 41, 1511. [Google Scholar] [CrossRef]
- Malla, B.R.; O’Brien, T.J.; Cascino, G.D.; So, E.L.; Radhakrishnan, K.; Silbert, P.; Marsh, W.R. Acute Postoperative Seizures Following Anterior Temporal Lobectomy for Intractable Partial Epilepsy. J. Neurosurg. 1998, 89, 177–182. [Google Scholar] [CrossRef]
- Commission on Neurosurgery of the International League Against Epilepsy (ILAE); Wieser, H.G.; Blume, W.T.; Fish, D.; Goldensohn, E.; Hufnagel, A.; King, D.; Sperling, M.R.; Lüders, H.; Pedley, T.A.; et al. ILAE Commission Report. Proposal for a New Classification of Outcome with Respect to Epileptic Seizures Following Epilepsy Surgery. Epilepsia 2001, 42, 282–286. [Google Scholar] [CrossRef]
- Giridharan, N.; Horn, P.S.; Greiner, H.M.; Holland, K.D.; Mangano, F.T.; Arya, R. Acute Postoperative Seizures as Predictors of Seizure Outcomes after Epilepsy Surgery. Epilepsy Res. 2016, 127, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Mani, J.; Gupta, A.; Mascha, E.; Lachhwani, D.; Prakash, K.; Bingaman, W.; Wyllie, E. Postoperative Seizures after Extratemporal Resections and Hemispherectomy in Pediatric Epilepsy. Neurology 2006, 66, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Moosa, A.N.V.; Gupta, A.; Jehi, L.; Marashly, A.; Cosmo, G.; Lachhwani, D.; Wyllie, E.; Kotagal, P.; Bingaman, W. Longitudinal Seizure Outcome and Prognostic Predictors after Hemispherectomy in 170 Children. Neurology 2013, 80, 253–260. [Google Scholar] [CrossRef]
- Park, K.; Buchhalter, J.; McClelland, R.; Raffel, C. Frequency and Significance of Acute Postoperative Seizures Following Epilepsy Surgery in Children and Adolescents. Epilepsia 2002, 43, 874–881. [Google Scholar] [CrossRef]
- Koh, S.; Nguyen, S.; Asarnow, R.F.; LoPresti, C.; Yudovin, S.; Shields, W.D.; Vinters, H.V.; Mathern, G.W. Five or More Acute Postoperative Seizures Predict Hospital Course and Long-Term Seizure Control after Hemispherectomy. Epilepsia 2004, 45, 527–533. [Google Scholar] [CrossRef]
- Tripathi, M.; Chandra, P.; Padma, V.; Shailesh, G.; Chandreshekar, B.; Sarkar, C. Hemispherotomy for Intractable Epilepsy. Neurol. India 2008, 56, 127. [Google Scholar] [CrossRef]
- Arifin, M.T.; Muttaqin, Z.; Hanaya, R.; Bakhtiar, Y.; Bintoro, A.C.; Iida, K.; Kurisu, K.; Arita, K.; Andar, E.B.P.S.; Priambada, D.; et al. Hemispherotomy for Drug-Resistant Epilepsy in an Indonesian Population. Epilepsy Behav. Rep. 2019, 12, 100337. [Google Scholar] [CrossRef] [PubMed]



| Delalande | Raftopoulos | |
|---|---|---|
| Incision | 1/3 anterior and 2/3 posterior to the coronal suture | Slightly anterior to the coronal suture |
| First step | Splenium disconnection | Anterior corpus callosotomy |
| Subrostral resection | Resection of the posterior part of the gyrus rectus | Resection of the posterior part of the gyrus rectus, of the cingulum, and of Brodmann area 25 |
| Splenium disconnection | Until the roof of the third ventricle | Down to the great cerebral vein of Galen |
| Posterior disconnection | Posterior column of the fornix disconnection | Ventricular trigone floor (posterior column of fornix but also the intralimbic and limbic gyri) |
| Lateral disconnection | Lateral to the thalamus, going through the globus pallidus | Sub-insular trans-claustral |
| Temporal disconnection | Anterior part of the temporal horn resection | Piriform lobe resection |
| Overall (25) No. of Patients (%) or Mean | |
|---|---|
| Age at | |
| Onset | 2.7 |
| Surgery | 7.3 |
| Sex M/F | 14/11 |
| Etiology | |
| Congenital | 6 (24%) |
| Cortical dysplasia | 2 (8%) |
| Hemimegalencephaly | 3 (12%) |
| Sturge–Weber | 1 (4%) |
| Acquired | 16 (64%) |
| Stroke (ischemic/hemorrhagic) | 13 (52%) |
| Post-traumatic | 1 (4%) |
| Gliosis (post-tumor resection) | 1 (4%) |
| Progressive (Rasmussen) | 4 (16%) |
| Hemispherotomy side (R/L) | 17/8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Gaudio, N.; Ferrao Santos, S.; Raftopoulos, C. Modified Vertical Parasagittal Sub-Insular Hemispherotomy—Case Series and Technical Note. Brain Sci. 2023, 13, 1395. https://doi.org/10.3390/brainsci13101395
Del Gaudio N, Ferrao Santos S, Raftopoulos C. Modified Vertical Parasagittal Sub-Insular Hemispherotomy—Case Series and Technical Note. Brain Sciences. 2023; 13(10):1395. https://doi.org/10.3390/brainsci13101395
Chicago/Turabian StyleDel Gaudio, Nicole, Susana Ferrao Santos, and Christian Raftopoulos. 2023. "Modified Vertical Parasagittal Sub-Insular Hemispherotomy—Case Series and Technical Note" Brain Sciences 13, no. 10: 1395. https://doi.org/10.3390/brainsci13101395
APA StyleDel Gaudio, N., Ferrao Santos, S., & Raftopoulos, C. (2023). Modified Vertical Parasagittal Sub-Insular Hemispherotomy—Case Series and Technical Note. Brain Sciences, 13(10), 1395. https://doi.org/10.3390/brainsci13101395







