Feasibility of Acquiring Neuroimaging Data from Adults with Acquired Brain Injuries before and after a Yoga Intervention
Abstract
:1. Introduction
2. Materials and Methods
Measures
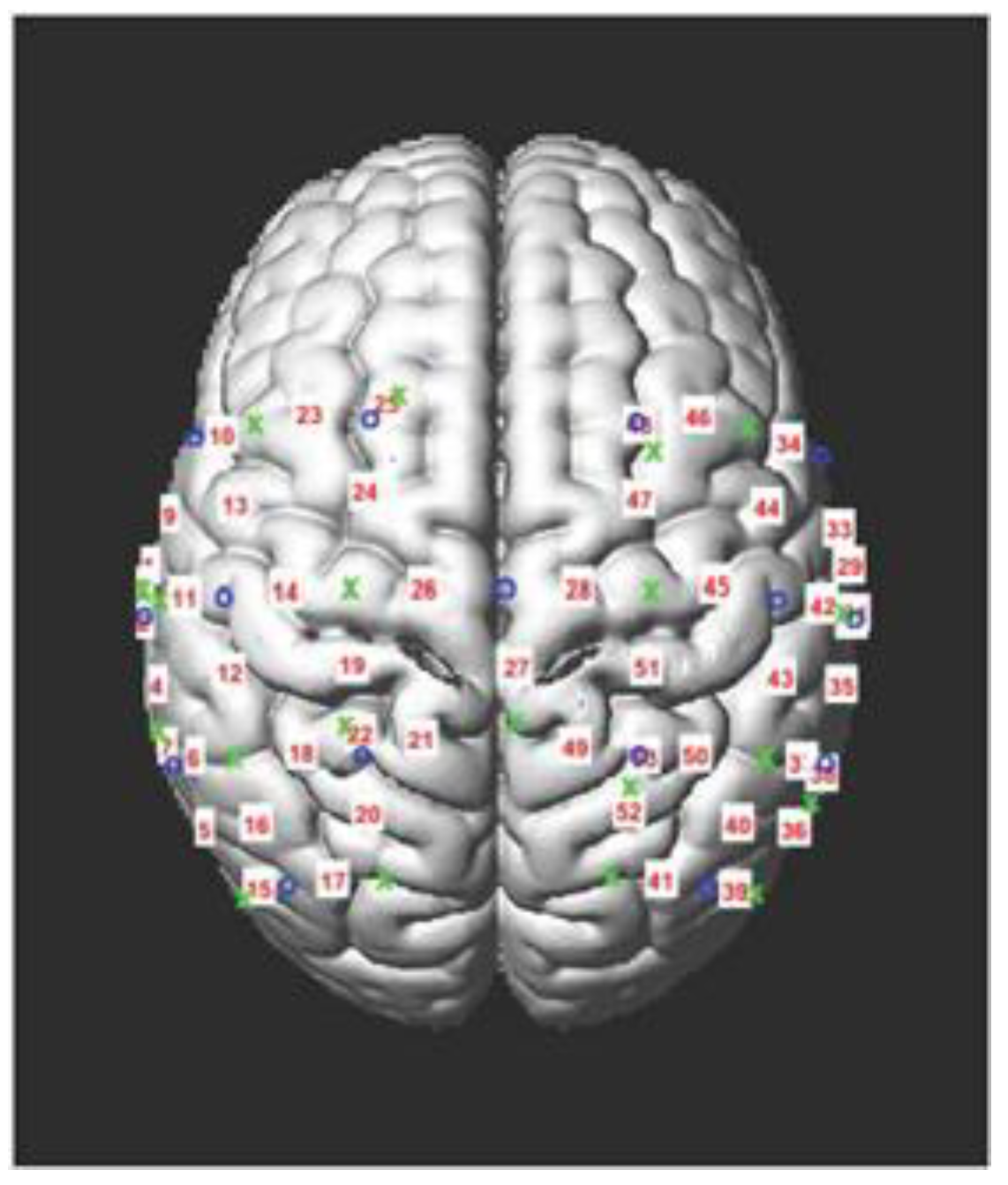
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Clinical Trial Registration
References
- Schiller, J.S.; Lucas, J.W.; Peregoy, J.A. Summary health statistics for U.S. Adults: National health interview survey, 2011. Vital Health Stat. 2012, 10, 1–218. [Google Scholar]
- Corrigan, J.D.; Hammond, F.M. Traumatic brain injury as a chronic health condition. Arch. Phys. Med. Rehabil. 2013, 94, 1199–1201. [Google Scholar] [CrossRef]
- Rutherford, G.W.; Corrigan, J.D. Long-term Consequences of Traumatic Brain Injury. J. Head Trauma Rehabil. 2009, 24, 421–423. [Google Scholar] [CrossRef]
- Whiteneck, G.G.; Cuthbert, J.P.; Corrigan, J.D.; Bogner, J.A. Prevalence of Self-Reported Lifetime History of Traumatic Brain Injury and Associated Disability: A Statewide Population-Based Survey. J. Head Trauma Rehabil. 2016, 31, E55–E62. [Google Scholar] [CrossRef]
- Wilson, L.; Stewart, W.; Dams-O’Connor, K.; Diaz-Arrastia, R.; Horton, L.; Menon, D.K.; Polinder, S. The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol. 2017, 16, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Klima, D.; Morgan, L.; Baylor, M.; Reilly, C.; Gladmon, D.; Davey, A. Physical Performance and Fall Risk in Persons With Traumatic Brain Injury. Percept. Mot. Ski. 2018, 126, 50–69. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, K.L.; Buxton, E.; Hackney, J.; Lowers, S. Balance, attention, and dual-task performance during walking after brain injury: Associations with falls history. J. Head Trauma Rehabil. 2010, 25, 155–163. [Google Scholar] [CrossRef]
- Perez, O.H.; Green, R.E.; Mochizuki, G. Characterization of Balance Control After Moderate to Severe Traumatic Brain Injury: A Longitudinal Recovery Study. Phys. Ther. 2018, 98, 786–795. [Google Scholar] [CrossRef]
- Schmid, A.A.; van Puymbroeck, M.; Koceja, D.M. Effect of a 12-Week Yoga Intervention on Fear of Falling and Balance in Older Adults: A Pilot Study. Arch. Phys. Med. Rehabil. 2010, 91, 576–583. [Google Scholar] [CrossRef]
- Schmid, A.A.; Van Puymbroeck, M.; Altenburger, P.A.; Schalk, N.L.; Dierks, T.A.; Miller, K.K.; Damush, T.M.; Bravata, D.M.; Williams, L.S. Poststroke balance improves with yoga: A pilot study. Stroke 2012, 43, 2402–2407. [Google Scholar] [CrossRef]
- Stephens, J.; Van Puymbroeck, M.; Sample, P.; Schmid, A. Yoga improves balance, mobility, and perceived occupational performance in adults with chronic brain injury: A preliminary investigation. Complement. Ther. Clin. Pract. 2020, 40, 101172. [Google Scholar] [CrossRef] [PubMed]
- Grimm, L.A.; Van Puymbroeck, M.; Miller, K.K.; Fisher, T.; Schmid, A.A. Yoga after Traumatic Brain Injury: Changes in Emotional Regulation and Health-Related Quality of Life in a Case-Study. Int. J. Complement. Altern. Med. 2017, 8, 00247. [Google Scholar]
- Donnelly, K.Z.; Goldberg, S.; Fournier, D. A qualitative study of LoveYourBrain Yoga: A group-based yoga with psychoeducation intervention to facilitate community integration for people with traumatic brain injury and their caregivers. Disabil. Rehabil. 2019, 42, 2482–2491. [Google Scholar] [CrossRef]
- Schmid, A.A.; Miller, K.K.; Van Puymbroeck, M.; Schalk, N. Feasibility and results of a case study of yoga to improve physical functioning in people with chronic traumatic brain injury. Disabil. Rehabilitation 2015, 38, 914–920. [Google Scholar] [CrossRef]
- Gothe, N.P.; Khan, I.; Hayes, J.; Erlenbach, E.; Damoiseaux, J.S. Yoga Effects on Brain Health: A Systematic Review of the Current Literature. Brain Plast. 2019, 5, 105–122. [Google Scholar] [CrossRef]
- Telles, S.; Gupta, R.K.; Singh, N.; Balkrishna, A. A Functional Near-Infrared Spectroscopy Study of High-Frequency Yoga Breathing Compared to Breath Awareness. Med. Sci. Monit. Basic Res. 2016, 22, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Sharp, D.J.; Beckmann, C.F.; Greenwood, R.; Kinnunen, K.M.; Bonnelle, V.; De Boissezon, X.; Powell, J.H.; Counsell, S.J.; Patel, M.C.; Leech, R. Default mode network functional and structural connectivity after traumatic brain injury. Brain 2011, 134, 2233–2247. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-Q.; Xiao, H.; Yang, Y.; Xi, J.-H. Structural and functional connectivity in traumatic brain injury. Neural Regen. Res. 2015, 10, 2062–2071. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, M.; Peart, T.; Perrier, L.; Lauzon, A.; Munce, S. Psychological Interventions for Individuals With Acquired Brain Injury, Cerebral Palsy, and Spina Bifida: A Scoping Review. Front. Pediatr. 2022, 10, 782104. [Google Scholar] [CrossRef]
- Kersey, J.; Hammel, J.; Baum, C.; Huebert, K.; Malagari, E.; Terhorst, L.; McCue, M.; Skidmore, E.R. Effect of interventions on activity and participation outcomes for adults with brain injury: A scoping review. Brain Inj. 2022, 36, 21–31. [Google Scholar] [CrossRef]
- Dever, A.; Powell, D.; Graham, L.; Mason, R.; Das, J.; Marshall, S.J.; Vitorio, R.; Godfrey, A.; Stuart, S. Gait Impairment in Traumatic Brain Injury: A Systematic Review. Sensors 2022, 22, 1480. [Google Scholar] [CrossRef]
- Medaglia, J.D. Functional Neuroimaging in Traumatic Brain Injury: From Nodes to Networks. Front. Neurol. 2017, 8, 407. [Google Scholar] [CrossRef]
- Pinti, P.; Aichelburg, C.; Gilbert, S.; Hamilton, A.; Hirsch, J.; Burgess, P.; Tachtsidis, I. A Review on the Use of Wearable Functional Near-Infrared Spectroscopy in Naturalistic Environments. Jpn. Psychol. Res. 2018, 60, 347–373. [Google Scholar] [CrossRef]
- Cox, R.W. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Comput. Biomed. Res. 1996, 29, 162–173. [Google Scholar] [CrossRef]
- Hariri, A.; Davis, C.; Jd, P.; Ka, B.; Az, S.; Bl, S.; Se, P. Faculty of 1000 evaluation for Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012, 59, 2142–2154. [Google Scholar]
- Surgent, O.J.; Dadalko, O.I.; Pickett, K.A.; Travers, B.G. Balance and the brain: A review of structural brain correlates of postural balance and balance training in humans. Gait Posture 2019, 71, 245–252. [Google Scholar] [CrossRef]
- Berg, K. Measuring balance in the elderly: Preliminary development of an instrument. Physiother. Can. 1989, 41, 304–311. [Google Scholar] [CrossRef]
- Feld, J.A.; Rabadi, M.H.; Blau, A.D.; Jordan, B.D. Berg Balance Scale and Outcome Measures in Acquired Brain Injury. Neurorehabilit. Neural Repair 2001, 15, 239–244. [Google Scholar] [CrossRef]
- Peirce, J.; Gray, J.R.; Simpson, S.; MacAskill, M.; Höchenberger, R.; Sogo, H.; Kastman, E.; Lindeløv, J.K. PsychoPy2: Experiments in behavior made easy. Behav. Res. Methods 2019, 51, 195–203. [Google Scholar] [CrossRef]
- Keith, R.A.; Granger, C.V.; Hamilton, B.B.; Sherwin, F.S. The functional independence measure: A new tool for rehabilitation. Adv. Clin. Rehabil. 1987, 1, 6–18. [Google Scholar] [PubMed]
- Zhao, J.; Zhang, T.; Xu, J.; Wang, M.; Zhao, S. Functional magnetic resonance imaging evaluation of brain function reorganization in cerebral stroke patients after constraint-induced movement therapy. Neural Regen. Res. 2012, 7, 1158–1163. [Google Scholar] [PubMed]
- Hall, K.M.; Mann, N.; High, W.M.J.; Wright, J.B.; Kreutzer, J.S.; Wood, D. Functional Measures After Traumatic Brain Injury: Ceiling Effects of FIM, FIM+FAM, DRS, and CIQ. J. Head Trauma Rehabil. 1996, 11, 27–39. [Google Scholar] [CrossRef]
- Lee, C.-H.; Sun, T.-L. Evaluation of postural stability based on a force plate and inertial sensor during static balance measurements. J. Physiol. Anthr. 2018, 37, 27. [Google Scholar] [CrossRef] [PubMed]




| Feasibility Objective | Feasibility Benchmark | Study Outcome |
|---|---|---|
| Recruit and retain adults with ABI for a longitudinal hatha yoga intervention with neuroimaging. | Recruit 12 adults with ABI Retain ≥ 80% (~10 of 12) over the course of the study. | √ Recruited 12 adults X Retained 75% |
| Safely acquire rs-fMRI data in participants with mobility limitations. | Detect 100% MRI contraindications during screening. Sustain zero instances of adverse events (e.g., pain or significant anxiety) or falls during data acquisition. | √ Detected all contraindications √ Zero adverse events or falls |
| Pre-process rs-fMRI and demonstrate acceptable rs-fMRI data quality indices. | Achieve time series quality indices < 3.5 * MAD in ≥ 80% of data time points. Observe similar quality indices as achieved in a normative sample. Achieve acceptable FD in the majority (4/6) participants. | √ Quality indices were observed in 86.4–100% of data time points. When compared to a normative sample, quality indices z-scores did not exceed 95%. Acceptable FD was reached in 4/6 participants. |
| Produce a valid group-based connectivity map from the precentral gyrus seed. | Complete visual inspection of the group seed-based connectivity map for the precentral gyrus and confirm strong connections between the premotor areas and other brain regions associated with motor function. | √The connectivity map is valid as visual inspection shows graded, transitional areas of connection rather than random pixelation. |
| Safely acquire fNIRS data during concurrent balance testing in participants with mobility limitations. | Have zero instances of adverse events (e.g., pain or anxiety) or falls during data acquisition. | √ Zero adverse events or falls |
| Acquire high-quality fNIRS data and successfully pre-process those data | Reach acceptable signal optimization and quality levels in ≥ 80% of participants assessed. Successfully detect and remove motion artifacts from data in ≥ 80% of participants assessed. | √ Acceptable signal and quality in data and all motion artifacts detected and removed in 90.9% of participants pre-yoga and 100% post-yoga |
| Detect yoga-induced improvements in balance with a modified balance measure when balance is assessed with simultaneous fNIRS. | Show a statistically significant improvement in post-yoga balance compared to pre-yoga balance performance. | √ Significant improvement in balance was detected while using a modified balance, as group-level improvements were observed post-yoga, p = 0.043. |
| ID | Age at Study Onset | Sex | Education | Type of ABI | Time since ABI | # of Yoga Sessions |
|---|---|---|---|---|---|---|
| 1 | 74 | Female | Did Not Report | Stroke | 13 years | 8 |
| 2 | 55 | Male | Doctorate | Severe TBI | 2 years | 7 |
| 3 | 66 | Male | Some College | Stroke | 2 years | 8 |
| 4 | 29 | Female | High School | TBI (severity not reported) | 4.5 years | 2 |
| 5 | 56 | Male | Bachelor’s Degree | Anoxic Brain Injury | 8 years | 8 |
| 6 | 57 | Female | Some College | Multiple Strokes | 20+ years (exact # unknown) | 7 |
| 7 | 29 | Female | Some College | TBI (severity not reported) | 9 years | Withdrew from study |
| 8 | 34 | Female | Some Graduate | TBI (severity not reported) | 3.5 years | 6 |
| 9 | 37 | Male | Some College | Multiple Concussions/mTBIs | 1.5 years since the most recent | 8 |
| 10 | 30 | Male | Some Graduate | Multiple Concussions/mTBIs | 13 years since the most recent | 7 |
| 11 | 33 | Female | Bachelor’s Degree | Hydrocephalus | 6 years | Withdrew from study |
| 12 | 40 | Female | Master’s Degree | Multiple Concussions/mTBIs | 1 year since the most recent | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stephens, J.A.; Press, D.; Atkins, J.; Duffy, J.R.; Thomas, M.L.; Weaver, J.A.; Schmid, A.A. Feasibility of Acquiring Neuroimaging Data from Adults with Acquired Brain Injuries before and after a Yoga Intervention. Brain Sci. 2023, 13, 1413. https://doi.org/10.3390/brainsci13101413
Stephens JA, Press D, Atkins J, Duffy JR, Thomas ML, Weaver JA, Schmid AA. Feasibility of Acquiring Neuroimaging Data from Adults with Acquired Brain Injuries before and after a Yoga Intervention. Brain Sciences. 2023; 13(10):1413. https://doi.org/10.3390/brainsci13101413
Chicago/Turabian StyleStephens, Jaclyn A., Denny Press, Jennifer Atkins, John R. Duffy, Michael L. Thomas, Jennifer A. Weaver, and Arlene A. Schmid. 2023. "Feasibility of Acquiring Neuroimaging Data from Adults with Acquired Brain Injuries before and after a Yoga Intervention" Brain Sciences 13, no. 10: 1413. https://doi.org/10.3390/brainsci13101413





