Low-Energy Transcranial Navigation-Guided Focused Ultrasound for Neuropathic Pain: An Exploratory Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Design and Ethics
2.2. Aims
2.3. Time Frame
2.4. Sample Size
2.5. Indications and the Patient Population
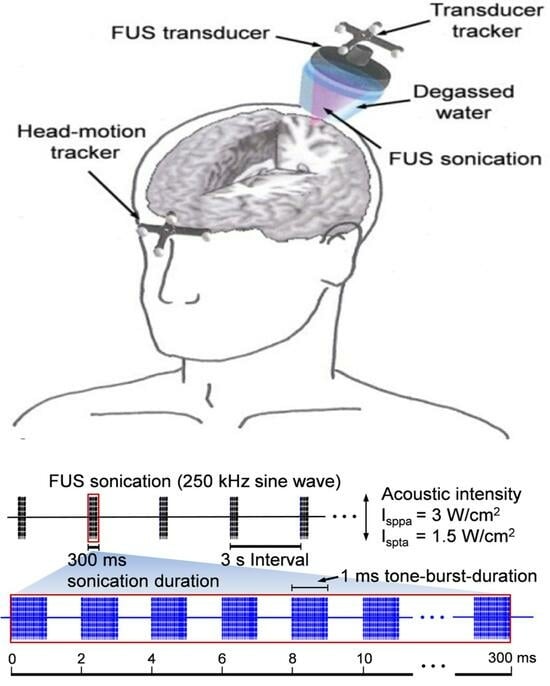
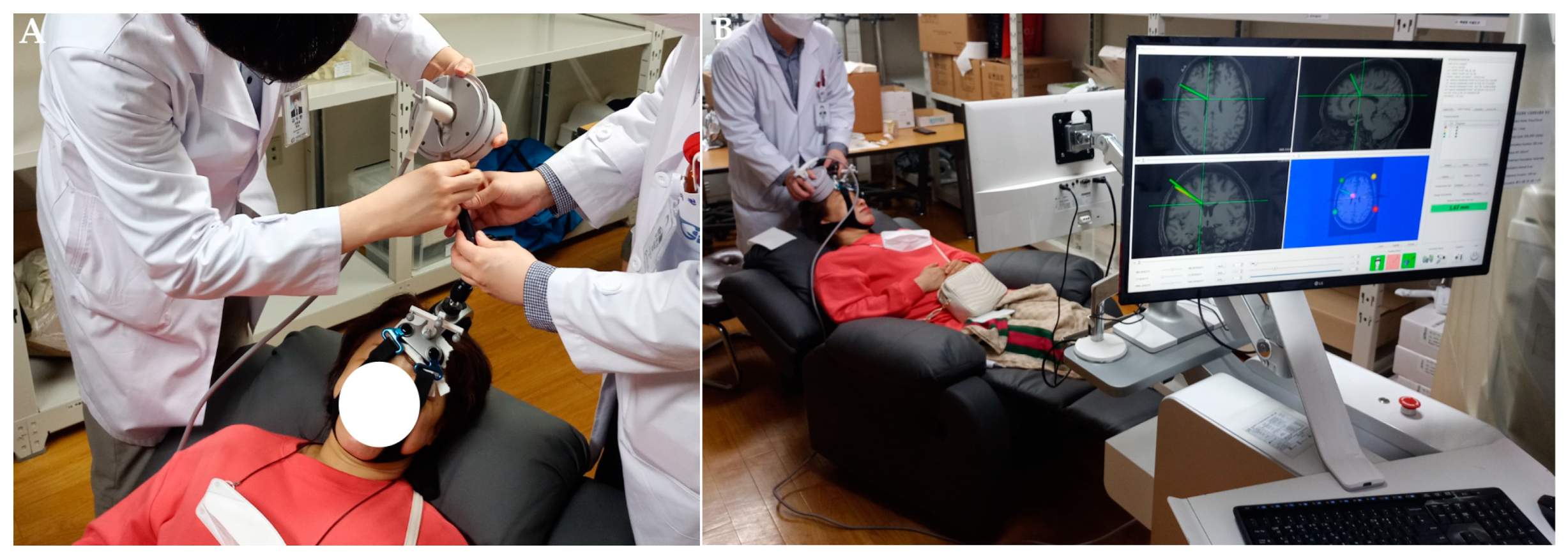
2.6. Procedure Protocol
2.7. Outcome Assessment
2.8. Statistical Analysis
3. Results
3.1. Demographic Data and Baseline Characteristics
3.2. Clinical Outcome Related to Pain Degree

3.3. Clinical Outcome Related to Daily Life Quality
3.4. The Complication and Follow-Up Brain MRI Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef] [PubMed]
- Bouhassira, D.; Attal, N.; Fermanian, J.; Alchaar, H.; Gautron, M.; Masquelier, E.; Rostaing, S.; Lanteri-Minet, M.; Collin, E.; Grisart, J.; et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain 2004, 108, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Treede, R.D.; Jensen, T.S.; Campbell, J.N.; Cruccu, G.; Dostrovsky, J.O.; Griffin, J.W.; Hansson, P.; Hughes, R.; Nurmikko, T.; Serra, J. Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology 2008, 70, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Boccard, S.G.; Pereira, E.A.; Aziz, T.Z. Deep brain stimulation for chronic pain. J. Clin. Neurosci. 2015, 22, 1537–1543. [Google Scholar] [CrossRef]
- Pereira, E.A.; Boccard, S.G.; Linhares, P.; Chamadoira, C.; Rosas, M.J.; Abreu, P.; Rebelo, V.; Vaz, R.; Aziz, T.Z. Thalamic deep brain stimulation for neuropathic pain after amputation or brachial plexus avulsion. Neurosurg. Focus 2013, 35, E7. [Google Scholar] [CrossRef]
- Finnerup, N.B. Nonnarcotic Methods of Pain Management. N. Engl. J. Med. 2019, 380, 2440–2448. [Google Scholar] [CrossRef]
- Levy, R.M. Deep brain stimulation for the treatment of intractable pain. Neurosurg. Clin. N. Am. 2003, 14, 389–399. [Google Scholar] [CrossRef]
- Boccard, S.G.; Pereira, E.A.; Moir, L.; Aziz, T.Z.; Green, A.L. Long-term outcomes of deep brain stimulation for neuropathic pain. Neurosurgery 2013, 72, 221–230, discussion 231. [Google Scholar] [CrossRef]
- Cruccu, G.; Aziz, T.Z.; Garcia-Larrea, L.; Hansson, P.; Jensen, T.S.; Lefaucheur, J.P.; Simpson, B.A.; Taylor, R.S. EFNS guidelines on neurostimulation therapy for neuropathic pain. Eur. J. Neurol. 2007, 14, 952–970. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.E.; Shin, J.; Jung, H.H.; Kim, S.J.; Chang, J.W. The neuromodulation of neuropathic pain by measuring pain response rate and pain response duration in animal. J. Korean Neurosurg. Soc. 2015, 57, 6–11. [Google Scholar] [CrossRef]
- Chung, M.; Huh, R. Neuromodulation for Trigeminal Neuralgia. J. Korean Neurosurg. Soc. 2022, 65, 640–651. [Google Scholar] [CrossRef] [PubMed]
- Hosobuchi, Y.; Adams, J.E.; Linchitz, R. Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science 1977, 197, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Morrell, M.J.; Vogt, B.A. Contributions of anterior cingulate cortex to behaviour. Brain 1995, 118 Pt 1, 279–306. [Google Scholar] [CrossRef]
- Vogt, B.A. Pain and emotion interactions in subregions of the cingulate gyrus. Nat. Rev. Neurosci. 2005, 6, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Melzack, R. From the gate to the neuromatrix. Pain 1999, 82 (Suppl. 6), S121–S126. [Google Scholar] [CrossRef] [PubMed]
- Boccard, S.G.J.; Prangnell, S.J.; Pycroft, L.; Cheeran, B.; Moir, L.; Pereira, E.A.C.; Fitzgerald, J.J.; Green, A.L.; Aziz, T.Z. Long-Term Results of Deep Brain Stimulation of the Anterior Cingulate Cortex for Neuropathic Pain. World Neurosurg. 2017, 106, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Boccard, S.G.; Pereira, E.A.; Moir, L.; Van Hartevelt, T.J.; Kringelbach, M.L.; FitzGerald, J.J.; Baker, I.W.; Green, A.L.; Aziz, T.Z. Deep brain stimulation of the anterior cingulate cortex: Targeting the affective component of chronic pain. Neuroreport 2014, 25, 83–88. [Google Scholar] [CrossRef]
- Giugno, A.; Maugeri, R.; Graziano, F.; Gagliardo, C.; Franzini, A.; Catalano, C.; Midiri, M.; Iacopino, D.G. Restoring Neurological Physiology: The Innovative Role of High-Energy MR-Guided Focused Ultrasound (HIMRgFUS). Preliminary Data from a New Method of Lesioning Surgery. Acta Neurochir. Suppl. 2017, 124, 55–59. [Google Scholar] [CrossRef]
- Tyler, W.J. Noninvasive neuromodulation with ultrasound? A continuum mechanics hypothesis. Neuroscientist 2011, 17, 25–36. [Google Scholar] [CrossRef]
- Bachu, V.S.; Kedda, J.; Suk, I.; Green, J.J.; Tyler, B. High-Intensity Focused Ultrasound: A Review of Mechanisms and Clinical Applications. Ann. Biomed. Eng. 2021, 49, 1975–1991. [Google Scholar] [CrossRef]
- di Biase, L.; Falato, E.; Caminiti, M.L.; Pecoraro, P.M.; Narducci, F.; Di Lazzaro, V. Focused Ultrasound (FUS) for Chronic Pain Management: Approved and Potential Applications. Neurol. Res. Int. 2021, 2021, 8438498. [Google Scholar] [CrossRef] [PubMed]
- Krishna, V.; Sammartino, F.; Rezai, A. A Review of the Current Therapies, Challenges, and Future Directions of Transcranial Focused Ultrasound Technology: Advances in Diagnosis and Treatment. JAMA Neurol. 2018, 75, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Gagliardo, C.; Geraci, L.; Napoli, A.; Giugno, A.; Cortegiani, A.; Canzio, D.; Giarratano, A.; Franzini, A.; Iacopino, D.G.; Catalano, C.; et al. Non-invasive trans-cranial magnetic resonance imaging-guided focused ultrasounds surgery to treat neurologic disorders. Recenti Prog. Med. 2016, 107, 242–245. [Google Scholar] [CrossRef]
- Yun, Y.H.; Mendoza, T.R.; Heo, D.S.; Yoo, T.; Heo, B.Y.; Park, H.A.; Shin, H.C.; Wang, X.S.; Cleeland, C.S. Development of a cancer pain assessment tool in Korea: A validation study of a Korean version of the brief pain inventory. Oncology 2004, 66, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Prévinaire, J.G.; Nguyen, J.P.; Perrouin-Verbe, B.; Fattal, C. Chronic neuropathic pain in spinal cord injury: Efficiency of deep brain and motor cortex stimulation therapies for neuropathic pain in spinal cord injury patients. Ann. Phys. Rehabil. Med. 2009, 52, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Frizon, L.A.; Yamamoto, E.A.; Nagel, S.J.; Simonson, M.T.; Hogue, O.; Machado, A.G. Deep Brain Stimulation for Pain in the Modern Era: A Systematic Review. Neurosurgery 2020, 86, 191–202. [Google Scholar] [CrossRef]
- Bittar, R.G.; Kar-Purkayastha, I.; Owen, S.L.; Bear, R.E.; Green, A.; Wang, S.; Aziz, T.Z. Deep brain stimulation for pain relief: A meta-analysis. J. Clin. Neurosci. 2005, 12, 515–519. [Google Scholar] [CrossRef]
- Galafassi, G.Z.; Simm Pires de Aguiar, P.H.; Simm, R.F.; Franceschini, P.R.; Filho, M.P.; Pagura, J.R.; Pires de Aguiar, P.H. Neuromodulation for Medically Refractory Neuropathic Pain: Spinal Cord Stimulation, Deep Brain Stimulation, Motor Cortex Stimulation, and Posterior Insula Stimulation. World Neurosurg. 2021, 146, 246–260. [Google Scholar] [CrossRef]
- Soer, R.; Köke, A.J.; Speijer, B.L.; Vroomen, P.C.; Smeets, R.J.; Coppes, M.H.; Reneman, M.F.; Gross, D.P. Reference Values of the Pain Disability Index in Patients with Painful Musculoskeletal and Spinal Disorders: A Cross-national Study. Spine 2015, 40, E545–E551. [Google Scholar] [CrossRef]
- Hori, H.; Iwamuro, H.; Nakano, M.; Ouchi, T.; Kawahara, T.; Taira, T.; Abe, K.; Iijima, K.; Yamaguchi, T. Correction of the skull density ratio for transcranial MRI-guided focused ultrasound thalamotomy: Clinical significance of predicting therapeutic temperature. J. Neurosurg. 2021, 135, 1436–1444. [Google Scholar] [CrossRef]
- Boutet, A.; Gwun, D.; Gramer, R.; Ranjan, M.; Elias, G.J.B.; Tilden, D.; Huang, Y.; Li, S.X.; Davidson, B.; Lu, H.; et al. The relevance of skull density ratio in selecting candidates for transcranial MR-guided focused ultrasound. J. Neurosurg. 2019, 132, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- McCormick, L.M.; Ziebell, S.; Nopoulos, P.; Cassell, M.; Andreasen, N.C.; Brumm, M. Anterior cingulate cortex: An MRI-based parcellation method. Neuroimage 2006, 32, 1167–1175. [Google Scholar] [CrossRef]
- Albe-Fessard, D.; Berkley, K.J.; Kruger, L.; Ralston, H.J., 3rd; Willis, W.D., Jr. Diencephalic mechanisms of pain sensation. Brain Res. 1985, 356, 217–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, P.; He, X.; Jiang, Z.; Wang, Q.; Gu, N.; Lu, Y. The PKCγ neurons in anterior cingulate cortex contribute to the development of neuropathic allodynia and pain-related emotion. Mol. Pain. 2021, 17, 17448069211061973. [Google Scholar] [CrossRef] [PubMed]
- Foltz, E.L.; White, L.E., Jr. Pain “relief” by frontal cingulumotomy. J. Neurosurg. 1962, 19, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Ballantine, H.T., Jr.; Cassidy, W.L.; Flanagan, N.B.; Marino, R., Jr. Stereotaxic anterior cingulotomy for neuropsychiatric illness and intractable pain. J. Neurosurg. 1967, 26, 488–495. [Google Scholar] [CrossRef]
- Park, S.I.; Oh, J.H.; Hwang, Y.S.; Kim, S.J.; Chang, J.W. Electrical stimulation of the anterior cingulate cortex in a rat neuropathic pain model. Acta Neurochir. Suppl. 2006, 99, 65–71. [Google Scholar] [CrossRef]
- Gray, A.M.; Pounds-Cornish, E.; Eccles, F.J.; Aziz, T.Z.; Green, A.L.; Scott, R.B. Deep brain stimulation as a treatment for neuropathic pain: A longitudinal study addressing neuropsychological outcomes. J. Pain 2014, 15, 283–292. [Google Scholar] [CrossRef]
- Spooner, J.; Yu, H.; Kao, C.; Sillay, K.; Konrad, P. Neuromodulation of the cingulum for neuropathic pain after spinal cord injury. Case report. J. Neurosurg. 2007, 107, 169–172. [Google Scholar] [CrossRef]
- Moon, H.C.; Park, Y.S. Optogenetic Stimulation of the Anterior Cingulate Cortex Modulates the Pain Processing in Neuropathic Pain: A Review. J. Mol. Neurosci. 2022, 72, 1–8. [Google Scholar] [CrossRef]
- Levi, V.; Cordella, R.; D’Ammando, A.; Tringali, G.; Dones, I.; Messina, G.; Franzini, A. Dorsal anterior cingulate cortex (ACC) deep brain stimulation (DBS): A promising surgical option for the treatment of refractory thalamic pain syndrome (TPS). Acta Neurochir. 2019, 161, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Leplus, A.; Lanteri-Minet, M.; Donnet, A.; Darmon, N.; Regis, J.; Fontaine, D. Treatment of Chronic Refractory Pain by Combined Deep Brain Stimulation of the Anterior Cingulum and Sensory Thalamus (EMOPAIN Study): Rationale and Protocol of a Feasibility and Safety Study. Brain Sci. 2022, 12, 1116. [Google Scholar] [CrossRef] [PubMed]
- Eames, M.D.; Hananel, A.; Snell, J.W.; Kassell, N.F.; Aubry, J.F. Trans-cranial focused ultrasound without hair shaving: Feasibility study in an ex vivo cadaver model. J. Ther. Ultrasound 2013, 1, 24. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Taira, T. Focused Ultrasound Treatment, Present and Future. Neurol. Med.-Chir. 2017, 57, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Jones, R.M.; Davidson, B.; Huang, Y.; Pople, C.B.; Surendrakumar, S.; Hamani, C.; Hynynen, K.; Lipsman, N. Technical Principles and Clinical Workflow of Transcranial MR-Guided Focused Ultrasound. Stereotact. Funct. Neurosurg. 2021, 99, 329–342. [Google Scholar] [CrossRef]
- Moser, D.; Zadicario, E.; Schiff, G.; Jeanmonod, D. MR-guided focused ultrasound technique in functional neurosurgery: Targeting accuracy. J. Ther. Ultrasound 2013, 1, 3. [Google Scholar] [CrossRef]
- Kim, S.; Jo, Y.; Kook, G.; Pasquinelli, C.; Kim, H.; Kim, K.; Hoe, H.S.; Choe, Y.; Rhim, H.; Thielscher, A.; et al. Transcranial focused ultrasound stimulation with high spatial resolution. Brain Stimul. 2021, 14, 290–300. [Google Scholar] [CrossRef]
- di Biase, L.; Falato, E.; Di Lazzaro, V. Transcranial Focused Ultrasound (tFUS) and Transcranial Unfocused Ultrasound (tUS) Neuromodulation: From Theoretical Principles to Stimulation Practices. Front. Neurol. 2019, 10, 549. [Google Scholar] [CrossRef]
- Jeanmonod, D.; Werner, B.; Morel, A.; Michels, L.; Zadicario, E.; Schiff, G.; Martin, E. Transcranial magnetic resonance imaging-guided focused ultrasound: Noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurg. Focus 2012, 32, E1. [Google Scholar] [CrossRef]
- Gallay, M.N.; Moser, D.; Jeanmonod, D. Safety and accuracy of incisionless transcranial MR-guided focused ultrasound functional neurosurgery: Single-center experience with 253 targets in 180 treatments. J. Neurosurg. 2018, 130, 1234–1243. [Google Scholar] [CrossRef]
- Kim, E.S.; Chang, S.Y. Patch Clamp Technology for Focused Ultrasonic (FUS) Neuromodulation. Methods Mol. Biol. 2022, 2393, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Tyler, W.J.; Tufail, Y.; Finsterwald, M.; Tauchmann, M.L.; Olson, E.J.; Majestic, C. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PLoS ONE 2008, 3, e3511. [Google Scholar] [CrossRef] [PubMed]
- Krasovitski, B.; Frenkel, V.; Shoham, S.; Kimmel, E. Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects. Proc. Natl. Acad. Sci. USA 2011, 108, 3258–3263. [Google Scholar] [CrossRef]
- Kim, H.; Chiu, A.; Lee, S.D.; Fischer, K.; Yoo, S.S. Focused ultrasound-mediated non-invasive brain stimulation: Examination of sonication parameters. Brain Stimul. 2014, 7, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kim, H.C.; Jung, Y.; Chung, Y.A.; Song, I.U.; Lee, J.H.; Yoo, S.S. Transcranial focused ultrasound stimulation of human primary visual cortex. Sci. Rep. 2016, 6, 34026. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kim, H.; Jung, Y.; Song, I.U.; Chung, Y.A.; Yoo, S.S. Image-guided transcranial focused ultrasound stimulates human primary somatosensory cortex. Sci. Rep. 2015, 5, 8743. [Google Scholar] [CrossRef] [PubMed]
- Fini, M.; Tyler, W.J. Transcranial focused ultrasound: A new tool for non-invasive neuromodulation. Int. Rev. Psychiatry 2017, 29, 168–177. [Google Scholar] [CrossRef]
- White, P.J. Transcranial focused ultrasound surgery. Top. Magn. Reson. Imaging 2006, 17, 165–172. [Google Scholar] [CrossRef]
- Kubanek, J. Neuromodulation with transcranial focused ultrasound. Neurosurg. Focus 2018, 44, E14. [Google Scholar] [CrossRef]
- Diaz, M.J.; Root, K.T.; Beneke, A.; Penev, Y.; Lucke-Wold, B. Neurostimulation for Traumatic Brain Injury: Emerging Innovation. OBM Neurobiol. 2023, 7, 161. [Google Scholar] [CrossRef]
- Auvichayapat, P.; Keeratitanont, K.; Janyachareon, T.; Auvichayapat, N. The effects of transcranial direct current stimulation on metabolite changes at the anterior cingulate cortex in neuropathic pain: A pilot study. J. Pain. Res. 2018, 11, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- Bing, C.; Ladouceur-Wodzak, M.; Wanner, C.R.; Shelton, J.M.; Richardson, J.A.; Chopra, R. Trans-cranial opening of the blood-brain barrier in targeted regions using a stereotaxic brain atlas and focused ultrasound energy. J. Ther. Ultrasound 2014, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Huang, Y.; Meng, Y.; Scantlebury, N.; Schwartz, M.L.; Lipsman, N.; Hynynen, K. Echo-Focusing in Transcranial Focused Ultrasound Thalamotomy for Essential Tremor: A Feasibility Study. Mov. Disord. 2020, 35, 2327–2333. [Google Scholar] [CrossRef]
- Iacopino, D.G.; Giugno, A.; Maugeri, R.; Gagliardo, C.; Franzini, A.; Catalano, C.; Midiri, M. Is there still a role for lesioning in functional neurosurgery? Preliminary Italian (and world-first) experience with a trans-cranial MRI-guided focused ultrasound surgery system operating at 1.5 Tesla. J. Neurosurg. Sci. 2017, 61, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Aubry, J.F.; Tanter, M. MR-Guided Transcranial Focused Ultrasound. Adv. Exp. Med. Biol. 2016, 880, 97–111. [Google Scholar] [CrossRef]
- van Hartevelt, T.J.; Cabral, J.; Deco, G.; Møller, A.; Green, A.L.; Aziz, T.Z.; Kringelbach, M.L. Neural plasticity in human brain connectivity: The effects of long term deep brain stimulation of the subthalamic nucleus in Parkinson’s disease. PLoS ONE 2014, 9, e86496. [Google Scholar] [CrossRef]
- Vion-Bailly, J.; Suarez-Castellanos, I.M.; Chapelon, J.Y.; Carpentier, A.; N’Djin, W.A. Neurostimulation success rate of repetitive-pulse focused ultrasound in an in vivo giant axon model: An acoustic parametric study. Med. Phys. 2022, 49, 682–701. [Google Scholar] [CrossRef]

| Characteristics | Patients (n = 11) |
|---|---|
| Age (years, mean ± SD) | 60.55 ± 13.18 (range, 44.00–81.00) |
| Men/Women | 6/5 |
| The median duration of symptoms (years) | 4.33 (range, 1.42–13.17) |
| Location of pain: Right/Left/Both | 4/1/6 |
| Diagnosis | |
| Spinal cord injury | 4 |
| Compressive myelopathy | 3 |
| Post spinal surgery syndrome | 3 |
| Cauda equina syndrome | 1 |
| Characteristics | Values | p Value |
|---|---|---|
| Current pain | 0.039 † | |
| Initial | 10.0 (range, 6.0–10.0) | |
| Immediately post-procedure | 8.0 (range, 3.0–10.0) | |
| 2 weeks | 7.0 (range, 3.0–9.0) | |
| 4 weeks | 7.0 (range, 4.0–9.0) | |
| Δ Current pain | ||
| Initial—Immediately post-procedure | 1.18 (95% CI, −0.40–2.77) | 0.509 † |
| Initial—2 weeks | 1.91 (95% CI, 0.36–3.45) | 0.048 † |
| Initial—4 weeks | 2.09 (95% CI, 0.64–3.55) | 0.021 † |
| Immediately post-procedure—2 weeks | 0.73 (95% CI, −0.13–1.58) | 0.186 † |
| Immediately post-procedure—4 weeks | 0.91 (95% CI, 0.09–1.73) | 0.099 † |
| 2 weeks–4 weeks | 0.18 (95% CI, −0.54–0.91) | 0.741 † |
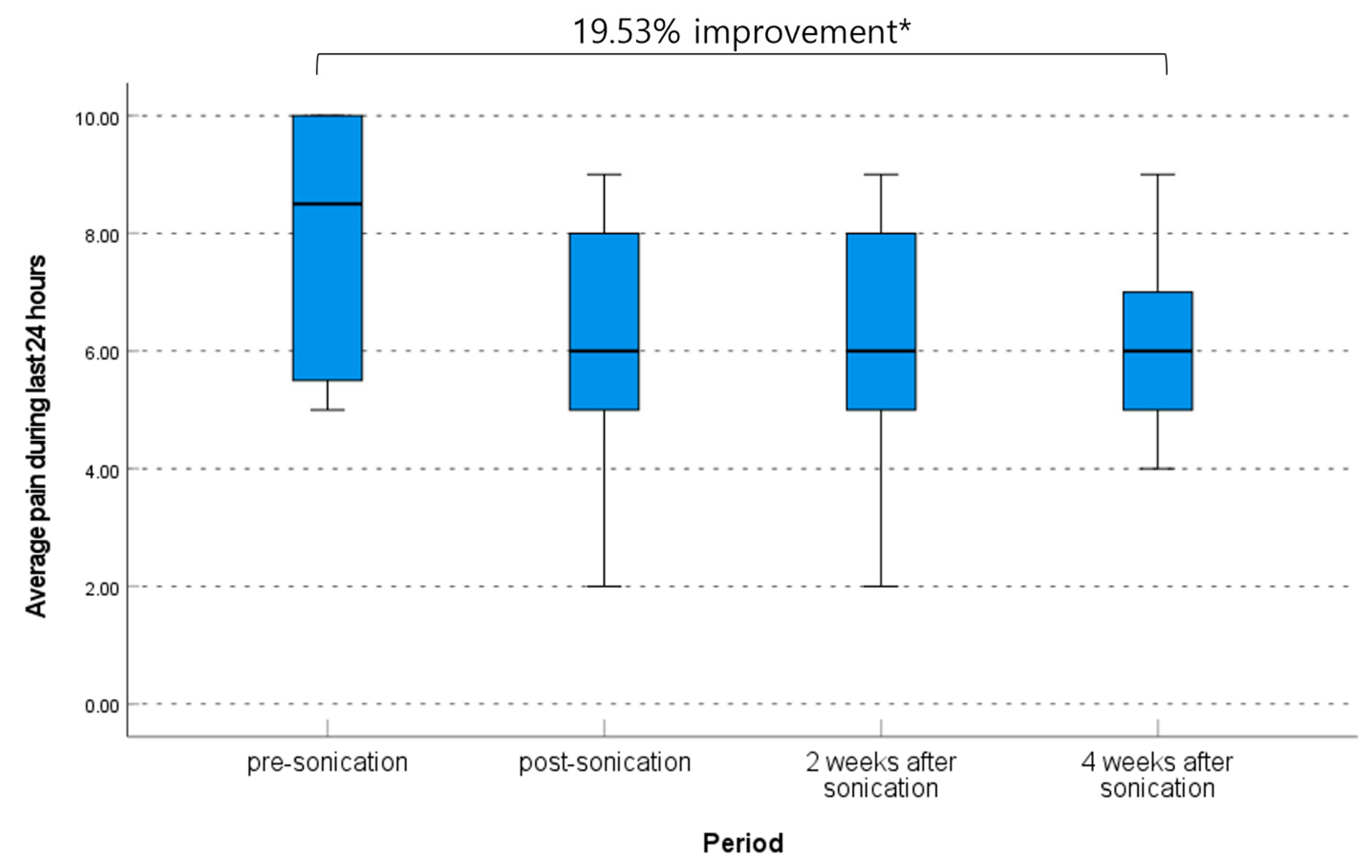
| Average pain during the last 24 h | 0.070 † | |
| Initial | 8.5 (range, 5.0–10.0) | |
| Immediately post-procedure | 6.0 (range, 2.0–9.0) | |
| 2 weeks | 6.0 (range, 2.0–9.0) | |
| 4 weeks | 6.0 (range, 4.0–9.0) | |
| Δ Average pain | ||
| Initial—Immediately post-procedure | 1.50 (95% CI, −0.21–3.21) | 0.079 † |
| Initial—2 weeks | 1.59 (95% CI, −0.05–3.23) | 0.056 † |
| Initial—4 weeks | 1.50 (95% CI, 0.41–2.59) | 0.027 † |
| Immediately post-procedure—2 weeks | 0.91 (95% CI, −0.27–0.45) | 0.588 † |
| Immediately post-procedure—4 weeks | (95% CI, −1.34–1.34) | 1.000 † |
| 2 weeks–4 weeks | −0.91 (95% CI, −1.42–1.23) | 0.882 † |
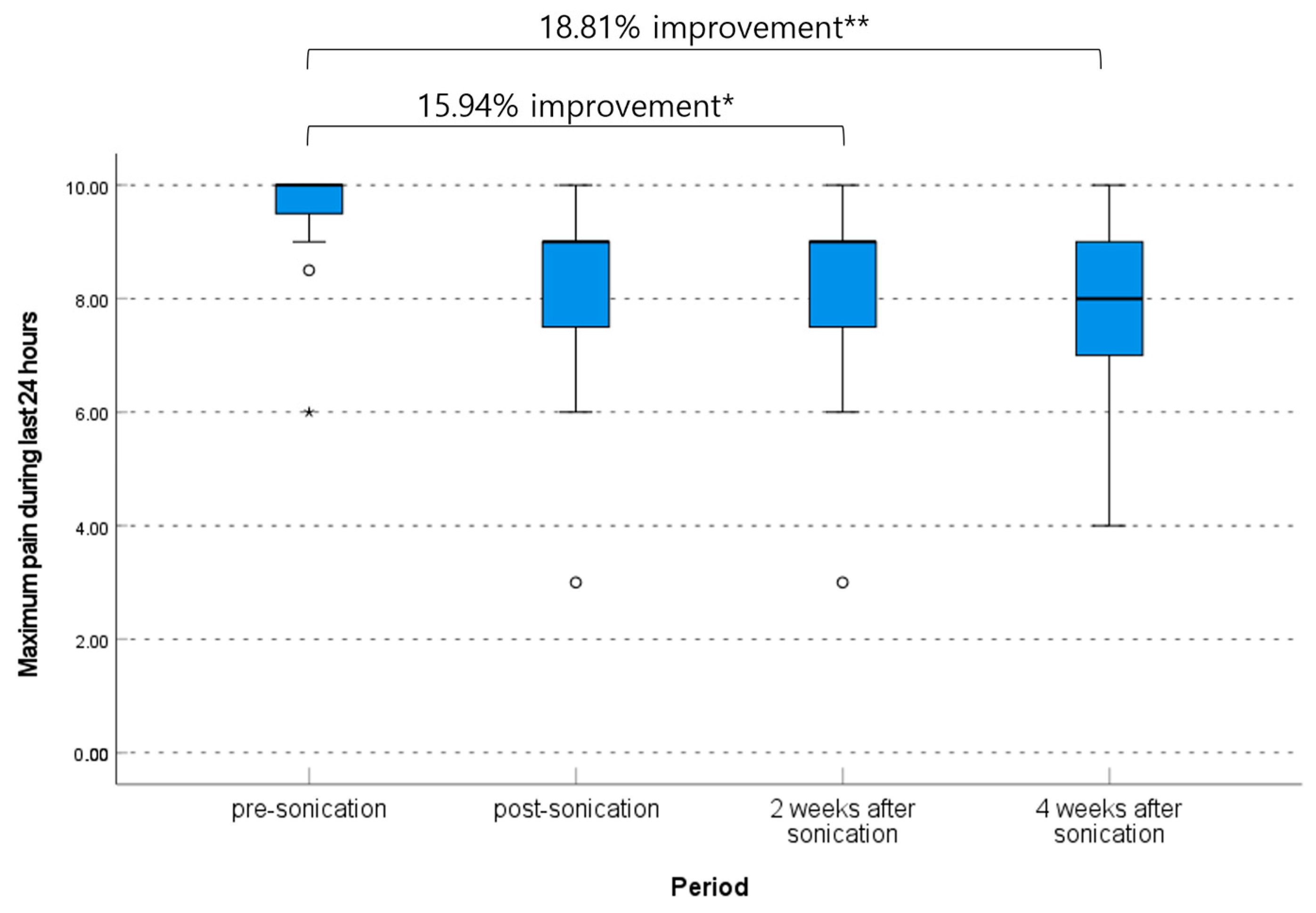
| Maximum pain during the last 24 h | 0.012 † | |
| Pre-procedure | 10.0 (range, 6.0–10.0) | |
| Immediately post-procedure | 9.0 (range, 3.0–10.0) | |
| 2 weeks | 9.0 (range, 3.0–10.0) | |
| 4 weeks | 8.0 (range, 4.0–10.0) | |
| Δ Maximum pain | ||
| Initial—Immediately post-procedure | 1.41 (95% CI, −0.38–2.86) | 0.069 † |
| Initial—2 weeks | 1.50 (95% CI, 0.83–2.92) | 0.032 † |
| Initial—4 weeks | 1.77 (95% CI, 0.56–2.99) | 0.008 † |
| Immediately post-procedure—2 weeks | 0.91 (95% CI, −0.54–0.71) | 0.741 † |
| Immediately post-procedure—4 weeks | 0.36 (95% CI, −0.65–1.37) | 0.409 † |
| 2 weeks–4 weeks | 0.27 (95% CI, −0.58–1.13) | 0.620 † |
| Characteristics | Values | p Value |
|---|---|---|
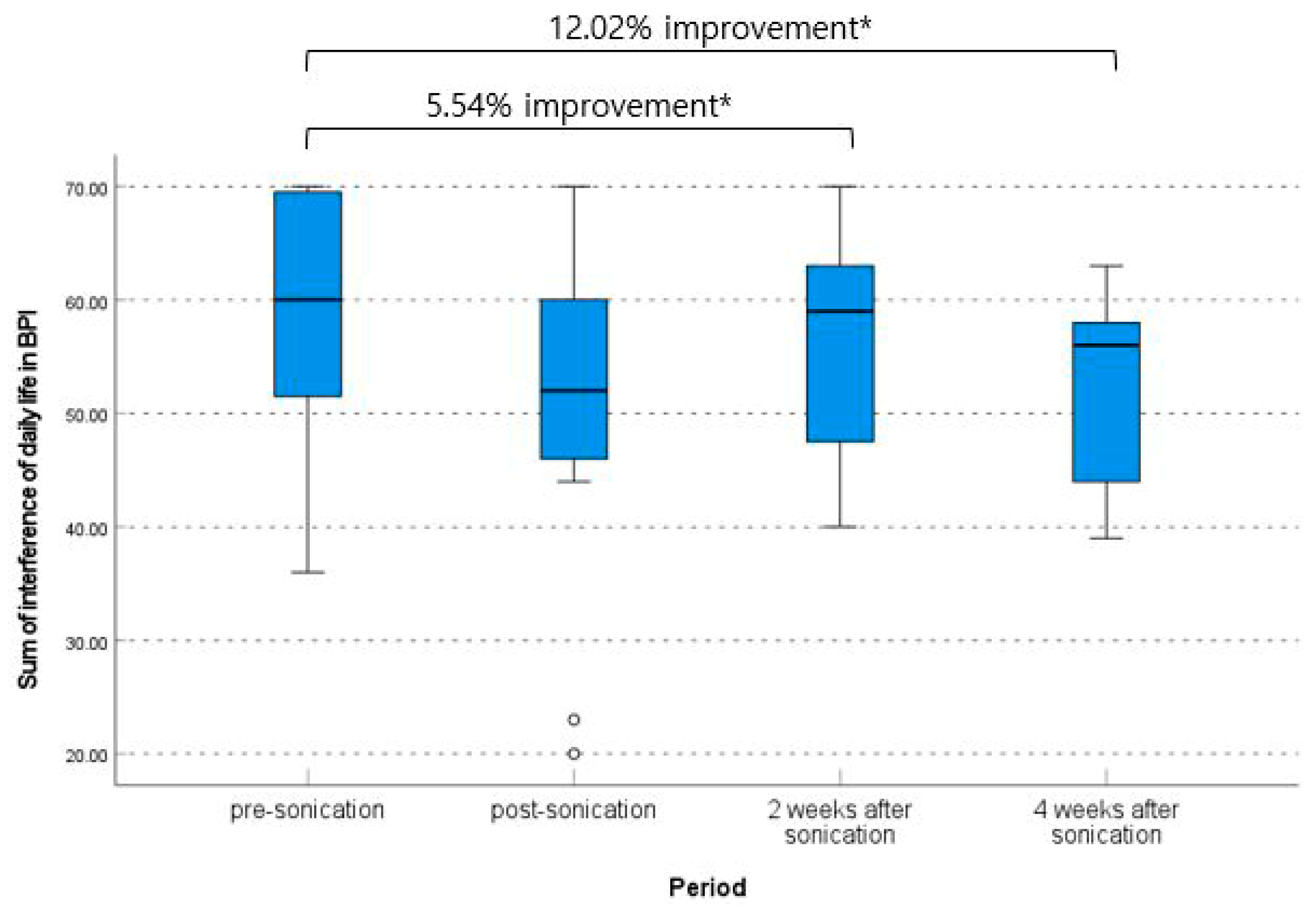
| Sum of interference of daily life in BPI (mean ± SD) | 0.318 † | |
| Pre-procedure | 59.00 ± 11.66 | |
| Immediately post-procedure | 50.09 ± 16.13 | |
| 2 weeks | 55.73 ± 9.75 | |
| 4 weeks | 51.91 ± 9.18 | |
| Δ Sum of interference of daily life in BPI | ||
| Initial—Immediately post-procedure | 8.91 (95% CI, 0.87–16.95) | 0.033 ‡ |
| Initial—2 weeks | 3.27 (95% CI, −1.97–8.51) | 0.194 ‡ |
| Initial—4 weeks | 7.09 (95% CI, 1.29–12.89) | 0.021 ‡ |
| Immediately post-procedure—2 weeks | −5.64 (95% CI, −12.25–0.98) | 0.087 ‡ |
| Immediately post-procedure—4 weeks | −1.82 (95% CI, −8.71–5.08) | 0.570 ‡ |
| 2 weeks–4 weeks | 3.82 (95% CI, −0.17–7.80) | 0.058 ‡ |
| The subjective pain-relieving effect of procedure (%) | 0.429 § | |
| Pre-procedure | 60.0 (range, 0.0–90.0) | |
| Immediately post-procedure | 50.0 (range, 0.0–90.0) | |
| 2 weeks | 50.0 (range, 0.0–80.0) | |
| 4 weeks | 50.0 (range, 0.0–80.0) | |
| Δ Pain-relieving effect (%) | ||
| Initial—Immediately post-procedure | 8.2 (95% CI, −4.1–20.5) | 0.172 § |
| Initial—2 weeks | 0.9 (95% CI, −11.6–20.5) | 0.783 § |
| Initial—4 weeks | 3.6 (95% CI, −5.5–12.8) | 0.395 § |
| Immediately post-procedure—2 weeks | −7.2 (95% CI, −17.2–2.7) | 0.121 § |
| Immediately post-procedure—4 weeks | −4.5 (95% CI, −14.7–5.6) | 0.357 § |
| 2 weeks–4 weeks | 2.7 (95% CI, −5.2–10.7) | 0.453 § |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, D.H.; Son, S.; Kim, E.Y. Low-Energy Transcranial Navigation-Guided Focused Ultrasound for Neuropathic Pain: An Exploratory Study. Brain Sci. 2023, 13, 1433. https://doi.org/10.3390/brainsci13101433
Shin DH, Son S, Kim EY. Low-Energy Transcranial Navigation-Guided Focused Ultrasound for Neuropathic Pain: An Exploratory Study. Brain Sciences. 2023; 13(10):1433. https://doi.org/10.3390/brainsci13101433
Chicago/Turabian StyleShin, Dong Hoon, Seong Son, and Eun Young Kim. 2023. "Low-Energy Transcranial Navigation-Guided Focused Ultrasound for Neuropathic Pain: An Exploratory Study" Brain Sciences 13, no. 10: 1433. https://doi.org/10.3390/brainsci13101433
APA StyleShin, D. H., Son, S., & Kim, E. Y. (2023). Low-Energy Transcranial Navigation-Guided Focused Ultrasound for Neuropathic Pain: An Exploratory Study. Brain Sciences, 13(10), 1433. https://doi.org/10.3390/brainsci13101433