A Review of the Potential of Nuclear Factor [Erythroid-Derived 2]-like 2 Activation in Autoimmune Diseases
Abstract
1. Introduction
- (a)
- (b)
- KEAP1, the protein sensor of these inducers;
- (c)
- The transcription factor NRF2, which regulates the transcriptional response to inducers and oxidative stress;
- (d)
- The target genes that supply the cytoprotective output of the path [20].
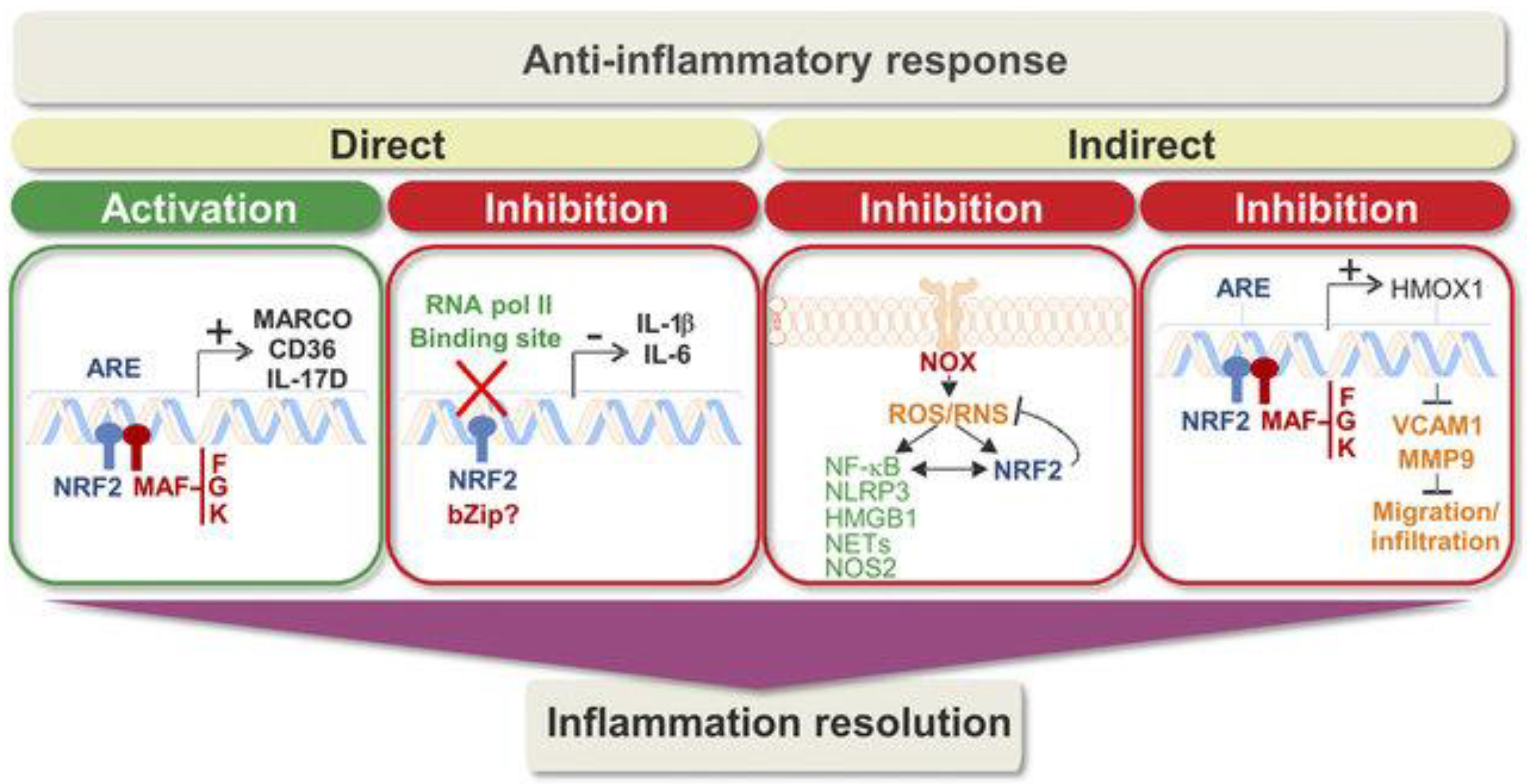
2. Nrf2 Regulation and Inflammation
3. Keap1/Nrf2/ARE Signaling Pathway: Possible Link to Anti-Inflammatory and Antioxidant Mechanisms
4. Diabetes Mellitus and Nrf2 Activation
5. Multiple Sclerosis and Nrf2 Activation
6. Systemic Lupus Erythematosus and Nrf2 Activation
7. Inflammatory Bowel Disease and Nrf2 Activation
8. Autoimmune Addison’s Disease and Nrf2 Activation
9. Graves’ Disease and Nrf2 Activation
10. Nrf2 and Rheumatoid Arthritis
11. Effects of Nrf2 Modulation on Brain Health
12. Nrf2 and Alzheimer’s Disease
13. Nrf2 and Parkinson’s Disease
14. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nicholson, L.B. The immune system. Essays Biochem. 2016, 60, 275–301. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.-J.; Wang, L.; Yang, Y.-Y.; Zhang, J.-J.; Liu, X.-H. Diabetes Aggravates Renal Ischemia and Reperfusion Injury in Rats by Exacerbating Oxidative Stress, Inflammation, and Apoptosis. Ren. Fail. 2019, 41, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Ramani, S.; Pathak, A.; Dalal, V.; Paul, A.; Biswas, S. Oxidative Stress in Autoimmune Diseases: An Under Dealt Malice. Curr. Protein Pept. Sci. 2020, 21, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Mehling, R.; Schwenck, J.; Lemberg, C.; Trautwein, C.; Zizmare, L.; Kramer, D.; Müller, A.; Fehrenbacher, B.; Gonzalez-Menendez, I.; Quintanilla-Martinez, L.; et al. Immunomodulatory role of reactive oxygen species and nitrogen species during T cell-driven neutrophil-enriched acute and chronic cutaneous delayed-type hypersensitivity reactions. Theranostics 2021, 11, 470–490. [Google Scholar] [CrossRef]
- Smallwood, M.J.; Nissim, A.; Knight, A.R.; Whiteman, M.; Haigh, R.; Winyard, P.G. Oxidative stress in autoimmune rheumatic diseases. Free Radic. Biol. Med. 2018, 125, 3–14. [Google Scholar] [CrossRef]
- Lin, W.; Shen, P.; Song, Y.; Huang, Y.; Tu, S. Reactive Oxygen Species in Autoimmune Cells: Function, Differentiation, and Metabolism. Front. Immunol. 2021, 25, 635021. [Google Scholar] [CrossRef]
- Taguchi, K.; Motohashi, H.; Yamamoto, M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells 2011, 16, 123–140. [Google Scholar] [CrossRef]
- Telkoparan-Akillilar, P.; Panieri, E.; Cevik, D.; Suzen, S.; Saso, L. Therapeutic Targeting of the NRF2 Signaling Pathway in Cancer. Molecules 2021, 26, 1417. [Google Scholar] [CrossRef]
- Motohashi, H.; Katsuoka, F.; Engel, J.D.; Yamamoto, M. Small Maf proteins serve as transcriptional cofactors for keratinocyte differentiation in the Keap1–Nrf2 regulatory pathway. Proc. Natl. Acad. Sci. USA 2004, 101, 6379–6384. [Google Scholar] [CrossRef]
- Canning, P.; Sorrell, F.J.; Bullock, A.N. Structural basis of Keap1 interactions with Nrf2. Free Radic. Biol. Med. 2015, 88, 101–107. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [PubMed]
- Barati, M.T.; Caster, D.J. The Potential of Nrf2 Activation as a Therapeutic Target in Systemic Lupus Erythematosus. Metabolites 2022, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, A.; Bramanti, P.; Mazzon, E. Activation of Nrf2 by Natural Bioactive Compounds: A Promising Approach for Stroke? Int. J. Mol. Sci. 2020, 21, 4875. [Google Scholar] [CrossRef]
- Bryan, H.K.; Olayanju, A.; Goldring, C.E.; Park, B.K. The Nrf2 cell defence pathway: Keap1-dependent and-independent mechanisms of regulation. Biochem. Pharmacol. 2013, 85, 705–717. [Google Scholar] [CrossRef]
- Hiemstra, S.; Fehling-Kaschek, M.; Kuijper, I.A.; Bischoff, L.J.M.; Wijaya, L.S.; Rosenblatt, M.; Esselink, J.; van Egmond, A.; Mos, J.; Beltman, J.B.; et al. Dynamic modeling of Nrf2 pathway activation in liver cells after toxicant exposure. Sci. Rep. 2022, 12, 7336. [Google Scholar] [CrossRef]
- Harder, B.; Jiang, T.; Wu, T.; Tao, S.; Rojo de la Vega, M.; Tian, W.; Chapman, E.; Zhang, D.D. Molecular mechanisms of Nrf2 regulation and how these influence chemical modulation for disease intervention. Biochem. Soc. Trans. 2015, 43, 680–686. [Google Scholar] [CrossRef]
- Dodson, M.; Rojo de la Vega, M.; Cholanians, A.B.; Schmidlin, C.J.; Chapman, E.; Zhang, D.D. Modulating NRF2 in disease: Timing is everything. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 555–575. [Google Scholar] [CrossRef]
- Tebay, L.E.; Robertson, H.; Durant, S.T.; Vitale, S.R.; Penning, T.M.; Dinkova-Kostova, A.T.; Hayes, J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 2015, 88, 108–146. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Rojo de la Vega, M.; Dodson, M.; Chapman, E.; Zhang, D.D. NRF2-targeted therapeutics: New targets and modes of NRF2 regulation. Curr. Opin. Toxicol. 2016, 1, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Surh, Y.J. The Role of Nrf2 in Cellular Innate Immune Response to Inflammatory Injury. Toxicol. Res. 2009, 25, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Suzen, S.; Tucci, P.; Profumo, E.; Buttari, B.; Saso, L. A Pivotal Role of Nrf2 in Neurodegenerative Disorders: A New Way for Therapeutic Strategies. Pharmaceuticals 2022, 15, 692. [Google Scholar] [CrossRef]
- Bullock, J.; Rizvi, S.A.A.; Saleh, A.M.; Ahmed, S.S.; Do, D.P.; Ansari, R.A.; Ahmed, J. Rheumatoid Arthritis: A Brief Overview of the Treatment. Med. Princ. Pract. 2018, 27, 501–507. [Google Scholar] [CrossRef]
- Fava, A.; Petri, M. Systemic lupus erythematosus: Diagnosis and clinical management. J. Autoimmun. 2019, 96, 1–13. [Google Scholar] [CrossRef]
- Suzuki, T.; Murakami, S.; Biswal, S.S.; Sakaguchi, S.; Harigae, H.; Yamamoto, M.; Motohashi, H. Systemic Activation of NRF2 Alleviates Lethal Autoimmune Inflammation in Scurfy Mice. Mol. Cell. Biol. 2017, 37, e00063-17. [Google Scholar] [CrossRef]
- Ferrándiz, M.L.; Nacher-Juan, J.; Alcaraz, M.J. Nrf2 as a therapeutic target for rheumatic diseases. Biochem. Pharmacol. 2018, 152, 338–346. [Google Scholar] [CrossRef]
- Tonelli, C.; Christine Chio, I.I.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Chen, Q.M.; Maltagliati, A.J. Nrf2 at the heart of oxidative stress and cardiac protection. Physiol. Genom. 2018, 50, 77–97. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress--implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Dodd, G.; Thomas, S.; Zhang, X.; Wasserman, M.A.; Rovin, B.H.; Kunsch, C. Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H1862–H1870. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Cuadrado, A.; Martín-Moldes, Z.; Ye, J.; Lastres-Becker, I. Transcription factors NRF2 and NF-kB are coordinated effectors of the Rho family, GTP-binding protein RAC1 during inflammation. J. Biol. Chem. 2014, 289, 15244–15258. [Google Scholar] [CrossRef]
- Thimmulappa, R.K.; Lee, H.; Rangasamy, T.; Reddy, S.P.; Yamamoto, M.; Kensler, T.W.; Biswal, S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J. Clin. Investig. 2006, 116, 984–995. [Google Scholar] [CrossRef]
- Sha, L.K.; Sha, W.; Kuchler, L.; Daiber, A.; Giegerich, A.K.; Weigert, A.; Knape, T.; Snodgrass, R.; Schröder, K.; Brandes, R.P.; et al. Loss of Nrf2 in bone marrow-derived macrophages impairs antigen-driven CD8(+) T cell function by limiting GSH and Cys availability. Free Radic. Biol. Med. 2015, 83, 77–88. [Google Scholar] [CrossRef]
- Rojo, A.I.; Innamorato, N.G.; Martín-Moreno, A.M.; De Ceballos, M.L.; Yamamoto, M.; Cuadrado, A. Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson’s disease. Glia 2010, 58, 588–598. [Google Scholar] [CrossRef]
- Rojo, A.I.; McBean, G.; Cindric, M.; Egea, J.; López, M.G.; Rada, P.; Zarkovic, N.; Cuadrado, A. Redox control of microglial function: Molecular mechanisms and functional significance. Antioxid. Redox Signal. 2014, 21, 1766–1801. [Google Scholar] [CrossRef]
- Brüne, B.; Dehne, N.; Grossmann, N.; Jung, M.; Namgaladze, D.; Schmid, T.; von Knethen, A.; Weigert, A. Redox control of inflammation in macrophages. Antioxid. Redox Signal. 2013, 19, 595–637. [Google Scholar] [CrossRef]
- Diotallevi, M.; Checconi, P.; Palamara, A.T.; Celestino, I.; Coppo, L.; Holmgren, A.; Abbas, K.; Peyrot, F.; Mengozzi, M.; Ghezzi, P. Glutathione fine-tunes the innate immune response toward antiviral pathways in a macrophage cell line independently of its antioxidant properties. Front. Immunol. 2017, 8, 1239. [Google Scholar] [CrossRef]
- Cuadrado, A.; Manda, G.; Hassan, A.; Alcaraz, M.J.; Barbas, C.; Daiber, A.; Ghezzi, P.; León, R.; López, M.G.; Oliva, B.; et al. Transcription factor NRF2 as a therapeutic target for chronic diseases: A systems medicine approach. Pharmacol. Rev. 2018, 70, 348–383. [Google Scholar] [CrossRef]
- Motterlini, R.; Foresti, R. Biological signaling by carbon monoxide and carbon monoxide-releasing molecules. Am. J. Physiol. Cell Physiol. 2017, 312, C302–C313. [Google Scholar] [CrossRef]
- Maines, M.D. The heme oxygenase system: A regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 517–554. [Google Scholar] [CrossRef]
- Gozzelino, R.; Jeney, V.; Soares, M.P. Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef]
- Ma, L.; Sun, L.; Wang, Y.; Sun, B.; Li, Y.; Jin, Y. Association between HO-1 gene promoter polymorphisms and diseases (Review). Mol. Med. Rep. 2022, 25, 29. [Google Scholar] [CrossRef]
- Wagener, F.A.; Volk, H.D.; Willis, D.; Abraham, N.G.; Soares, M.P.; Adema, G.J.; Figdor, C.G. Different faces of the heme-heme oxygenase system in inflammation. Pharmacol. Rev. 2003, 55, 551–571. [Google Scholar] [CrossRef]
- Kim, H.P.; Ryter, S.W.; Choi, A.M. CO as a cellular signaling molecule. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 411–449. [Google Scholar] [CrossRef]
- Zhou, J.; Terluk, M.R.; Basso, L.; Mishra, U.R.; Orchard, P.J.; Cloyd, J.C.; Schröder, H.; Kartha, R.V. N-acetylcysteine provides cytoprotection in murine oligodendrocytes through heme oxygenase-1 activity. Biomedicines 2020, 8, 240. [Google Scholar] [CrossRef]
- Jazwa, A.; Cuadrado, A. Targeting heme oxygenase-1 for neuroprotection and neuroinflammation in neurodegenerative diseases. Curr. Drug Targets 2010, 11, 1517–1531. [Google Scholar] [CrossRef]
- Alam, J.; Stewart, D.; Touchard, C.; Boinapally, S.; Choi, A.M.; Cook, J.L. Nrf2, a Cap’n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 1999, 274, 26071–26078. [Google Scholar] [CrossRef]
- Balogun, E.; Hoque, M.; Gong, P.; Killeen, E.; Green, C.J.; Foresti, R.; Alam, J.; Motterlini, R. Curcumin activates the heme oxygenase-1 gene via regulation of Nrf2 and the antioxidant responsive element. Biochem. J. 2003, 371, 887–895. [Google Scholar] [CrossRef]
- Ishii, T.; Itoh, K.; Takahashi, S.; Sato, H.; Yanagawa, T.; Katoh, Y.; Bannai, S.; Yamamoto, M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000, 275, 16023–16029. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Cole, R.N.; Itoh, K.; Wakabayashi, N.; Katoh, Y.; Yamamoto, M.; Talalay, P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA 2002, 99, 11908–11913. [Google Scholar] [CrossRef]
- Baird, L.; Dinkova-Kostova, A.T. The cytoprotective role of the Keap1-Nrf2 pathway. Arch. Toxicol. 2011, 85, 241–272. [Google Scholar] [CrossRef]
- Rangasamy, T.; Cho, C.Y.; Thimmulappa, R.K.; Zhen, L.; Srisuma, S.S.; Kensler, T.W.; Yamamoto, M.; Petrache, I.; Tuder, R.M.; Biswal, S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J. Clin. Investig. 2004, 114, 1248–1259. [Google Scholar] [CrossRef]
- Motterlini, R.; Foresti, R. Heme oxygenase-1 as a target for drug discovery. Antioxid. Redox Signal. 2014, 20, 1810–1826. [Google Scholar] [CrossRef]
- Fagone, P.; Piombino, E.; Mangano, K.; De Pasquale, R.; Nicoletti, F.; Caltabiano, R. Evaluation of the involvement of Heme Oxygenase-1 expression in discoid Lupus Erythematosus lesions. Antioxidants 2023, 12, 1352. [Google Scholar] [CrossRef]
- Li, J.; Stein, T.D.; Johnson, J.A. Genetic dissection of systemic autoimmune disease in Nrf2-deficient mice. Physiol. Genom. 2004, 18, 261–272. [Google Scholar] [CrossRef]
- Bayo Jimenez, M.T.; Frenis, K.; Hahad, O.; Steven, S.; Cohen, G.; Cuadrado, A.; Münzel, T.; Daiber, A. Protective actions of nuclear factor erythroid 2-related factor 2 (NRF2) and downstream pathways against environmental stressors. Free Radic. Biol. Med. 2022, 187, 72–91. [Google Scholar] [CrossRef]
- Mangano, K.; Cavalli, E.; Mammana, S.; Basile, M.S.; Caltabiano, R.; Pesce, A.; Puleo, S.; Atanasov, A.G.; Magro, G.; Nicoletti, F.; et al. Involvement of the Nrf2/HO-1/CO axis and therapeutic intervention with the CO-releasing molecule CORM-A1, in a murine model of autoimmune hepatitis. J. Cell. Physiol. 2018, 233, 4156–4165. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, I.; Saksida, T.; Mangano, K.; Vujicic, M.; Stojanovic, I.; Nicoletti, F.; Stosic-Grujicic, S. Pharmacological application of carbon monoxide ameliorates islet-directed autoimmunity in mice via anti-inflammatory and anti-apoptotic effects. Diabetologia 2014, 57, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, M.; Patil, J.; D’Angelo, B.; Weber, S.G.; Mallard, C. NRF2-regulation in brain health and disease: Implication of cerebral inflammation. Neuropharmacology 2014, 79, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohe, R.; Flohe, L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid. Redox Signal. 2011, 15, 2335–2381. [Google Scholar] [CrossRef]
- Ahn, K.S.; Aggarwal, B.B. Transcription Factor NF-κB: A sensor for smoke and stress signals. Ann. N. Y. Acad. Sci. 2005, 1056, 218. [Google Scholar] [CrossRef]
- Rushworth, S.A.; Chen, X.L.; Mackman, N.; Ogborne, R.M.; O’Connell, M.A. Lipopolysaccharide-induced heme oxygenase-1 expression in human monocytic cells is mediated via Nrf2 and protein kinase C. J. Immunol. 2005, 175, 4408. [Google Scholar] [CrossRef]
- Ates, I.; Suzen, H.S.; Yucesoy, B.; Ozel Tekin, I.; Karakaya, A. Association of cytokine gene polymorphisms in CWP and its severity in Turkish coal workers. Am. J. Ind. Med. 2008, 51, 741–747. [Google Scholar] [CrossRef]
- Mills, E.L.; Ryan, D.G.; Prag, H.A.; Dikovskaya, D.; Menon, D.; Zaslona, Z.; Jedrychowski, M.P.; Costa, A.S.H.; Higgins, M.; Hams, E.; et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 2018, 556, 113–117. [Google Scholar] [CrossRef]
- Yan, N.; Xu, Z.; Qu, C.; Zhang, J. Dimethyl fumarate improves cognitive deficits in chronic cerebral hypoperfusion rats by alleviating inflammation, oxidative stress, and ferroptosis via NRF2/ARE/NF-κB signal pathway. Int. Immunopharmacol. 2021, 98, 107844. [Google Scholar] [CrossRef]
- Ding, L.; Yuan, X.; Yan, J.; Huang, Y.; Xu, M.; Yang, Z.; Yang, N.; Wang, M.; Zhang, C.; Zhang, L. Nrf2 exerts mixed inflammation and glucose metabolism regulatory effects on murine RAW264.7 macrophages. Int. Immunopharmacol. 2019, 71, 198–204. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Sehgal, A.; Sharma, E.; Kumar, A.; Grover, M.; Bungau, S. Unfolding Nrf2 in diabetes mellitus. Mol. Biol. Rep. 2021, 48, 927–939. [Google Scholar] [CrossRef]
- Zheng, H.; Whitman, S.A.; Wu, W.; Wondrak, G.T.; Wong, P.K.; Fang, D.; Zhang, D.D. Therapeutic potential of Nrf2 activators in streptozotoc ininduced diabetic nephropathy. Diabetes 2011, 60, 3055–3066. [Google Scholar] [CrossRef]
- El-Bab, M.F.; Zaki, N.S.; Mojaddidi, M.A.; Al-Barry, M.; El-Beshbishy, H.A. Diabetic retinopathy is associated with oxidative stress and mitigation of gene expression of antioxidant enzymes. Int. J. Gen. Med. 2013, 6, 799. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ghosh, P.; Sahoo, R.; Vaidya, A.; Chorev, M.; Halperin, J.A. Role of complement and complement regulatory proteins in the complications of diabetes. Endocr. Rev. 2015, 36, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, Y.; Kanda, H.; Shinkai, Y.; Toyama, T. The role of the Keap1/Nrf2 pathway in the cellular response to methylmercury. Oxidative Med. Cell. Longev. 2013, 2013, 848279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, X.; Jin, S.; Chen, Y.; Guo, R. Ferroptosis in cancer therapy: A novel approach to reversing drug resistance. Mol. Cancer 2022, 21, 47. [Google Scholar] [CrossRef]
- Li, S.; Zheng, L.; Zhang, J.; Liu, X.; Wu, Z. Inhibition of ferroptosis by up-regulating Nrf2 delayed the progression of diabetic nephropathy. Free Radic. Biol. Med. 2021, 162, 435–449. [Google Scholar] [CrossRef]
- Cao, Z.; Cooper, M.E. Pathogenesis of diabetic nephropathy. J. Diabetes Investig. 2011, 2, 243–247. [Google Scholar] [CrossRef]
- Seldon, M.P.; Silva, G.; Pejanovic, N.; Larsen, R.; Gregoire, I.P.; Filipe, J.; Anrather, J.; Soares, M.P. Heme oxygenase-1 inhibits the expression of adhesion molecules associated with endothelial cell activation via inhibition of NF-kappa B RelA phosphorylation at serine 276. J. Immunol. 2007, 179, 7840–7851. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Tohidi, M.; Nazeri, P.; Mehran, M.; Azizi, F.; Mirmiran, P. Effect of broccoli sprouts on insulin resistance in type 2 diabetic patients: A randomized double-blind clinical trial. Int. J. Food Sci. Nutr. 2012, 63, 767–771. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Sun, W.; Tan, Y.; Liu, Y.; Zheng, Y.; Liu, Q.; Cai, L.; Sun, J. Sulforaphane attenuation of type 2 diabetes-induced aortic damage was associated with the upregulation of Nrf2 expression and function. Oxidative Med. Cell. Longev. 2014, 2014, 123963. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Fernandes, R.; Crisostomo, J.; Seiça, R.M.; Sena, C.M. The Sulforaphane and pyridoxamine supplementation normalize endothelial dysfunction associated with type 2 diabetes. Sci. Rep. 2017, 7, 14357. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Mann, G.E.; Chapple, S.J. Concerted redox modulation by sulforaphane alleviates diabetes and cardiometabolic syndrome. Free Radic. Biol. Med. 2018, 122, 150–160. [Google Scholar] [CrossRef]
- Ellison, D.H. Bardoxolone methyl in type 2 diabetes and advanced chronic kidney disease. N. Eng. J. Med. 2014, 370, 1768. [Google Scholar]
- Tan, S.M.; Sharma, A.; Stefanovic, N.; Yuen, D.Y.C.; Karagiannis, T.C.; Meyer, C.; Ward, K.W.; Cooper, M.E.; de Haan, J.B. Derivative of bardoxolone methyl, dh404, in an inverse dose-dependent manner lessens diabetes-associated atherosclerosis and improves diabetic kidney disease. Diabetes 2014, 63, 3091–3103. [Google Scholar] [CrossRef]
- Ha, C.M.; Park, S.; Choi, Y.K.; Jeong, J.-Y.; Oh, C.J.; Bae, K.-H.; Lee, S.J.; Kim, J.-H.; Park, K.-G.; Jun, D.Y.; et al. Activation of Nrf2 by dimethyl fumarate improves vascular calcification. Vasc. Pharmacol. 2014, 63, 29–36. [Google Scholar] [CrossRef]
- Lazaro, I.; Lopez-Sanz, L.; Bernal, S.; Oguiza, A.; Recio, C.; Melgar, A.; Jimenez-Castilla, L.; Egido, J.; Madrigal-Matute, J.; Gomez-Guerrero, C. Nrf2 activation provides atheroprotection in diabetic mice through concerted upregulation of antioxidant, anti-inflammatory, and autophagy mechanisms. Front. Pharmacol. 2018, 9, 819. [Google Scholar] [CrossRef]
- Yu, W.; Liu, W.; Xie, D.; Wang, Q.; Xu, C.; Zhao, H.; Lv, J.; He, F.; Chen, B.; Yamamoto, T.; et al. High Level of Uric Acid Promotes Atherosclerosis by Targeting NRF2-Mediated Autophagy Dysfunction and Ferroptosis. Oxidative Med. Cell. Longev. 2022, 2022, 9304383. [Google Scholar] [CrossRef]
- Li, H.; Zhuang, W.; Xiong, T.; Park, W.S.; Zhang, S.; Zha, Y.; Yao, J.; Wang, F.; Yang, Y.; Chen, Y.; et al. Nrf2 deficiency attenuates atherosclerosis by reducing LOX-1-mediated proliferation and migration of vascular smooth muscle cells. Atherosclerosis 2022, 347, 1–16. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, X.; Zhang, R.; Ma, B.; Niu, S.; Di, X.; Ni, L.; Liu, C. Melatonin attenuates smoking-induced atherosclerosis by activating the Nrf2 pathway via NLRP3 inflammasomes in endothelial cells. Aging 2021, 13, 11363–11380. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, C.; Jin, Y.; Meng, Q.; Wu, J.; Sun, H. Kaempferol-induced GPER upregulation attenuates atherosclerosis via the PI3K/AKT/Nrf2 pathway. Pharm. Biol. 2021, 59, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Dreger, H.; Westphal, K.; Wilck, N.; Baumann, G.; Stangl, V.; Stangl, K.; Meiners, S. Protection of vascular cells from oxidative stress by proteasome inhibition depends on Nrf2. Cardiovasc. Res. 2009, 85, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Kovac, S.; Angelova, P.R.; Holmström, K.M.; Zhang, Y.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim. Biophys. Acta 2015, 1850, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Ashford, M.L. Nrf2 Orchestrates Fuel Partitioning for Cell Proliferation. Cell Metab. 2012, 16, 139–141. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of Medical Care in Diabetes-2022. Abridged for Primary Care Providers. Clin. Diabetes 2022, 40, 10–38. [Google Scholar] [CrossRef]
- Jia, Q.; Yang, R.; Liu, X.; Ma, S.; Wang, L. Genistein Attenuates Renal Fibrosis in Streptozotocin-induced Diabetic Rats. Mol. Med. Rep. 2019, 1, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Civantos, E.; Bosch, E.; Ramírez Bustillo, E.; Zhenyukh, O.; Egido, J.; Lorenzo, O.; Mas, S. Sitagliptin Ameliorates Oxidative Stress in Experimental Diabetic Nephropathy by Diminishing the MiR-200a/Keap-1/Nrf2 Antioxidant Pathway. Diabetes Metab. Syndr. Obes. 2017, 10, 207–222. [Google Scholar] [CrossRef]
- Sakashita, M.; Tanaka, T.; Inagi, R. Metabolic Changes and Oxidative Stress in Diabetic Kidney Disease. Antioxidants 2021, 10, 1143. [Google Scholar] [CrossRef]
- Yamazaki, T.; Mimura, I.; Tanaka, T.; Nangaku, M. Treatment of Diabetic Kidney Disease: Current and Future. Diabetes Metab. J. 2021, 45, 11–26. [Google Scholar] [CrossRef]
- Alaofi, A.L. Sinapic Acid Ameliorates the Progression of Streptozotocin (STZ)-Induced Diabetic Nephropathy in Rats via NRF2/HO-1 Mediated Pathways. Front. Pharmacol. 2020, 11, 1119. [Google Scholar] [CrossRef]
- Arellano-Buendía, A.S.; Castañeda-Lara, L.G.; Loredo-Mendoza, M.L.; García-Arroyo, F.E.; Rojas-Morales, P.; Argüello-García, R.; Juárez-Rojas, J.G.; Tapia, E.; Pedraza-Chaverri, J.; Sánchez-Lozada, L.G.; et al. Effects of Allicin on Pathophysiological Mechanisms during the Progression of Nephropathy Associated to Diabetes. Antioxidants 2020, 9, 1134. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, X.; Ma, F.; Sun, W.; Wang, W.; Yu, J.; Shi, Y.; Cai, L.; Xu, Z. The Role of Akt2 in the Protective Effect of Fenofibrate against Diabetic Nephropathy. Int. J. Biol. Sci. 2020, 16, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhu, H.; Wang, X.; Gao, Q.; Li, Z.; Huang, H. CoQ10 ameliorates mitochondrial dysfunction in diabetic nephropathy through mitophagy. J. Endocrinol. 2019, 240, 445–465. [Google Scholar] [CrossRef] [PubMed]
- Tanase, D.M.; Gosav, E.M.; Anton, M.I.; Floria, M.; Isac, P.N.S.; Hurjui, L.L.; Tarniceriu, C.C.; Costea, C.F.; Ciocoiu, M.; Rezus, C. Oxidative Stress and NRF2/KEAP1/ARE Pathway in Diabetic Kidney Disease (DKD): New Perspectives. Biomolecules 2022, 12, 1227. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, X.; Jiang, Z.; Jiang, J.; Xu, L.; Tian, A.; Sun, X.; Meng, H.; Li, Y.; Huang, W.; et al. Protective role of NRF2 in macrovascular complications of diabetes. J. Cell. Mol. Med. 2020, 24, 8903–8917. [Google Scholar] [CrossRef]
- Rampin, A.; Carrabba, M.; Mutoli, M.; Eman, C.L.; Testa, G.; Madeddu, P.; Spinetti, G. Recent Advances in KEAP1/NRF2-Targeting Strategies by Phytochemical Antioxidants, Nanoparticles, and Biocompatible Scaffolds for the Treatment of Diabetic Cardiovascular Complications. Antioxid. Redox Signal. 2022, 36, 707–728. [Google Scholar] [CrossRef]
- Frank, R.N. Diabetic retinopathy. N. Eng. J. Med. 2004, 350, 48–58. [Google Scholar] [CrossRef]
- Kowluru, R.A. Diabetes-induced elevations in retinal oxidative stress, protein kinase C and nitric oxide are inter-related. Acta Diabetol. 2001, 38, 179–185. [Google Scholar] [CrossRef]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Uruno, A.; Furusawa, Y.; Yagishita, Y.; Fukutomi, T.; Muramatsu, H.; Negishi, T.; Sugawara, A.; Kensler, T.W.; Yamamoto, M. The Keap1-Nrf2 system prevents onset of diabetes mellitus. Mol. Cell. Biol. 2013, 33, 2996–3010. [Google Scholar] [CrossRef]
- Shin, S.; Wakabayashi, J.; Yates, M.S.; Wakabayashi, N.; Dolan, P.M.; Aja, S.; Liby, K.T.; Sporn, M.B.; Yamamoto, M.; Kensler, T.W. Role of Nrf2 in prevention of high-fat diet-induced obesity by synthetic triterpenoid CDDO-imidazolide. Eur. J. Pharmacol. 2009, 620, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Shao, W.; Chiang, Y.; Foltz, W.; Zhang, Z.; Ling, W.; Fantus, I.G.; Jin, T. Oltipraz upregulates the nuclear factor (erythroid-derived 2)-like 2 [corrected](NRF2) antioxidant system and prevents insulin resistance and obesity induced by a high-fat diet in C57BL/6J mice. Diabetologia 2011, 54, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.K.; Reddy, V.T.; Konopleva, M.; Andreeff, M.; Chan, L. The triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic-acid methyl ester has potent anti-diabetic effects in diet-induced diabetic mice and Lepr(db/db) mice. J. Biol. Chem. 2010, 285, 40581–40592. [Google Scholar] [CrossRef] [PubMed]
- He, H.J.; Wang, G.Y.; Gao, Y.; Ling, W.H.; Yu, Z.W.; Jin, T.R. Curcumin attenuates Nrf2 signaling defect, oxidative stress in muscle and glucose intolerance in high fat diet-fed mice. World J. Diabetes 2012, 3, 94–104. [Google Scholar] [CrossRef]
- Xu, J.; Kulkarni, S.R.; Donepudi, A.C.; More, V.R.; Slitt, A.L. Enhanced Nrf2 activity worsens insulin resistance, impairs lipid accumulation in adipose tissue, and increases hepatic steatosis in leptin-deficient mice. Diabetes 2012, 61, 3208–3218. [Google Scholar] [CrossRef]
- More, V.R.; Xu, J.; Shimpi, P.C.; Belgrave, C.; Luyendyk, J.P.; Yamamoto, M.; Slitt, A.L. Keap1 knockdown increases markers of metabolic syndrome after long-term high fat diet feeding. Free Radic. Biol. Med. 2013, 61, 85–94. [Google Scholar] [CrossRef]
- de Zeeuw, D.; Akizawa, T.; Audhya, P.; Bakris, G.L.; Chin, M.; Christ-Schmidt, H.; Goldsberry, A.; Houser, M.; Krauth, M.; Lambers Heerspink, H.J.; et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Engl. J. Med. 2013, 369, 2492–2503. [Google Scholar] [CrossRef]
- Zhang, D.D. Bardoxolone brings Nrf2-based therapies to light. Antioxid. Redox Signal. 2013, 19, 517–518. [Google Scholar] [CrossRef]
- Haider, L. Inflammation, iron, energy failure, and oxidative stress in the pathogenesis of Multiple Sclerosis. Oxidative Med. Cell. Longev. 2015, 2015, 725370. [Google Scholar] [CrossRef]
- Ohl, K.; Tenbrock, K.; Kipp, M. Oxidative stress in multiple sclerosis: Central and peripheral mode of action. Exp. Neurol. 2016, 277, 58–67. [Google Scholar] [CrossRef]
- Neves Carvalho, A.; Firuzi, O.; Joao Gama, M.; van Horssen, J.; Saso, L. Oxidative stress and antioxidants in neurological diseases: Is there still hope? Curr. Drug Targets 2017, 18, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, B.; Adamczyk-Sowa, M. New insights into the role of oxidative stress mechanisms in the pathophysiology and treatment of Multiple Sclerosis. Oxidative Med. Cell. Longev. 2016, 2016, 1973834. [Google Scholar] [CrossRef] [PubMed]
- Fetisova, E.; Chernyak, B.; Korshunova, G.; Muntyan, M.; Skulachev, V. Mitochondria-targeted antioxidants as a prospective therapeutic strategy for Multiple Sclerosis. Curr. Med. Chem. 2017, 24, 2086–2114. [Google Scholar] [CrossRef]
- Solleiro-Villavicencio, H.; Rivas-Arancibia, S. Effect of chronic oxidative stress on neuroinflammatory response mediated by CD4+T cells in neurodegenerative diseases. Front. Cell. Neurosci. 2018, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Virgili, F.; Cervelatti, C.; Pecorelli, A. OxInflammation: From subclinical condition to pathological biomarker. Front. Physiol. 2018, 9, 858. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Kostov, R.V.; Kazantsev, A.G. The role of Nrf2 signaling in counteracting neurodegenerative diseases. FEBS J. 2018, 285, 3576–3590. [Google Scholar] [CrossRef]
- Vasconcelos, B.H.B.; Callegari, B.; Costa, K.H.A.; Barroso, T.G.C.P.; Sousa, R.C.M.; Saunier, G.; Xavier, M.B.; Souza, G.S. Balance impairments in patients with human T-Cell lymphotropic virus Type 1 infection. Sci. Rep. 2019, 9, 11456. [Google Scholar] [CrossRef]
- Tsokos, G.C. Systemic lupus erythematosus. N. Engl. J. Med. 2011, 365, 2110–2121. [Google Scholar] [CrossRef]
- Perl, A. Oxidative stress in the pathology and treatment of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2013, 9, 674–686. [Google Scholar] [CrossRef]
- Bona, N.; Pezzarini, E.; Balbi, B.; Daniele, S.M.; Rossi, M.F.; Monje, A.L.; Basiglio, C.L.; Pelusa, H.F.; Arriaga, S.M.M. Oxidative stress, inflammation and disease activity biomarkers in lupus nephropathy. Lupus 2020, 29, 311–323. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [PubMed]
- Ohl, K.; Tenbrock, K. Oxidative stress in SLE T cells, Is NRF2 really the target to treat? Front. Immunol. 2021, 12, 633845. [Google Scholar] [PubMed]
- Yoh, K.; Itoh, K.; Enomoto, A.; Hirayama, A.; Yamaguchi, N.; Kobayashi, M.; Morito, N.; Koyama, A.; Yamamoto, M.; Takahashi, S. Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int. 2001, 60, 1343–1353. [Google Scholar] [PubMed]
- Jiang, T.; Tian, F.; Zheng, H.; Whitman, S.A.; Lin, Y.; Zhang, Z.; Zhang, N.; Zhang, D.D. Nrf2 suppresses lupus nephritis through inhibition of oxidative injury and the NF-κB-mediated inflammatory response. Kidney Int. 2014, 85, 333–343. [Google Scholar] [PubMed]
- Wen, Z.; Liu, W.; Li, X.; Chen, W.; Liu, Z.; Wen, J.; Liu, Z. A protective role of the NRF2-Keap 1 pathway in maintaining intestinal barrier function. Oxid. Med. Cell. Longev. 2019, 2019, 1759149. [Google Scholar] [CrossRef] [PubMed]
- Pierik, M.; Yang, H.; Barmada, M.M.; Cavanaugh, J.A.; Annese, V.; Brant, S.R.; Cho, J.H.; Duerr, R.H.; Hugot, J.P.; McGovern, D.P.; et al. IBD International Genetics Consortium, the IBD international genetics consortium provides further evidence for linkage to IBD4 and shows gene-environment interaction. Inflamm. Bowel Dis. 2005, 11, 1–7. [Google Scholar] [CrossRef]
- Ye, Y.; Pang, Z.; Chen, W.; Ju, S.; Zhou, C. The epidemiology and risk factors of inflammatory bowel disease. Int. J. Clin. Exp. Med. 2015, 8, 22529–22542. [Google Scholar]
- Sairenji, T.; Collins, K.L.; Evans, D.V. An update on inflammatory bowel disease. Prim. Care 2017, 44, 673–692. [Google Scholar]
- Khor, T.O.; Huang, M.T.; Prawan, A.; Liu, Y.; Hao, X.; Yu, S.; Cheung, W.K.L.; Chan, J.Y.; Reddy, B.S.; Yang, C.S.; et al. Increased susceptibility of Nrf2 knockout mice to colitis-associated colorectal Cancer. Cancer Prev. Res. 2008, 1, 187–191. [Google Scholar]
- Lu, M.C.; Ji, J.A.; Jiang, Y.L.; Chen, Z.Y.; Yuan, Z.W.; You, Q.D.; Jiang, Z.Y. An inhibitor of the Keap1-Nrf2 protein-protein interaction protects NCM460 colonic cells and alleviates experimental colitis. Sci. Rep. 2016, 6, 26585. [Google Scholar]
- Farquhar, M.G.; Palade, G.E. Junctional complexes in various epithelia. J. Cell Biol. 1963, 17, 375–412. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Staitieh, B.S.; Jensen, J.S.; Mould, K.J.; Greenberg, J.A.; Joshi, P.C.; Koval, M.; Guidot, D.M. Activating the Nrf2-mediated antioxidant response element restores barrier function in the alveolar epithelium of HIV-1 transgenic rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L267–L277. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hu, Y.; Fang, Y.; Djukic, Z.; Yamamoto, M.; Shaheen, N.J.; Orlando, R.C.; Chen, X. Nrf2 deficiency impairs the barrier function of mouse oesophageal epithelium. Gut 2014, 63, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bao, Z.; Xu, X.; Chao, H.; Lin, C.; Li, Z.; Liu, Y.; Wang, X.; You, Y.; Liu, J.; et al. Extracellular signal-regulated kinase/nuclear factor-erythroid2-like2/heme oxygenase-1 pathway-mediated mitophagy alleviates traumatic brain injury-induced intestinal mucosa damage and epithelial barrier dysfunction. J. Neurotrauma 2017, 34, 2119–2131. [Google Scholar] [CrossRef]
- Hu, Q.; Ren, J.; Li, G.; Wu, J.; Wu, X.; Wang, G.; Gu, G.; Ren, H.; Hong, Z.; Li, J. The mitochondrially targeted antioxidant MitoQ protects the intestinal barrier by ameliorating mitochondrial DNA damage via the Nrf2/ARE signaling pathway. Cell Death Dis. 2018, 9, 403. [Google Scholar] [PubMed]
- Durante, W. Protective role of heme oxygenase-1 against inflammation in atherosclerosis. Front. Biosci. Landmark Ed. 2011, 16, 2372–2388. [Google Scholar] [CrossRef] [PubMed]
- Awuh, J.A.; Haug, M.; Mildenberger, J.; Marstad, A.; Do, C.P.N.; Louet, C.; Stenvik, J.; Steigedal, M.; Damås, J.K.; Halaas, Q.; et al. Keap1 regulates inflammatory signaling in Mycobacterium avium-infected human macrophages. Proc. Natl. Acad. Sci. USA 2015, 112, E4272. [Google Scholar] [CrossRef]
- Lee, S.H.; Sohn, D.H.; Jin, X.Y.; Kim, S.W.; Choi, S.C.; Seo, G.S. 2′4′,6′-Tris (methoxymethoxy) chalcone protects against trinitrobenzene sulfonic acid-induced colitis and blocks tumor necrosis factor-α-induced intestinal epithelial inflammation via heme oxygenase 1-dependent and independent pathways. Biochem. Pharmacol. 2007, 74, 870–880. [Google Scholar] [CrossRef]
- Trivedi, P.P.; Jena, G.B. Role of α-lipoic acid in dextran sulfate sodium-induced ulcerative colitis in mice: Studies on inflammation, oxidative stress, DNA damage and fibrosis. Food Chem. Toxicol. 2013, 59, 339–355. [Google Scholar] [CrossRef]
- Trivedi, P.P.; Jena, G.B.; Tikoo, K.B.; Kumar, V. Melatonin modulated autophagy and Nrf2 signaling pathways in mice with colitis-associated colon carcinogenesis. Mol. Carcinog. 2016, 55, 255–267. [Google Scholar] [CrossRef]
- Arisawa, T.; Tahara, T.; Shibata, T.; Nagasaka, M.; Nakamura, M.; Kamiya, Y.; Fujita, H.; Yoshioka, D.; Okubo, M.; Sakata, M.; et al. Nrf2 gene promoter polymorphism is associated with ulcerative colitis in a Japanese population. Hepatogastroenterology 2008, 55, 394–397. [Google Scholar] [PubMed]
- Sabzevary-Ghahfarokhi, M.; Shohan, M.; Shirzad, H.; Rahimian, G.; Soltani, A.; Ghatreh-Samani, M.; Deris, F.; Bagheri, N.; Shafigh, M.; Tahmasbi, K. The regulatory role of Nrf2 in antioxidants phase2 enzymes and IL-17A expression in patients with ulcerative colitis. Pathol. Res. Pract. 2018, 214, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, M.; Swierczynski, M.; Fichna, J.; Piechota-Polanczyk, A. The Nrf2 in the pathophysiology of the intestine: Molecular mechanisms and therapeutic implications for inflammatory bowel diseases. Pharmacol. Res. 2021, 163, 105243. [Google Scholar] [CrossRef] [PubMed]
- Stachel, I.; Geismann, C.; Aden, K.; Deisinger, F.; Rosenstiel, P.; Schreiber, S.; Sebens, S.; Arlt, A.; Schäfer, H. Modulation of nuclear factor E2-related factor-2 (NRF2) activation by the stress response gene immediate early response-3 (IER3) in colonic epithelial cells: A novel mechanism of cellular adaption to inflammatory stress. J. Biol. Chem. 2014, 289, 1917–1929. [Google Scholar] [CrossRef]
- Myers, J.N.; Schäffer, M.W.; Korolkova, O.Y.; Williams, A.D.; Gangula, P.R.; M’Koma, A.E. Implications of the colonic deposition of free hemoglobin-α chain: A previously unknown tissue by-product in inflammatory bowel disease. Inflamm. Bowel Dis. 2014, 20, 1530–1547. [Google Scholar] [CrossRef]
- Lin, H.; Johnston, L.J.; Wang, F.; Ma, X. Triggers for the Nrf2/ARE signaling pathway and its nutritional regulation: Potential therapeutic applications of ulcerative colitis. Int. J. Mol. Sci. 2021, 22, 11411. [Google Scholar]
- Khor, T.O.; Huang, M.T.; Kwon, K.H.; Chan, J.Y.; Reddy, B.S.; Kong, A.N. NRF2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 2006, 66, 11580–11584. [Google Scholar] [CrossRef]
- Osburn, W.O.; Karim, B.; Dolan, P.M.; Liu, G.; Yamamoto, M.; Huso, D.L.; Kensler, T.W. Increased colonic inflammatory injury and formation of aberrant crypt foci in NRF2-deficient mice upon dextran sulfate treatment. Int. J. Cancer 2007, 121, 1883–1891. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Kloska, D.; Grochot-Przęczek, A.; Feelisch, M.; Cuadrado, A.; van Goor, H. Personalized redox medicine in inflammatory bowel diseases: An emerging role for HIF-1α and NRF2 as therapeutic targets. Redox Biol. 2023, 60, 102603. [Google Scholar] [CrossRef]
- Yagishita, Y.; McCallum, M.L.; Kensler, T.W.; Wakabayashi, N. Constitutive activation of NRF2 in mice expands enterogenesis in small intestine through negative regulation of Math1. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 503–524. [Google Scholar] [CrossRef]
- Eriksson, D.; Royrvik, E.C.; Aranda-Guillen, M.; Berger, A.H.; Landegren, N.; Artaza, H.; Hallgren, A.; Grytaas, M.A.; Ström, S.; Bratland, E.; et al. GWAS for autoimmune Addison’s disease identifies multiple risk loci and highlights AIRE in disease susceptibility. Nat. Commun. 2021, 12, 959. [Google Scholar] [CrossRef]
- Husebye, E.S.; Allolio, B.; Arlt, W.; Badenhoop, K.; Bensing, S.; Betterle, C.; Falorni, A.; Gan, E.H.; Hulting, A.L.; Kasperlik-Zaluska, A.; et al. Consensus statement on the diagnosis, treatment and follow-up of patients with primary adrenal insufficiency. J. Intern. Med. 2014, 275, 104–115. [Google Scholar] [CrossRef]
- Husebye, E.S.; Anderson, M.S.; Kämpe, O. Autoimmune polyendocrine syndromes. N. Engl. J. Med. 2018, 378, 2543–2544. [Google Scholar] [CrossRef]
- Winqvist, O.; Karlsson, F.A.; Kämpe, O. 21-Hydroxylase, a major autoantigen in idiopathic Addison’s disease. Lancet 1992, 339, 1559–1562. [Google Scholar] [CrossRef]
- Magomedova, L.; Cummins, C.L. Glucocorticoids and metabolic control. Handb. Exp. Pharmacol. 2016, 233, 73–93. [Google Scholar]
- Giudice, A.; Aliberti, S.M.; Barbieri, A.; Pentangelo, P.; Bisogno, I.; D’Arena, G.; Cianciola, E.; Caraglia, M.; Capunzo, M. Potential mechanisms by which glucocorticoids induce breast carcinogenesis through Nrf2 inhibition. Front. Biosci. Landmark Ed. 2022, 27, 223. [Google Scholar] [CrossRef] [PubMed]
- Giudice, A.; Arra, C.; Turco, M.C. Review of molecular mechanisms involved in the activation of the Nrf2-ARE signaling pathway by chemopreventive agents. Methods Mol. Biol. 2010, 647, 37–74. [Google Scholar] [PubMed]
- Rojo de la Vega, M.; Chapman, E.; Zhang, D.D. NRF2 and the hallmarks of cancer. Cancer Cell 2018, 34, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Ziros, P.G.; Habeos, I.G.; Chartoumpekis, D.V.; Ntalampyra, E.; Somm, E.; Renaud, C.O.; Bongiovanni, M.; Trougakos, I.P.; Yamamoto, M.; Kensler, T.W.; et al. NFE2-Related transcription factor 2 coordinates antioxidant defense with thyroglobulin production and iodination in the thyroid gland. Thyroid 2018, 28, 780–798. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yehuda Greenwald, M.; Frusic-Zlotkin, M.; Soroka, Y.; Ben-Sasson, S.; Bianco-Peled, H.; Kohen, R. A novel role of topical iodine in skin: Activation of the Nrf2 pathway. Free Radic. Biol. Med. 2017, 104, 238–248. [Google Scholar] [CrossRef]
- Chartoumpekis, D.V.; Ziros, P.G.; Habeos, I.G.; Sykiotis, G.P. Emerging roles of Keap1/Nrf2 signaling in the thyroid gland and perspectives for bench-to-bedside translation. Free Radic. Biol. Med. 2022, 190, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Sultana, D.R.; Shahin, A.D.; Md Jawadul, H. Measurement of oxidative stress and total antioxidant capacity in hyperthyroid patients following treatment with carbimazole and antioxidant. Heliyon 2021, 30, e08651. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.S.; Hu, L.B.; Zhang, H.; Shan, W.X.; Wang, Y.; Li, X.; Liu, T.; Zhao, J.; You, Q.D.; Jiang, Z.Y. Design, synthesis, and structure-activity relationships of Indoline-Based Kelch-like ECH-Associated Protein 1-Nuclear Factor (Erythroid-Derived 2)-Like 2 (Keap1-Nrf2) Protein-Protein interaction inhibitors. J. Med. Chem. 2020, 63, 11149–11168. [Google Scholar] [CrossRef]
- Bellucci, E.; Terenzi, R.; La Paglia, G.M.C.; Gentileschi, S.; Tripoli, A.; Tani, C.; Alunno, A. One year in review 2016: Pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 2016, 34, 793–801. [Google Scholar] [PubMed]
- Ito, H.; Moritoshi, F.; Hashimoto, M.; Tanaka, M.; Matsuda, S. Control of articular synovitis for bone and cartilage regeneration in rheumatoid arthritis. Inflamm. Regen. 2018, 38, 7. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos Nathan, J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 107–120. [Google Scholar] [CrossRef]
- Quinonez-Flores, C.M.; Gonzalez-Chavez, S.A.; Del Rio Najera, D.; Pacheco-Tena, C. Oxidative stress relevance in the pathogenesis of the Rheumatoid Arthritis: A Systematic Review. BioMed Res. Int. 2016, 2016, 6097417. [Google Scholar] [CrossRef]
- Chadha, S.; Behl, T.; Kumar, A.; Khullar, G.; Arora, S. Role of Nrf2 in rheumatoid arthritis. Curr. Res. Transl. Med. 2020, 68, 171–181. [Google Scholar] [CrossRef]
- Holmstrom, K.M.; Kostov, R.V.; Dinkova-Kostova, A.T. The multifaceted role of Nrf2 in mitochondrial function. Curr. Opin. Toxicol. 2016, 1, 80–91. [Google Scholar] [CrossRef]
- Zhai, Z.; Huang, Y.; Zhang, Y.; Zhao, L.; Li, W. Clinical research progress of small molecule compounds targeting Nrf2 for treating inflammation-related diseases. Antioxidants 2022, 11, 1564. [Google Scholar] [CrossRef]
- Feigin, V.L.; Vos, T.; Nichols, E.; Owolabi, M.O.; Carroll, W.M.; Dichgans, M.; Deuschl, G.; Parmar, P.; Brainin, M.; Murray, C. The global burden of neurological disorders: Translating evidence into policy. Lancet Neurol. 2020, 19, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, M.; Jurajda, M.; Duris, K. Oxidative Stress in the Brain: Basic Concepts and Treatment Strategies in Stroke. Antioxidants 2021, 25, 1886. [Google Scholar] [CrossRef] [PubMed]
- Paladino, S.; Conte, A.; Caggiano, R.; Pierantoni, G.M.; Faraonio, R. Nrf2 pathway in age-related neurological disorders: Insights into microRNAs. Cell. Physiol. Biochem. 2018, 47, 1951–1976. [Google Scholar] [CrossRef] [PubMed]
- Gergues, M.M.; Moiseyenko, A.; Saad, S.Z.; Kong, A.N.; Wagner, G.C. Nrf2 deletion results in impaired performance in memory tasks and hyperactivity in mature and aged mice. Brain Res. 2018, 1701, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Rojo, A.I.; Pajares, M.; Rada, P.; Nunez, A.; Nevado-Holgado, A.J.; Killik, R.; Van Leuven, F.; Ribe, E.; Lovestone, S.; Yamamoto, M.; et al. NRF2 deficiency replicates transcriptomic changes in Alzheimer’s patients and worsens APP and TAU pathology. Redox Biol. 2017, 13, 444–451. [Google Scholar] [CrossRef]
- Cuadrado, A. Brain-Protective Mechanisms of Transcription Factor NRF2: Toward a Common Strategy for Neurodegenerative Diseases. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 255–277. [Google Scholar] [CrossRef]
- Esteras, N.; Blacker, T.S.; Zherebtsov, E.A.; Stelmashuk, O.A.; Zhang, Y.; Wigley, W.C.; Duchen, M.R.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 regulates glucose uptake and metabolism in neurons and astrocytes. Redox Biol. 2023, 62, 102672. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, G.; Ting, S.-M.; Song, S.; Zhang, J.; Edwards, N.J.; Aronowski, J. Cleaning up after ICH: The role of Nrf2 in modulating microglia function and hematoma clearance. J. Neurochem. 2015, 133, 144–152. [Google Scholar] [CrossRef]
- Pajares, M.; Cuadrado, A.; Rojo, A.I. Modulation of proteostasis by transcription factor NRF2 and impact in neurodegenerative diseases. Redox Biol. 2017, 11, 543–553. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Profumo, E.; Tucci, P.; Saso, L. A perspective on Nrf2 signaling pathway for neuroinflammation: A potential therapeutic target in Alzheimer’s and Parkinson’s Diseases. Front. Cell. Neurosci. 2022, 15, 787258. [Google Scholar] [CrossRef]
- Zhang, X.X.; Tian, Y.; Wang, Z.T.; Ma, Y.H.; Tan, L.; Yu, J.-T. The epidemiology of Alzheimer’s Disease modifiable risk factors and prevention. J. Prev. Alzheimer’s Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Bahn, G.; Park, J.S.; Yun, U.J.; Lee, Y.J.; Choi, Y.; Park, J.S.; Baek, S.H.; Choi, B.Y.; Cho, Y.S.; Kim, H.K.; et al. NRF2/ARE pathway negatively regulates BACE1 expression and ameliorates cognitive deficits in mouse Alzheimer’s models. Proc. Natl. Acad. Sci. USA 2019, 116, 12516–12523. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.; Gundemir, S.; Pritchard, S.; Jin, Y.N.; Rahman, I.; Johnson, G.V. Nrf2 reduces levels of phosphorylated tau protein by inducing autophagy adaptor protein NDP52. Nat. Commun. 2014, 5, 3496. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, M.; Wood, S.A.; Mellick, G.D. Nrf2: A modulator of Parkinson’s disease? J. Neural. Transm. 2016, 123, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Kasen, A.; Houck, C.; Burmeister, A.R.; Sha, Q.; Brundin, L.; Brundin, P. Upregulation of alpha-synuclein following immune activation: Possible trigger of Parkinson’s disease. Neurobiol. Dis. 2022, 166, 105654. [Google Scholar] [CrossRef]
- Latif, S.; Jahangeer, M.; Maknoon Razia, D.; Ashiq, M.; Ghaffar, A.; Akram, M.; El Allam, A.; Bouyahya, A.; Garipova, L.; Ali Shariati, M.; et al. Dopamine in Parkinson’s disease. Clin. Chim. Acta 2021, 522, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Din, J.U.; Zhao, F.; Liu, X. Effect of soybean peptides against hydrogen peroxide induced oxidative stress in HepG2 cells via Nrf2 signaling. Food Funct. 2020, 11, 2725–2737. [Google Scholar] [CrossRef]
- Paraidathathu, T.; Degroot, H.; Kehrer, J.P. Production of reactive oxygen by mitochondria fromnormoxic and hypoxic rat-heart tissue. Free Radic. Biol. Med. 1992, 13, 289–297. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zhang, Q.; Xun, Z.; Yuan, L.; Li, R.; Li, X.; Tian, S.Y.; Xin, N.; Xu, Y. Increases of iASPP-Keap1 interaction mediated by syringin enhance synaptic plasticity and rescue cognitive impairments via stabilizing Nrf2 in Alzheimer’s models. Redox Biol. 2020, 36, 101672. [Google Scholar] [CrossRef]
- Nesi, G.; Sestito, S.; Digiacomo, M.; Rapposelli, S. Oxidative stress, mitochondrial abnormalities and proteins deposition: Multitarget approaches in Alzheimer’s Disease. Curr. Top. Med. Chem. 2017, 17, 3062–3079. [Google Scholar]
- Guo, C.J.; Zhang, Y.; Nie, Q.; Cao, D.D.; Wang, X.X.; Wan, X.K.; Liu, M.; Cui, J.; Sun, J.; Bai, Y.F.; et al. SQSTM1/ p62 oligomerization contributes to AO-induced inhibition of Nrf2 signaling. Neurobiol. Aging 2021, 98, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.Z.A.; Zhao, D.; Hussain, T.; Sabir, N.; Mangi, M.H.; Yang, L. p62-Keap1-NRF2-ARE pathway: A contentious player for selective targeting of autophagy, oxidative stress and mitochondrial dysfunction in prion diseases. Front. Mol. Neurosci. 2018, 11, 310. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, S.; Schirinzi, T.; Di Lazzaro, G.; D’Amico, J.; Colona, V.L.; Bertini, E.; Pierantozzi, M.; Mari, L.; Mercuri, N.B.; Piemonte, F.; et al. Systemic activation of Nrf2 pathway in Parkinson’s disease. Mov. Disord. 2020, 35, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Sivandzade, F.; Prasad, S.; Bhalerao, A.; Cucullo, L. NRF2 and NF-B interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019, 21, 101059. [Google Scholar] [CrossRef]
- Buhlman, L.M. Parkin loss-of-function pathology: Premature neuronal senescence induced by high levels of reactive oxygen species? Mech. Ageing Dev. 2017, 161, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Xu, Y.; Wang, X.; Guo, C.; Wang, T.; Wang, Z.Y. Dl-3-n-Butylphthalide inhibits NLRP3 inflammasome and mitigates Alzheimer’s-Like pathology via Nrf2-TXNIP-TrX axis. Antioxid. Redox Signal. 2019, 30, 1411–1431. [Google Scholar] [CrossRef]
- Schepici, G.; Bramanti, P.; Mazzon, E. Efficacy of sulforaphane in neurodegenerative diseases. Int. J. Mol. Sci. 2020, 21, 8637. [Google Scholar] [CrossRef]
- Liu, L.; Vollmer, M.K.; Fernandez, V.M.; Dweik, Y.; Kim, H.; Dore, S. Korean Red Ginseng Pretreatment Protects Against Long-Term Sensorimotor Deficits After Ischemic Stroke Likely Through Nrf2. Front. Cell. Neurosci. 2018, 12, 74. [Google Scholar] [CrossRef]
- Hu, Y.; Luo, Y.; Zheng, Y. Nrf2 Pathway and Autophagy Crosstalk: New Insights into Therapeutic Strategies for Ischemic Cerebral Vascular Diseases. Antioxidants 2022, 11, 1747. [Google Scholar] [CrossRef]
- Innamorato, N.G.; Rojo, A.I.; Garcia-Yague, A.J.; Yamamoto, M.; de Ceballos, M.L.; Cuadrado, A. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J. Immunol. 2008, 181, 680–689. [Google Scholar] [CrossRef]
- Panieri, E.; Telkoparan-Akillilar, P.; Suzen, S.; Saso, L. The NRF2/KEAP1 Axis in the Regulation of Tumor Metabolism: Mechanisms and Therapeutic Perspectives. Biomolecules 2020, 20, 791. [Google Scholar] [CrossRef] [PubMed]
- Wani, R.; Nagata, A.; Murray, B.W. Protein redox chemistry: Post-translational cysteine modifications that regulate signal transduction and drug pharmacology. Front. Pharmacol. 2014, 5, 224. [Google Scholar] [CrossRef] [PubMed]
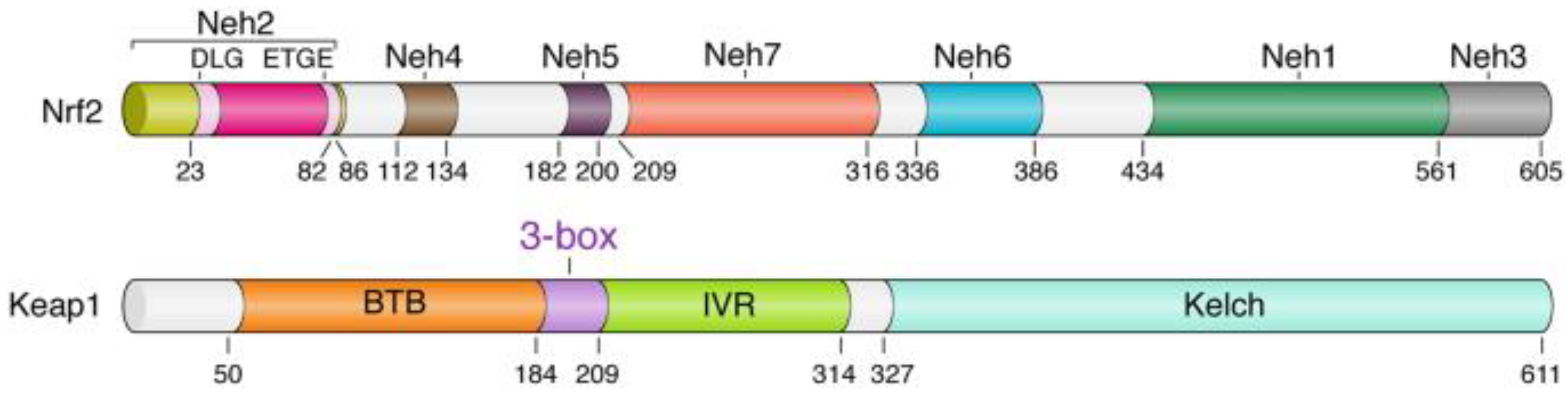
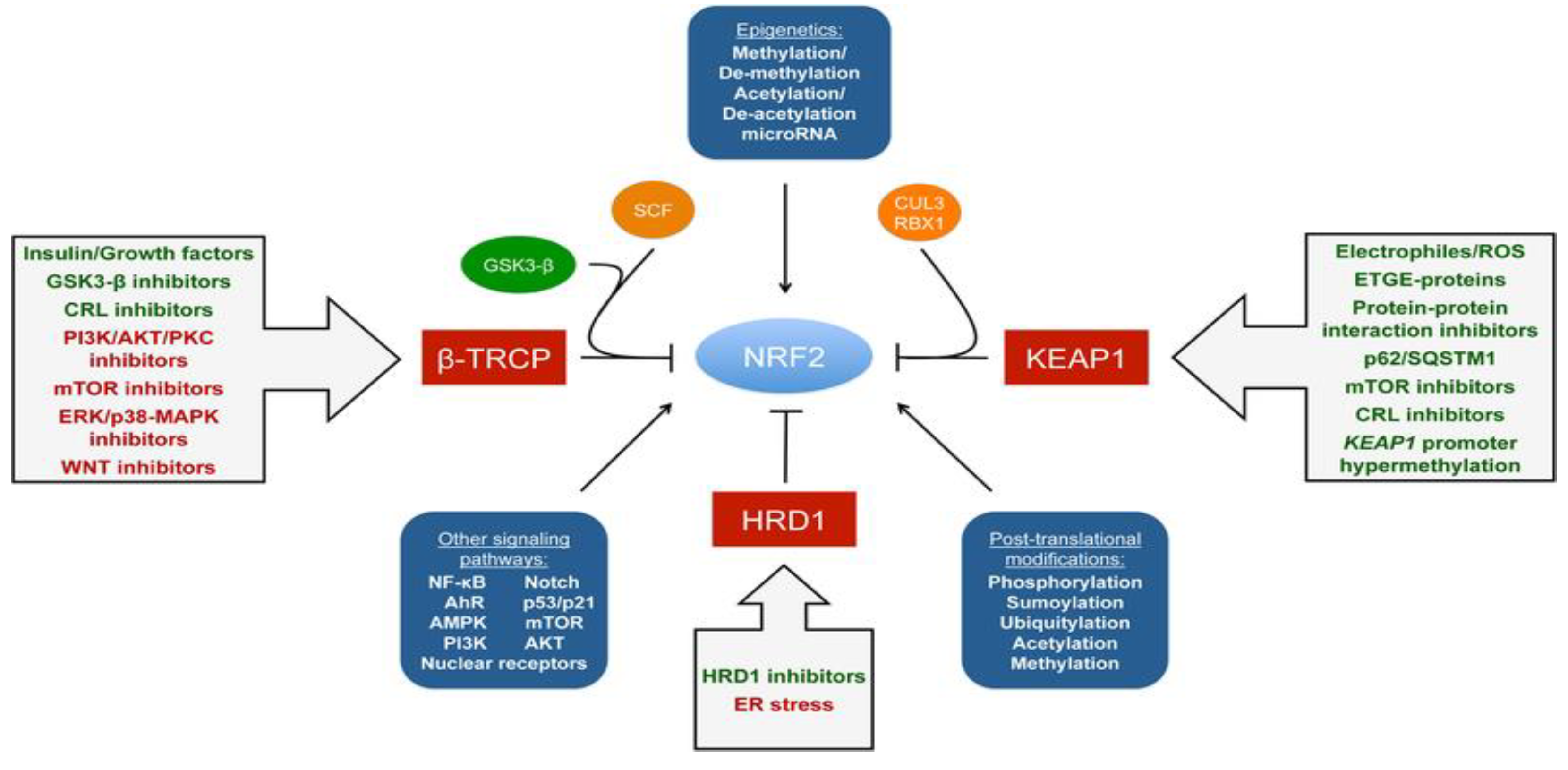

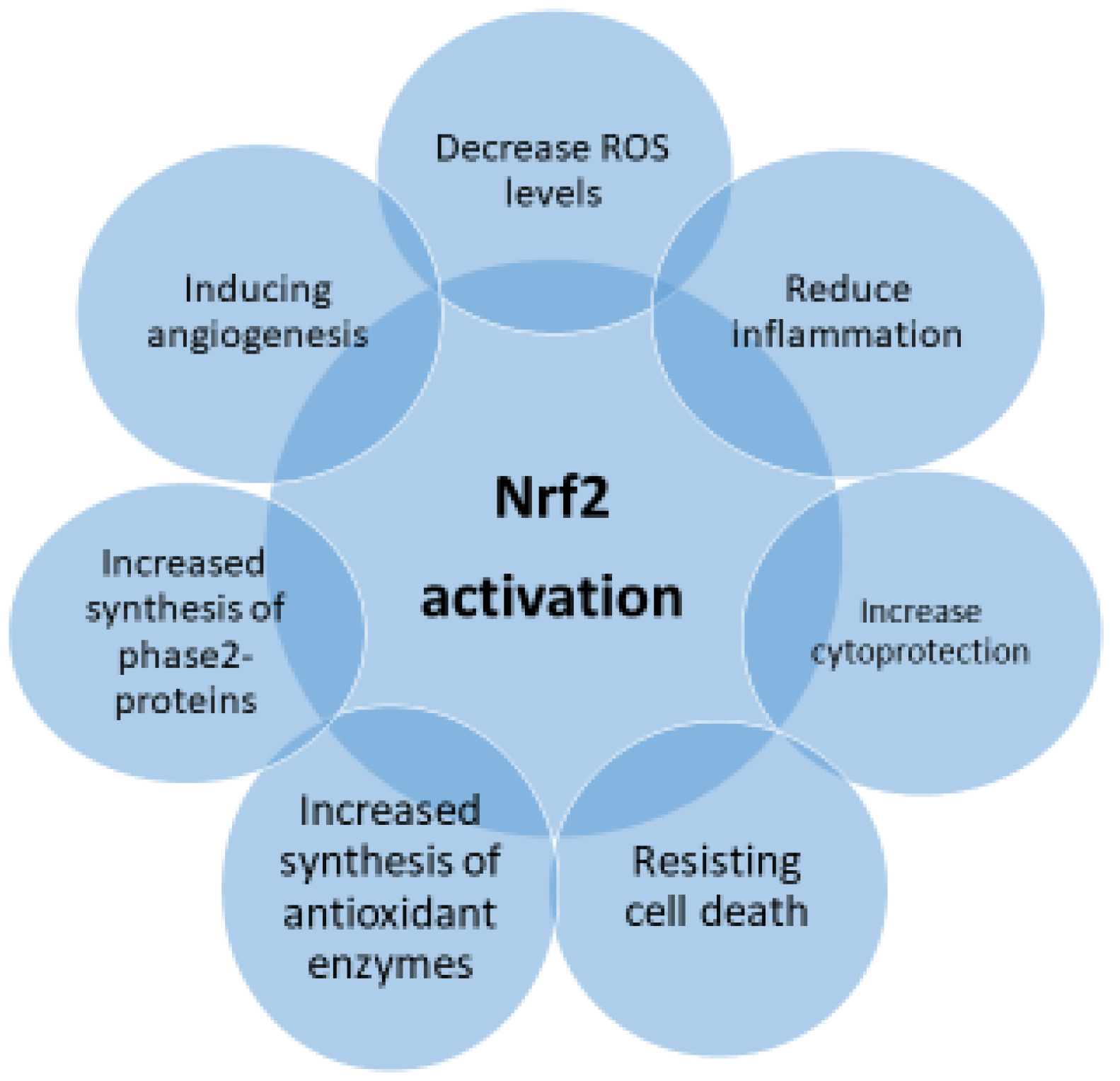
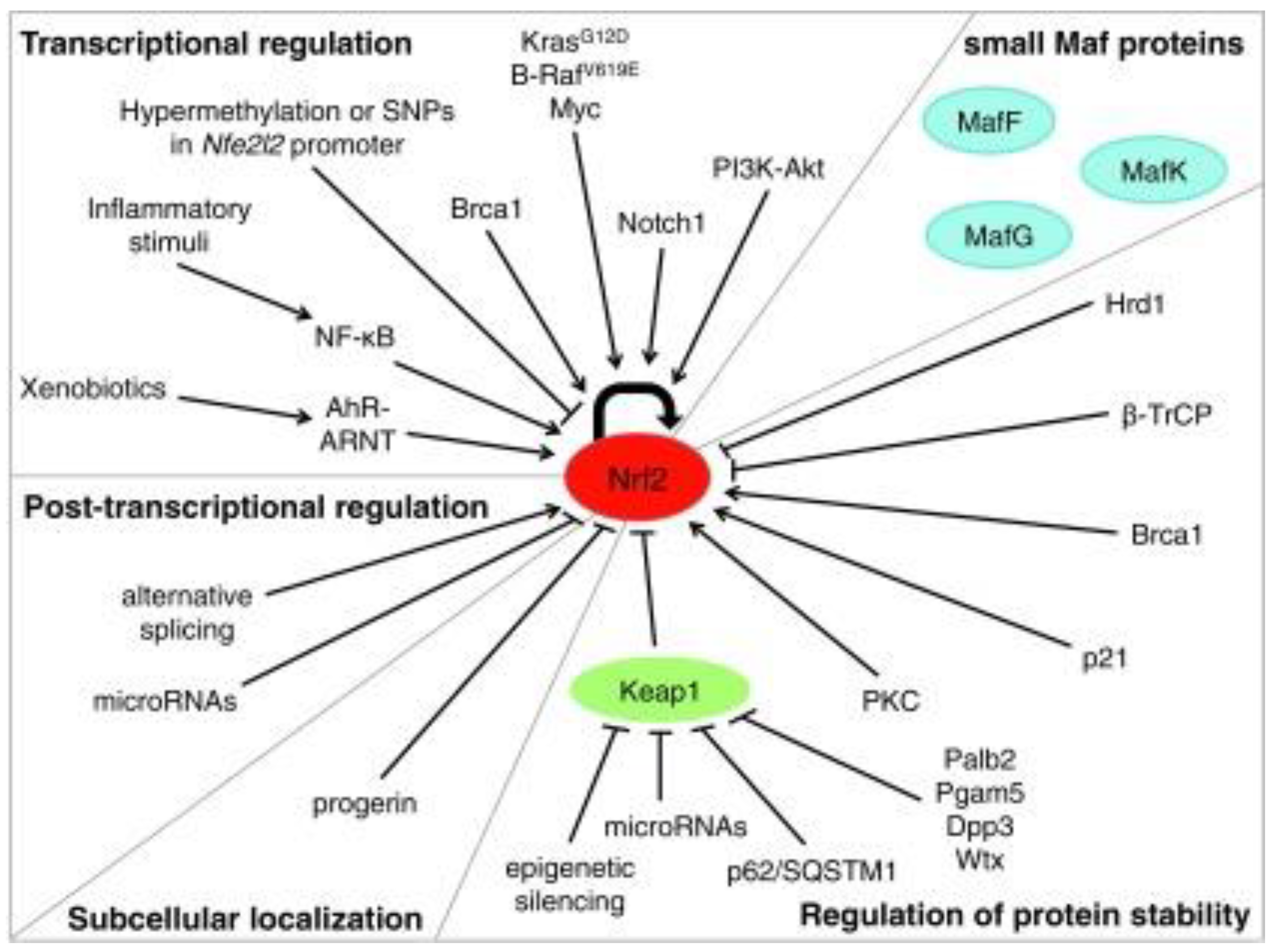

| Inducer | Example |
|---|---|
| Endogenous signaling compounds/metabolites | H2O2 Lipid peroxidation products Nitric oxide 8-Nitro-cGMP Hydrogen sulfide Methylglyoxal |
| Oncometabolites | Fumarate Succinylacetone |
| Immunometabolite | Itaconate |
| Dietary compounds | Sulforaphane Curcumin |
| Pharmaceuticals | Dimethyl fumarate Bardoxylone |
| Microorganisms | Bacteria/lipopolysaccharide Marburg virus Plasmodium infection |
| Extracellular inducers | Heat Laminar flow UVA radiation Exercise |
| Therapeutics | Mechanism of Action | Molecules | |
|---|---|---|---|
| Electrophilic NRF2 activators | Natural products | Electrophilic modification of KEAP1-C151 | Sulphorapane Bixin |
| Natural product-derived | Electrophilic modification of KEAP1-C151 | Dimethyl fumarate Bardoxolone methyl | |
| Pro-electrophilic NRF2 activators | Natural products | Electrophilic modification of KEAP1-C151 | Carnosic acid Carnosol |
| Non-electrophilic compounds | Peptides | Binding to KEAP1 Kelch domain | Ac-DPETGEL-OH (7mer) FITC-β-DEETGEF-OH (7mer) FITC-LDEETGEFL-NH2 (9mer) FAM-LDEETGELP-OH (9mer) |
| Small molecules | Binding to KEAP1 Kelch domain | Compound 2 Cpd15 Cpd16 (SRS)-5 AN-465/144580 | |
| KEAP1-independent NRF2 activators | Natural products | GSK-3 inhibition | Nordihydroguaiaretic acid |
| Synthetic | HRD1 inhibition | LS-102 | |
| NRF2 inhibitors | Natural products | Global protein translation inhibitor | Brusatol |
| Intervention | Topic | Phase | Trial Country | Primary Endpoints | Dose | Subjects |
|---|---|---|---|---|---|---|
| Curcumin | Effect of curcumin on systemic lupus erythematosus | 2 | California, United States | Change in SLEDAI. | Intervention: 2 g of curcumin supplement per day. | Sample size: 23; Gender: all; Ages: 18 years and older. |
| Vitamin D and curcumin piperine attenuates disease activity and cytokine levels in systemic lupus erythematosus patients | 2 | Indonesia | 1. Disease activity from the SLE patients after the treatments; 2. fatigue assessment from the SLE patients after the treatments; and 3. comparison of cytokine levels before and after the treatments. | The third group received 400 IU cholecalciferol (Nature Plus) t.i.d and curcumin (600 mg)–piperine (15,800 mg) (Bioglan) one time daily. | Sample size: 45; Gender: all; Ages: 18 years to 45 years. | |
| Vitamin D3 | Vitamin D3 treatment in pediaric systemic lupus erythematosus | 2 | California | Change in average IFN module expression level. Percentage of subjects by treatment arm experiencing any adverse event (AE) ≥ grade 3. | Supplementation of 6000 IU vitamin D3 by mouth daily until the subject’s serum 25 (OH) level was ≥40 ng/mL, at which point the supplementation dose was reduced to 4000 IU/day. Note: subjects weighing < 40 kg (kg) at study entry received their dose five days a week, and all other subjects received their dose seven days a week. | Sample size: 7; Gender: all; Ages: 5 years to 20 years. |
| Vitamin D3 in systemic lupus erythematosus | 2 | United States | Percent of patients with an IFN alpha signature response at week 12. | Dose of 8% vitamin D3 powder, 84% microcrystalline cellulose, 8% fumed silica by weight. | Sample size: 57; Gender: all; Ages: 18 years and older. | |
| Vitamin D to improve endothelial function in SLE | 2 | United States | Change at week 16 in % flow-mediated dilation in those who did and did not replete vitamin D. | Dose of 5000 international units versus 400 international units as an active comparator. | Sample size: 9; Gender: all; Ages: 18 years and older. | |
| Vitamin D therapy in patients with systemic lupus erythematosus (SLE) | 1 | United States | Hypercalcuria. | Cholecalciferol 800 IU oral daily. Cholecalciferol 2000 IU oral daily. Cholecalciferol 4000 IU oral daily. | Sample size: 18; Gender: all; Ages: 18 years to 85 years. | |
| Vitamin D and curcumin piperine attenuates disease activity and cytokine levels in systemic lupus erythematosus patients | 2 | Indonesia | 1. Disease activity in SLE patients after the treatments; 2. fatigue assessment of SLE patients after the treatments; and 3. comparison of cytokine levels before and after the treatments. | The second group received a tablet containing curcumin (632 mg)–piperine (15,800 mg) (Bioglan) one time daily and a placebo (Saccharum lactis) t.i.d. | Sample size: 45; Gender: all; Ages:18 years to 45 years. | |
| Effect of vitamin Dsupplement on disease activity in SLE | Not applicable | Thailand | The effect of vitamin D supplementation on SLE disease activity. | Add on vitamin D2 (calciferol) 40,000 IU/wk (2 cap) for 12 weeks. | Sample size: 100; Gender: all; Ages: 18 years and older. | |
| The effect of vitamin D supplementation on disease activity markers in systemic lupus erythematosus (SLE) | Not applicable | Egypt | Decrease in SLE disease activity. | 2000 IU/day for 12 months. | Sample size: 248; Gender: all; Ages: 30 years and older. | |
| SM934 | Safety and efficacy of SM934 compared to placebo in adult subjects with active systemic lupus erythematosus | 2 | China | 1. Percentage of subjects with lupus low disease activity score (LLDAS) in each group; 2. percentage of subjects with systemic lupus erythematosus responder index 4 (SRI-4) response in each group; and 3. percentage of subjects with treatment-emergent adverse events (TEAEs) in each group. | SM934 10 mg (five tablets) p.o. qd in combination with steroids. | Sample size: 48; Gender: all; Ages: 30 years and older. |
| Intervention | Topic | Phase | Trial Country | Primary Endpoints | Dose | Subjects |
|---|---|---|---|---|---|---|
| Dimethyl fumarate | Efficacy and safety study of BG00012 with methotrexate in patients with active rheumatoid arthritis | 2 | Australia | The primary objective is the proportion of subjects with ACR20 response in their RA at week 12. | Dose of 480 mg/day, oral, and 720 mg/day, oral. | Sample size: 153; Gender: all; Ages: 18 years to 75 years. |
| Curcumin | Curcuma Longa L in rheumatoid arthritis | 1; terminated (insufficient enrollment) | United States | Number of participants with adverse events as a measure of safety and tolerability. | Four 250 mg curcumin capsules twice a day for one month. | Sample size: 3; Gender: all; Ages: 18 years and older. |
| Curcumin in rheumatoid arthritis | Early phase 1 | United States | American College of Rheumatology 20%. Time frame: 4-month period. | Four capsules once a day for 2 weeks, and then the dose was increased to four capsules twice a day beginning at week 3. Subjects remained at this dose for an additional 13 weeks for a total 16 weeks. After 16 weeks, the same procedures were repeated for another 16 weeks. | Sample size: 40; Gender: all; Ages: 18 years to 75 years. |
| Intervention | Topic | Phase | Trial Country | Primary Endpoints | Dose | Subjects |
|---|---|---|---|---|---|---|
| EGCG | Prevention of cognitive decline in ApoE4 carriers with subjective cognitive decline after EGCG and a multimodal intervention | N/A | Spain | Preclinical Alzheimer cognitive composite plus exe-like score (ADCS-PACC-like). | Oral 532 mg/day (weight > 50 kg). Oral 266 mg/day (weight < 50 kg). | Sample size: 200. Gender: all. Ages: 60 years to 80 years old. |
| Sunphenon EGCg (epigallocatechin-gallate) in the Early stage of Alzheimer’s disease | 2/3 | Germany | ADAS-COG (score 0–70) (baseline to treatment). Time frame: 18 months. | Months 1–3: 200 mg/day (200-0-0 mg); months 4–6: 400 mg/day (200-0-200 mg); months 7–9: 600 mg/day (400-0–200 mg); and months 10–18: 800 mg/day (400-0-400 mg). | Sample size: 21. Gender: all. Ages: 60 years and older. | |
| Sunphenon EGCg (epigallocatechin-gallat) in the early stage of Alzheimer’s disease—SUN-AK | 2 | Germany | Sample size: 50. Gender: all. Ages: 18 years and older. | |||
| Sulforaphane | Effects of sulforaphane in patients with prodromal to mild Alzheimer’s disease | N/A | China | The Alzheimer’s Disease Assessment Scale. | Oral 2550 mg once a day for 24 weeks. | Sample size: 160. Gender: all. Ages: 50 years to 75 years old. |
| Resveratrol | BDPP treatment for mild cognitive impairment (MCI) and prediabetes or type 2 diabetes mellitus (T2DM) | 1 | United States | Assessment of AEs and SAEs. Brain penetrance of BDPP. Neuropsychiatric Inventory and Cornell Scale for Depression in Dementia. Memory, executive function, and attention measures (composite). | N/A | Sample size: 14. Gender: all. Ages: 50 years to 90 years old. |
| Short-term efficacy and safety of perispinal administration of etanercept in mild to moderate Alzheimer’s disease | 1 | United States | Difference in effects of treatment for 6 weeks with etanercept + nutritional supplements versus nutritional supplements alone on the mini-mental status examination (MMSE) score. | N/A | Sample size: 12. Gender: all. Ages: 60 years to 85 years old. | |
| Resveratrol for Alzheimer’s disease | 2 | United States | Number of adverse events. Change from baseline in volumetric magnetic resonance imaging (MRI). | Begin at 500 mg taken once daily and increase after 13 weeks to 1 g taken by mouth twice daily. | Sample size: 119. Gender: all. Ages: 50 years and older. | |
| Pilot study on the effects of resveratrol supplement in mild to moderate Alzheimer’s disease | 3; withdrawn (PI left institution) | United States | Cognition. Time frame: 52 weeks. | Oral 215 mg once a day for 52 weeks. | Sample size: 0. | |
| Randomized trial of a nutritional supplement in Alzheimer’s disease | 3 | United States | Alzheimer’s Disease Assessment Scale (ADAScog). Time frame: one year. | N/A | Sample size: 27. Gender: all. Ages: 50 years to 90 years old. | |
| Curcumin | KARVIAH_XTND: Longitudinal follow-up study examining the health and well-being of participants for identifying new biomarkers and the impact of lifestyle (following a 12-month intervention of curcumin for the prevention of Alzheimer’s disease) | N/A | Australia | Blood biomarker compared with brain amyloid levels. Blood biomarkers and PET imaging results. | N/A | Sample size: 100. Gender: all. Ages: 65 years and older. |
| Curcumin and yoga therapy for those at risk of Alzheimer’s disease | 2 | United States | Curcumin effects (first six-month period) or curcumin and aerobic yoga effects (second six-month period) on the changes in the levels of blood biomarkers for mild cognitive impairment relative to baseline or relative to placebo or non-aerobic yoga. | Oral 800 mg curcumin in four capsules BID per day prior to meals. | Sample size: 80. Gender: all. Ages: 50 years to 90 years old. | |
| KARVIAH Sub-study: Examining the use of curcumin on cognition and mood in an older population | 2 | Australia | Attention tasks and working memory as measured using a computerized cognitive battery (CogState). | Oral 500 mg three times daily. | Sample size: 40. Gender: all. Ages: 65 years to 90 years old. | |
| Effect of curcumin (tumeric) in Alzheimer’s disease | N/A | Iran (Islamic Republic of) | MMSE and quality of life questionnaires. Time frame: before and after intervention (12 weeks). | Oral 500 mg twice a day for 12 weeks. | Sample size: 70. Gender: all. Ages: no age limit. | |
| The epigenetic effect of curcumin as measured in the blood and observed with lifestyle for the prevention of Alzheimer’s disease | 2 | Australia | Measurement of blood biomarkers within healthy and MCI groups. | Oral 1.5 mg daily (×3 divided doses) for a period of 3 or 6 months. | Sample size: 60. Gender: all. Ages: 65 years to 90 years old. | |
| McCusker KARVIAH: Curcumin in Alzheimer’s disease prevention | 2 | Australia | AD-related blood biomarker profiles. Pib PET imaging. Neuropsychological tests. Time frame: up to 12 months. | Dose of 500 mg daily for 2 weeks, progressing to 500 mg twice daily (1000 mg/daily) for 2 weeks, and then 500 mg three times daily (1500 mg) for a period of 12 months in total. | Sample size: 134. Gender: all. Ages: 65 years to 90 years old. | |
| Biocurcumax from curry spice turmeric in retaining cognitive function | N/A | Australia | Psychometric testing using mini-mental state examination (MMSE), CAMDEX-R, (CAMCOG)-R, etc. | Oral 500 mg three times daily (total 1500 mg/day). | Sample size: 134. Gender: all. Ages: 65 years to 90 years old. | |
| Efficacy and safety of curcumin formulation in Alzheimer’s disease | 2 | India | To determine if curcumin formulation affects mental capacity in Alzheimer’s patients based on mental exams. | Oral 2000 mg or 3000 mg daily BID. | Sample size: 26. Gender: all. Ages: 50 years to 80 years old. | |
| A pilot study on curcumin and ginkgo for treating Alzheimer’s disease | 1/2 | Hong Kong, China | Change in isoprostane level in plasma. Change in A-beta level in serum. | Oral 1 g/4 g once daily. | Sample size: 36. Gender: all. Ages: 50 years and older. | |
| Curcumin in patients with mild to moderate Alzheimer’s disease | 2 | United States | Side effect checklist. | N/A | Sample size: 33. Gender: all. Ages: 50 years and older. |
| Intervention | Topic | Phase | Trial Country | Primary Endpoints | Dose | Subjects |
|---|---|---|---|---|---|---|
| Vitamin D3 | The effects of vitamin D and bone loss in Parkinson’s disease | 2 | United States | Direct changes in bone formation and resorption were investigated by measuring serum 25-hydroxyvitamin D [25(OH)D] level, serum parathyroid hormone (PTH) levels, serum osteocalcin, and serum n-telopeptides (N-Tx). Time frame: 12 months. | Dose of 1000 IU/day of vitamin D3. | Sample size: 23; Gender: all; Ages: 18 years and older. |
| Clinical effects of vitamin D repletion in patients with Parkinson’s disease | 4 | United State | Change from baseline visit to 3 months (treatment visit #1) in the TUG, timed walking task (8 m), and UPDRS III subscore. Time frame: 6 months. | Dose of 600 IU vitamin D3 capsule daily. | Sample size: 31; Gender: all; Ages: 18 years to 89 years. | |
| Twelve weeks of vitamin D supplementation and physical activity in PD patients with DBS | Not Applicable | Poland | The effects of vitamin D supplementation and physical activity on the concentration of vitamin D3 in serum and the evaluation of changes before and after 12 weeks of supplementation and physical activity. Time frame: the outcome was assessed up to 1 year after the last collection of blood. | Dosage based on BMI as follows: for BMI under 25–4000 IU/day, for BMI between 25 and 30–5000 IU/day, and for BMI over 30–6000 IU/day. | Sample size: 72; Gender: all; Ages: 40 years to 90 years. | |
| Effects of vitamin D in Parkinson’s disease (PD) | 2 | United States | Change in static balance as recorded using dynamic posturography with the sensory organization test (SOT 1–3). | Drug: vitamin D3 Vitamin D3 at 10,000 IU a day. Dietary supplement: calcium Calcium at 1000 mg a day. | Sample size: 101; Gender: all; Ages: 50 years to 99 years. | |
| Resveratrol | Tolerability, safety, and pharmacokinetics of four single doses of BIA 6-512 (Trans-resveratrol) and their effect on the levodopa pharmacokinetics | 1 | Portugal | 1. Maximum observed plasma drug concentration (Cmax) post-dose—levodopa. Time of occurrence of Cmax (tmax)—levodopa. 2. Area under the plasma concentration–time curve (AUC) from time zero to the last sampling time at which concentrations were at or above the limit of quantification (AUC0-t), calculated using the linear trapezoidal rule—evodopa. 3. Area under the plasma concentration versus time curve from time zero to infinity (AUC0-∞), calculated from AUC0-t + (Clast/λz), where Clast is the last quantifiable concentration and λz is the apparent terminal rate constant—levodopa. 4. Apparent terminal half-life, calculated from ln 2/λz (t1/2)—levodopa. 5. Maximum observed plasma drug concentration (Cmax) post-dose—BIA 6-512. 6. Time of occurrence of Cmax (tmax)—BIA 6-512. 7. Area under the plasma concentration–time curve (AUC) from time zero to the last sampling time at which concentrations were at or above the limit of quantification (AUC0-t), calculated using the linear trapezoidal rule—BIA 6-512. 8. Area under the plasma concentration versus time curve from time zero to infinity (AUC0-∞), calculated from AUC0-t + (Clast/λz), where Clast is the last quantifiable concentration and λz the apparent terminal rate constant—BIA 6-512. 9. Apparent terminal half-life calculated from ln 2/λz (t1/2)—BIA 6-512. | One capsule of Madopar® HBS 125 ROCHE, Basel, Switzerland (levodopa 100 mg/benserazide 25 mg) in an open label manner, concomitantly with BIA 6-512/placebo. | Sample size: 20; Gender: all; Ages: 18 years to 45 years. |
| Effect of BIA 6-512 at steady-state on levodopa pharmacokinetics with a single dose of levodopa/benserazide 200/50 mg or with a single dose of levodopa/benserazide 200/50 mg plus a single-dose of nebicapone 150 mg | 1 | Portugal | 1. Day 4—Maximum observed plasma drug concentration (Cmax). 2. Day 4—Time of occurrence of Cmax (tmax). 3. Day 4—Area under the plasma concentration–time curve (AUC) from time zero to the last sampling time at which concentrations were at or above the limit of quantification (AUC0-t). 4. Day 4—AUC from time zero to 8 h post-dose (AUC0-τ). 5. Day 4—Area under the plasma concentration versus time curve from time zero to infinity (AUC0-∞). 6. Day 4—Apparent terminal elimination half-life, calculated from ln 2/λz (t1/2). 7. Day 5—Maximum observed plasma drug concentration (Cmax). 8. Day 5—Time of occurrence of Cmax (tmax). 9. Day 5—Area under the plasma concentration–time curve (AUC) from time zero to the last sampling time at which concentrations were at or above the limit of quantification (AUC0-t). 10. Day 5—AUC from time zero to 8 h post-dose (AUC0-τ). 11. Day 5—Area under the plasma concentration versus time curve from time zero to infinity (AUC0-∞). 12. Day 5—Apparent terminal elimination half-life, calculated from ln 2/λz (t1/2). | The investigational products consisted of capsules containing BIA 6-512 25 mg, 50 mg, 75 mg, and 100 mg. Oral doses with 240 mL of potable water. | Sample size: 38; Gender: all; Ages: 18 years to 45 years. | |
| EGCG | Efficacy and safety of green tea polyphenol in de novo Parkinson’s disease patients | 2 | China | Delay of progression of motor dysfunction. | N/A | Sample size: 480; Gender: all; Ages: 30 years and older. |
| Sulforaphane | A 6-month study to evaluate sulforaphane effects in PD patients | 2 | China | Cognitive improvement assessed using the MATRICS Consensus Cognitive Battery (MCCB) composite score. | N/A | Sample size: 100; Gender: all; Ages: 40 years to 75 years. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ates, I.; Yılmaz, A.D.; Buttari, B.; Arese, M.; Saso, L.; Suzen, S. A Review of the Potential of Nuclear Factor [Erythroid-Derived 2]-like 2 Activation in Autoimmune Diseases. Brain Sci. 2023, 13, 1532. https://doi.org/10.3390/brainsci13111532
Ates I, Yılmaz AD, Buttari B, Arese M, Saso L, Suzen S. A Review of the Potential of Nuclear Factor [Erythroid-Derived 2]-like 2 Activation in Autoimmune Diseases. Brain Sciences. 2023; 13(11):1532. https://doi.org/10.3390/brainsci13111532
Chicago/Turabian StyleAtes, Ilker, Ayşe Didem Yılmaz, Brigitta Buttari, Marzia Arese, Luciano Saso, and Sibel Suzen. 2023. "A Review of the Potential of Nuclear Factor [Erythroid-Derived 2]-like 2 Activation in Autoimmune Diseases" Brain Sciences 13, no. 11: 1532. https://doi.org/10.3390/brainsci13111532
APA StyleAtes, I., Yılmaz, A. D., Buttari, B., Arese, M., Saso, L., & Suzen, S. (2023). A Review of the Potential of Nuclear Factor [Erythroid-Derived 2]-like 2 Activation in Autoimmune Diseases. Brain Sciences, 13(11), 1532. https://doi.org/10.3390/brainsci13111532








