Prefrontal Glutathione Levels in Major Depressive Disorder Are Linked to a Lack of Positive Affect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Acquisition and Analysis
2.2. Clinical Assessments
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lim, G.Y.; Tam, W.W.; Lu, Y.; Ho, C.S.; Zhang, M.W.; Ho, R.C. Prevalence of Depression in the Community from 30 Countries between 1994 and 2014. Sci. Rep. 2018, 8, 2861. [Google Scholar] [CrossRef] [PubMed]
- Shorey, S.; Ng, E.D.; Wong, C.H.J. Global prevalence of depression and elevated depressive symptoms among adolescents: A systematic review and meta-analysis. Br. J. Clin. Psychol. 2022, 61, 287–305. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.H. Core symptoms of major depressive disorder: Relevance to diagnosis and treatment. Dialogues Clin. Neurosci. 2008, 10, 271–277. [Google Scholar] [CrossRef] [PubMed]
- O’Keane, V.; Frodl, T.; Dinan, T.G. A review of Atypical depression in relation to the course of depression and changes in HPA axis organization. Psychoneuroendocrinology 2012, 37, 1589–1599. [Google Scholar] [CrossRef]
- Köhler, C.A.; Freitas, T.H.; Maes, M.; de Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef]
- Luykx, J.J.; Laban, K.G.; van den Heuvel, M.P.; Boks, M.P.; Mandl, R.C.; Kahn, R.S.; Bakker, S.C. Region and state specific glutamate downregulation in major depressive disorder: A meta-analysis of (1)H-MRS findings. Neurosci. Biobehav. Rev. 2012, 36, 198–205. [Google Scholar] [CrossRef]
- Ritter, C.; Buchmann, A.; Muller, S.T.; Volleberg, M.; Haynes, M.; Ghisleni, C.; Noeske, R.; Tuura, R.; Hasler, G. Evaluation of Prefrontal gamma-Aminobutyric Acid and Glutamate Levels in Individuals With Major Depressive Disorder Using Proton Magnetic Resonance Spectroscopy. JAMA Psychiatry 2022, 79, 1209–1216. [Google Scholar] [CrossRef]
- Rae, C.D.; Williams, S.R. Glutathione in the human brain: Review of its roles and measurement by magnetic resonance spectroscopy. Anal. Biochem. 2017, 529, 127–143. [Google Scholar] [CrossRef]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef]
- Terpstra, M.; Henry, P.G.; Gruetter, R. Measurement of reduced glutathione (GSH) in human brain using LCModel analysis of difference-edited spectra. Magn. Reson. Med. 2003, 50, 19–23. [Google Scholar] [CrossRef]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L.; et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Caraci, F.; Tascedda, F.; Merlo, S.; Benatti, C.; Spampinato, S.F.; Munafo, A.; Leggio, G.M.; Nicoletti, F.; Brunello, N.; Drago, F.; et al. Fluoxetine Prevents Abeta(1-42)-Induced Toxicity via a Paracrine Signaling Mediated by Transforming-Growth-Factor-beta1. Front. Pharmacol. 2016, 7, 389. [Google Scholar] [CrossRef] [PubMed]
- Hannestad, J.; DellaGioia, N.; Bloch, M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: A meta-analysis. Neuropsychopharmacology 2011, 36, 2452–2459. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Zhang, G.Z.; Li, B.; Li, M.; Woelfer, M.; Walter, M.; Wang, L. Role of inflammation in depression relapse. J. Neuroinflamm. 2019, 16, 90. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Fisar, Z.; Medina, M.; Scapagnini, G.; Nowak, G.; Berk, M. New drug targets in depression: Inflammatory, cell-mediated immune, oxidative and nitrosative stress, mitochondrial, antioxidant, and neuroprogressive pathways. And new drug candidates--Nrf2 activators and GSK-3 inhibitors. Inflammopharmacology 2012, 20, 127–150. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Caraci, F.; Spampinato, S.F.; Morgese, M.G.; Tascedda, F.; Salluzzo, M.G.; Giambirtone, M.C.; Caruso, G.; Munafo, A.; Torrisi, S.A.; Leggio, G.M.; et al. Neurobiological links between depression and AD: The role of TGF-beta1 signaling as a new pharmacological target. Pharmacol. Res. 2018, 130, 374–384. [Google Scholar] [CrossRef]
- Wadley, A.J.; Veldhuijzen van Zanten, J.J.; Aldred, S. The interactions of oxidative stress and inflammation with vascular dysfunction in ageing: The vascular health triad. Age 2013, 35, 705–718. [Google Scholar] [CrossRef]
- Bakunina, N.; Pariante, C.M.; Zunszain, P.A. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology 2015, 144, 365–373. [Google Scholar] [CrossRef]
- Satoh, T.; Yoshioka, Y. Contribution of reduced and oxidized glutathione to signals detected by magnetic resonance spectroscopy as indicators of local brain redox state. Neurosci. Res. 2006, 55, 34–39. [Google Scholar] [CrossRef]
- Srinivasan, R.; Ratiney, H.; Hammond-Rosenbluth, K.E.; Pelletier, D.; Nelson, S.J. MR spectroscopic imaging of glutathione in the white and gray matter at 7 T with an application to multiple sclerosis. Magn. Reson. Imaging 2010, 28, 163–170. [Google Scholar] [CrossRef]
- Freed, R.D.; Hollenhorst, C.N.; Weiduschat, N.; Mao, X.; Kang, G.; Shungu, D.C.; Gabbay, V. A pilot study of cortical glutathione in youth with depression. Psychiatry Res. Neuroimaging 2017, 270, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Shungu, D.C.; Weiduschat, N.; Murrough, J.W.; Mao, X.; Pillemer, S.; Dyke, J.P.; Medow, M.S.; Natelson, B.H.; Stewart, J.M.; Mathew, S.J. Increased ventricular lactate in chronic fatigue syndrome. III. Relationships to cortical glutathione and clinical symptoms implicate oxidative stress in disorder pathophysiology. NMR Biomed. 2012, 25, 1073–1087. [Google Scholar] [CrossRef]
- Lapidus, K.A.B.; Gabbay, V.; Mao, X.L.; Johnson, A.; Murrough, J.W.; Mathew, S.J.; Shungu, D.C. In vivo H-1 MRS study of potential associations between glutathione, oxidative stress and anhedonia in major depressive disorder. Neurosci. Lett. 2014, 569, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Gawryluk, J.W.; Wang, J.F.; Andreazza, A.C.; Shao, L.; Young, L.T. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int. J. Neuropsychopharmacol. 2011, 14, 123–130. [Google Scholar] [CrossRef]
- Erbay, M.F.; Zayman, E.P.; Erbay, L.G.; Unal, S. Evaluation of Transcranial Magnetic Stimulation Efficiency in Major Depressive Disorder Patients: A Magnetic Resonance Spectroscopy Study. Psychiatry Investig. 2019, 16, 745–750. [Google Scholar] [CrossRef]
- Duffy, S.L.; Lagopoulos, J.; Cockayne, N.; Hermens, D.F.; Hickie, I.B.; Naismith, S.L. Oxidative stress and depressive symptoms in older adults: A magnetic resonance spectroscopy study. J. Affect. Disord. 2015, 180, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Das, T.K.; Javadzadeh, A.; Dey, A.; Sabesan, P.; Theberge, J.; Radua, J.; Palaniyappan, L. Antioxidant defense in schizophrenia and bipolar disorder: A meta-analysis of MRS studies of anterior cingulate glutathione. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 91, 94–102. [Google Scholar] [CrossRef]
- Michels, L.; Schulte-Vels, T.; Schick, M.; O’Gorman, R.L.; Zeffiro, T.; Hasler, G.; Mueller-Pfeiffer, C. Prefrontal GABA and glutathione imbalance in posttraumatic stress disorder: Preliminary findings. Psychiatry Res. 2014, 224, 288–295. [Google Scholar] [CrossRef]
- Charney, M.F.; Howell, D.R.; Lanois, C.; Starr, T.C.; Liao, H.J.; Coello, E.; Breedlove, K.M.; Meehan, W.P.; Koerte, I.; Lin, A.P. Associations Between Neurochemistry and Gait Performance Following Concussion in Collegiate Athletes. J. Head Trauma Rehabil. 2020, 35, 342–353. [Google Scholar] [CrossRef]
- Joyce, J.M.; Mercier, L.J.; Stokoe, M.; La, P.L.; Bell, T.; Batycky, J.M.; Debert, C.T.; Harris, A.D. Glutamate, GABA and glutathione in adults with persistent post-concussive symptoms. Neuroimage Clin. 2022, 36, 103152. [Google Scholar] [CrossRef] [PubMed]
- Koenigs, M.; Grafman, J. The functional neuroanatomy of depression: Distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav. Brain Res. 2009, 201, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, F.A.; O’Gorman, R.L.; Nashef, L.; Elwes, R.D.; Edden, R.A.; Murdoch, J.B.; Barker, G.J.; Richardson, M.P. Investigation of glutamine and GABA levels in patients with idiopathic generalized epilepsy using MEGAPRESS. J. Magn. Reson. Imaging 2015, 41, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Michels, L.; Martin, E.; Klaver, P.; Edden, R.; Zelaya, F.; Lythgoe, D.J.; Luchinger, R.; Brandeis, D.; O’Gorman, R.L. Frontal GABA levels change during working memory. PLoS ONE 2012, 7, e31933. [Google Scholar] [CrossRef]
- Edden, R.A.; Puts, N.A.; Harris, A.D.; Barker, P.B.; Evans, C.J. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J. Magn. Reson. Imaging 2014, 40, 1445–1452. [Google Scholar] [CrossRef]
- First, M.B.; Gibbon, M. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II). In Comprehensive Handbook of Psychological Assessment, Vol. 2: Personality Assessment; John Wiley & Sons: Hoboken, NJ, USA, 2004; pp. 134–143. [Google Scholar]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, S.A.; Asberg, M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry 1979, 134, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef]
- Hautzinger, M.; Bailer, M.; Worall, H.; Keller, F. Beck- Depressions-Inventar (BDI): Bearbeitung der Deutschen Ausgabe; Hans Huber: Bern, Switzerland, 1994. [Google Scholar]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Patil, I. Visualizations with statistical details: The ‘ggstatsplot’ approach. J. Open Source Softw. 2021, 6(61), 3167. [Google Scholar] [CrossRef]
- Lehrl, S.; Triebig, G.; Fischer, B. Multiple-Choice Vocabulary-Test Mwt as a Valid and Short Test to Estimate Premorbid Intelligence. Acta Neurol. Scand. 1995, 91, 335–345. [Google Scholar] [CrossRef]
- Maher, P. The effects of stress and aging on glutathione metabolism. Ageing Res. Rev. 2005, 4, 288–314. [Google Scholar] [CrossRef]
- Cui, J.; Shao, L.; Young, L.T.; Wang, J.F. Role of glutathione in neuroprotective effects of mood stabilizing drugs lithium and valproate. Neuroscience 2007, 144, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Cortez, I.; Brocardo, P.S.; Leasure, J.L. Changes in Affective Behavior and Oxidative Stress after Binge Alcohol in Male and Female Rats. Brain Sci. 2021, 11, 1250. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Carvey, P.M.; Ling, Z. Age-related changes in glutathione and glutathione-related enzymes in rat brain. Brain Res. 2006, 1090, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Emir, U.E.; Raatz, S.; McPherson, S.; Hodges, J.S.; Torkelson, C.; Tawfik, P.; White, T.; Terpstra, M. Noninvasive quantification of ascorbate and glutathione concentration in the elderly human brain. NMR Biomed. 2011, 24, 888–894. [Google Scholar] [CrossRef]
- Zalachoras, I.; Hollis, F.; Ramos-Fernandez, E.; Trovo, L.; Sonnay, S.; Geiser, E.; Preitner, N.; Steiner, P.; Sandi, C.; Morato, L. Therapeutic potential of glutathione-enhancers in stress-related psychopathologies. Neurosci. Biobehav. Rev. 2020, 114, 134–155. [Google Scholar] [CrossRef]
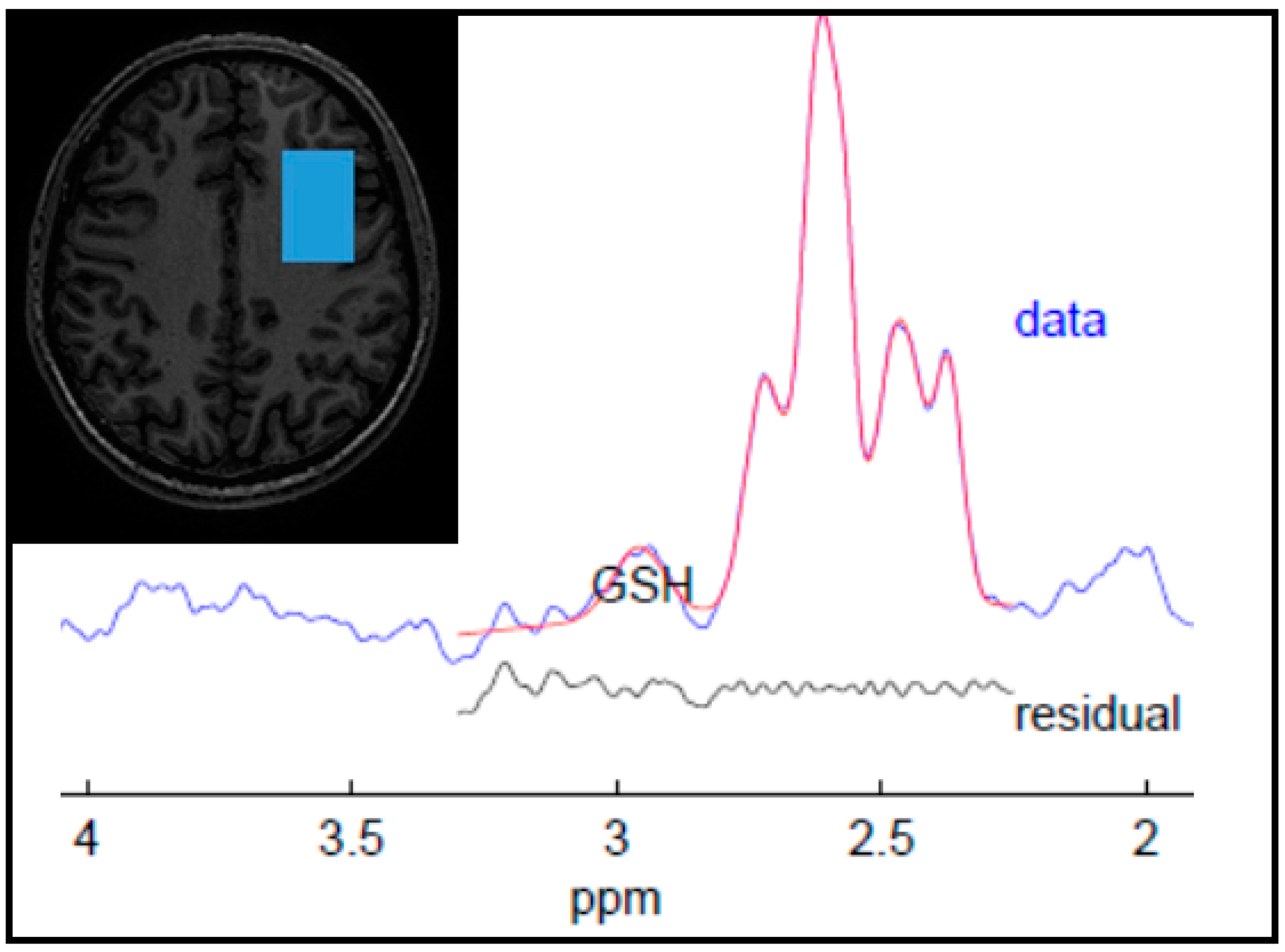
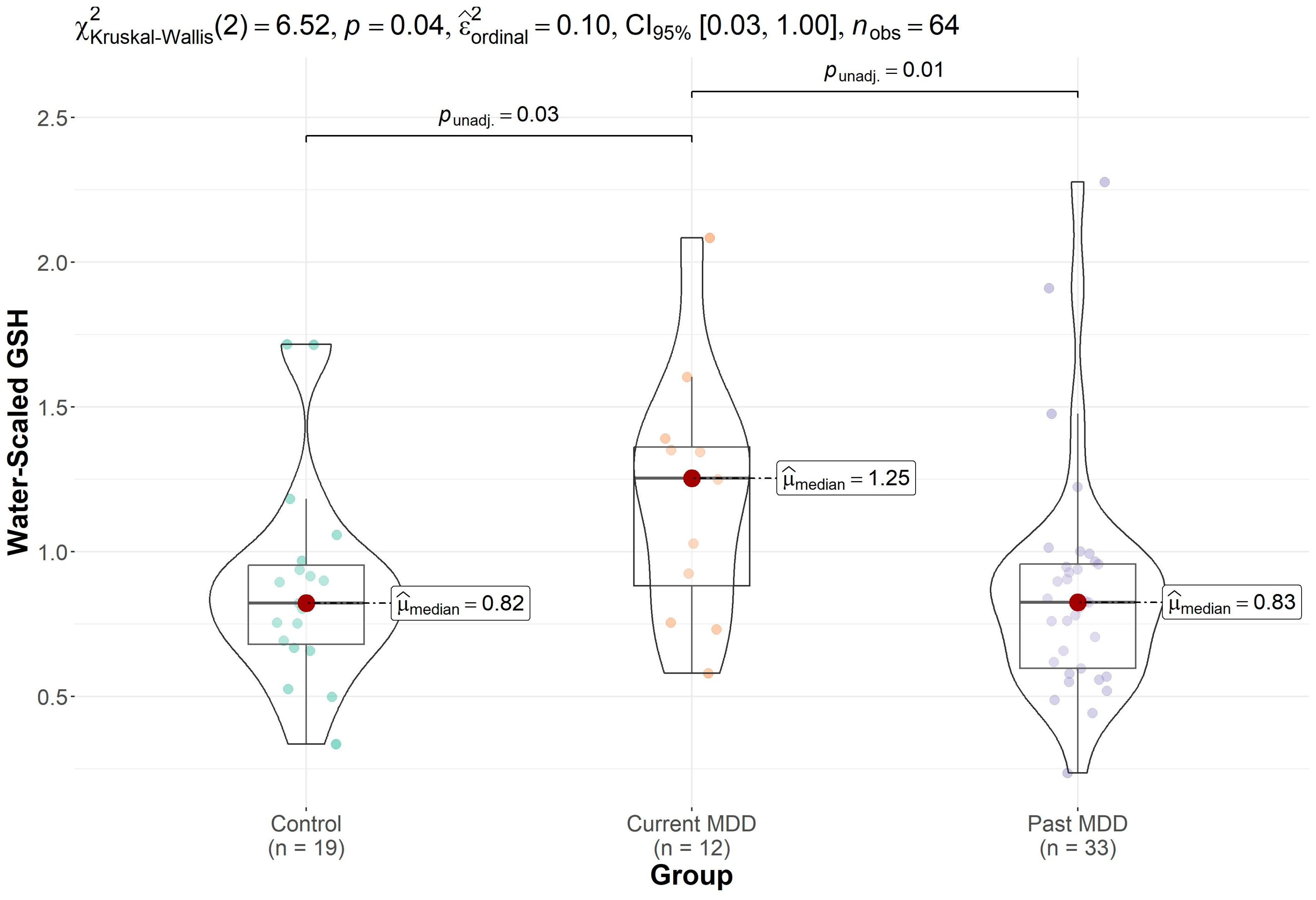
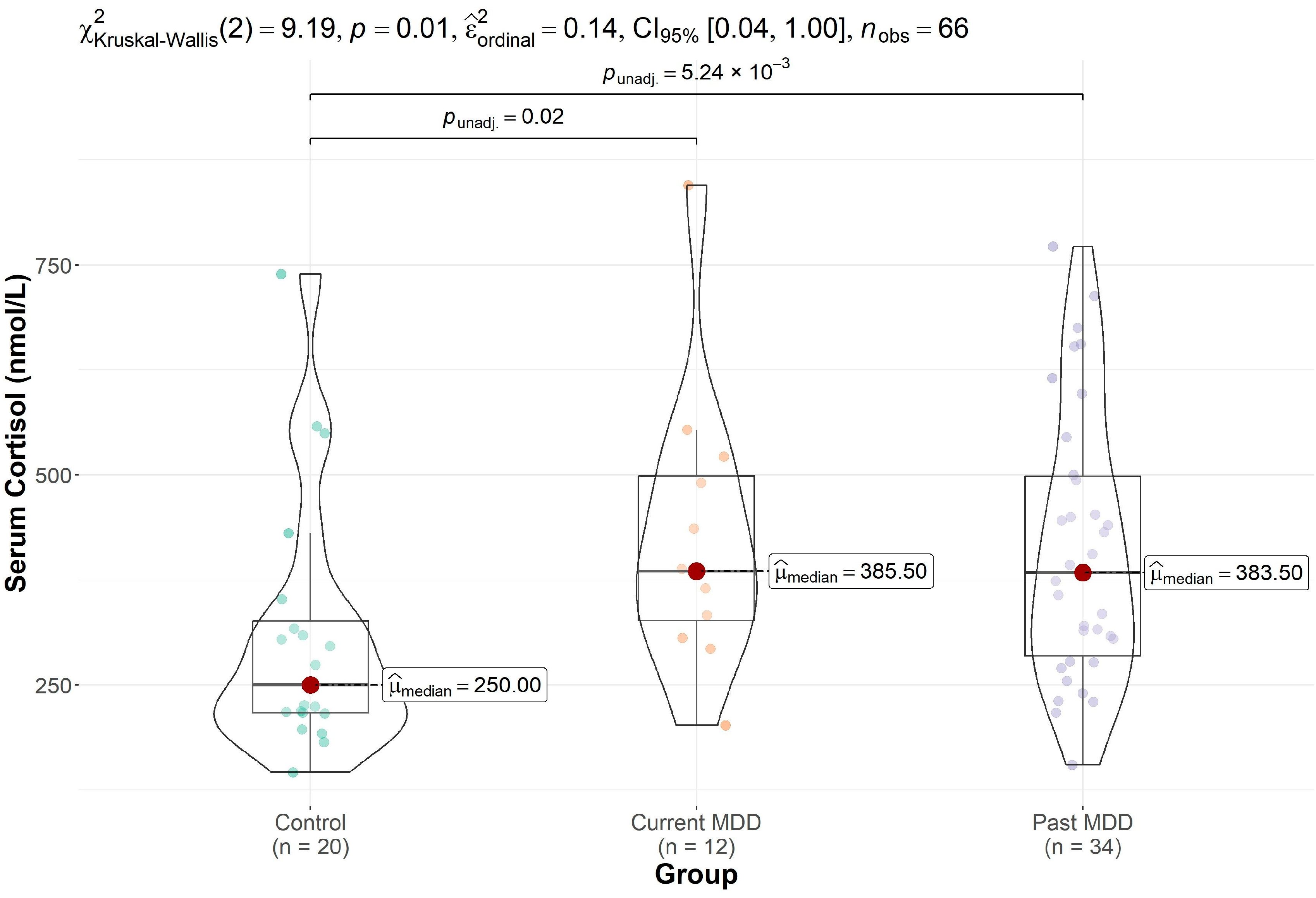
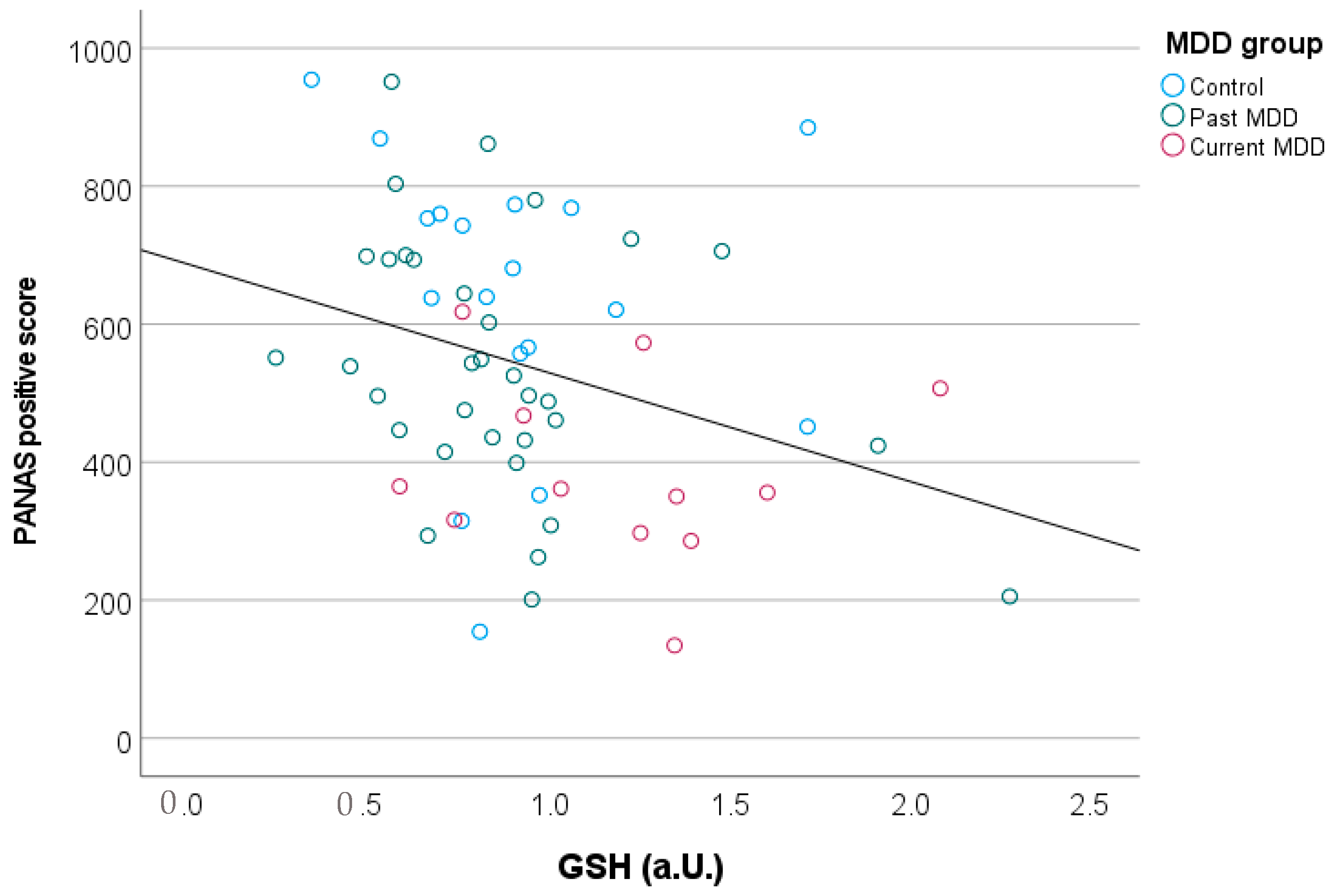
| Control (n = 20) | Past MDD (n = 34) | Current MDD (n = 12) | p-Value | |
|---|---|---|---|---|
| Age | 27.5 (5.2) | 24.7 (4.7) | 22.3 (2.8) | 0.010 |
| Male sex | 37% | 30% | 42% | 0.753 |
| GSH | 0.89 (0.36) (n = 19) * | 0.87 (0.40) (n = 33) * | 1.19 (0.42) (n = 12) | 0.038 |
| BDI | 4.1 (4.3) | 8.3 (5.2) | 23.1 (9.5) | <0.001 |
| HAM-D (range) | 1.83 (4.6) (0–17) | 5.42 (4.9) (0–17) | 16.3 (9.2) (1–31) | <0.001 |
| MADRS (range) | 1.33 (3.8) (0–16) | 6.94 (6.1) (0–16) | 22.5 (6.6) (13–34) | <0.001 |
| PSS | 18.8 (6.1) | 25.8 (6.3) | 35.7 (5.2) | <0.001 |
| PANAS-positive | 638 (211) | 540 (185) | 386 (135) | 0.003 |
| PANAS-negative | 53 (80) | 83 (102) | 222 (171) | <0.001 |
| cortisol | 308 (160) | 417 (162) | 427 (166) | 0.013 |
| Voxel GM fraction | 0.31 (0.03) | 0.32 (0.04) | 0.34 (0.03) | 0.017 |
| Voxel WM fraction | 0.66 (0.04) | 0.65 (0.05) | 0.63 (0.03) | 0.055 |
| Voxel CSF fraction | 0.03 (0.01) | 0.03 (0.02) | 0.03 (0.01) | 0.601 |
| Education (years) | 13.1 (3.82) | 14.0 (3.12) | 12.7 (2.16) | 0.549 |
| MWT-A | 29.2 (4.6) | 31.0 (4.6) | 29.5 (2.6) | 0.280 |
| Comorbid anxiety disorder | 21% | 55% | 75% | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuura, R.O.; Buchmann, A.; Ritter, C.; Hase, A.; Haynes, M.; Noeske, R.; Hasler, G. Prefrontal Glutathione Levels in Major Depressive Disorder Are Linked to a Lack of Positive Affect. Brain Sci. 2023, 13, 1475. https://doi.org/10.3390/brainsci13101475
Tuura RO, Buchmann A, Ritter C, Hase A, Haynes M, Noeske R, Hasler G. Prefrontal Glutathione Levels in Major Depressive Disorder Are Linked to a Lack of Positive Affect. Brain Sciences. 2023; 13(10):1475. https://doi.org/10.3390/brainsci13101475
Chicago/Turabian StyleTuura, Ruth O’Gorman, Andreas Buchmann, Christopher Ritter, Adrian Hase, Melanie Haynes, Ralph Noeske, and Gregor Hasler. 2023. "Prefrontal Glutathione Levels in Major Depressive Disorder Are Linked to a Lack of Positive Affect" Brain Sciences 13, no. 10: 1475. https://doi.org/10.3390/brainsci13101475
APA StyleTuura, R. O., Buchmann, A., Ritter, C., Hase, A., Haynes, M., Noeske, R., & Hasler, G. (2023). Prefrontal Glutathione Levels in Major Depressive Disorder Are Linked to a Lack of Positive Affect. Brain Sciences, 13(10), 1475. https://doi.org/10.3390/brainsci13101475






