Surgical Clipping of Intracranial Aneurysms Using a Transcranial Neuroendoscopic Approach
Abstract
:1. Introduction
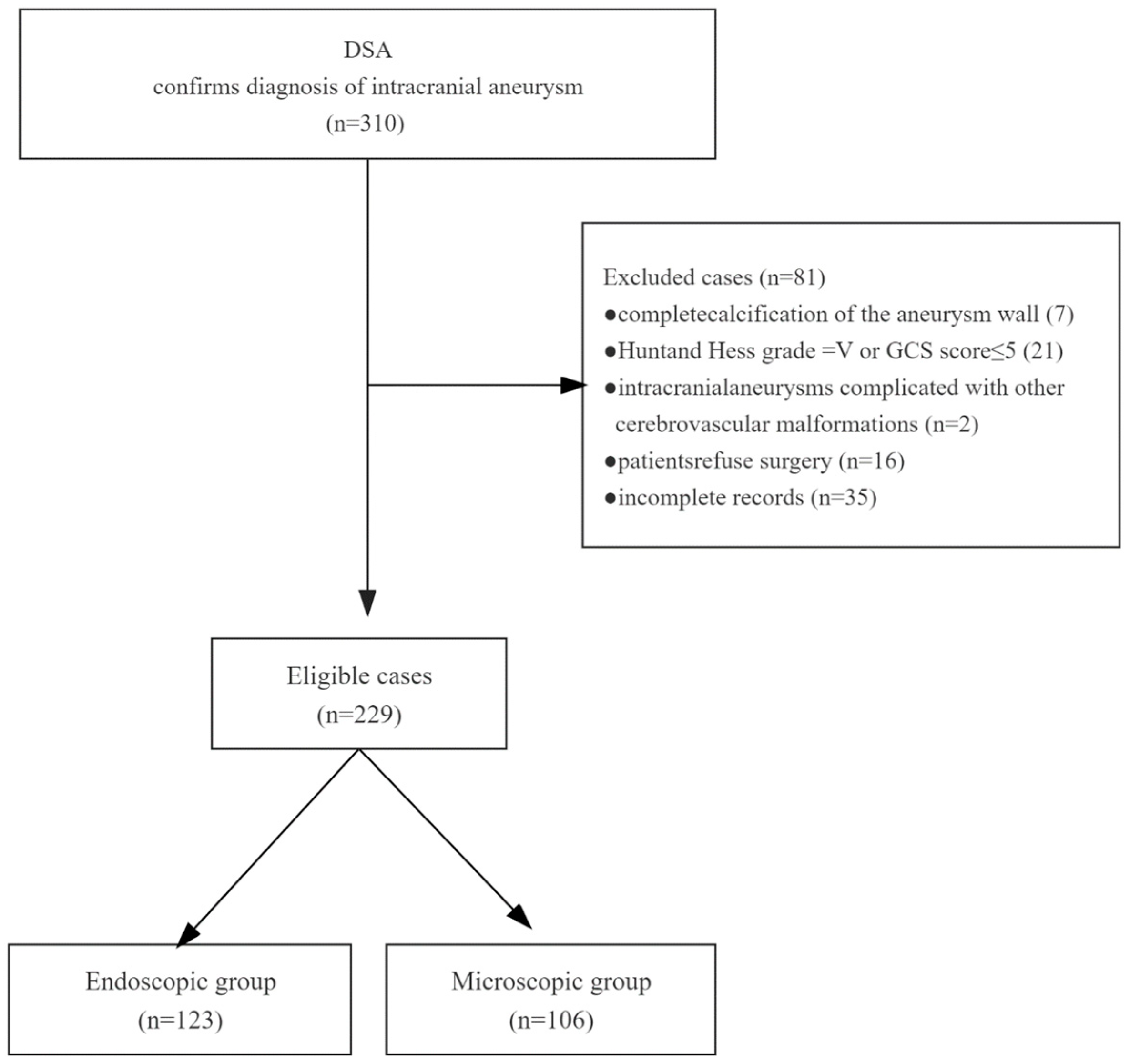
2. Patients and Methods
2.1. Patient Population
2.2. Data Collection
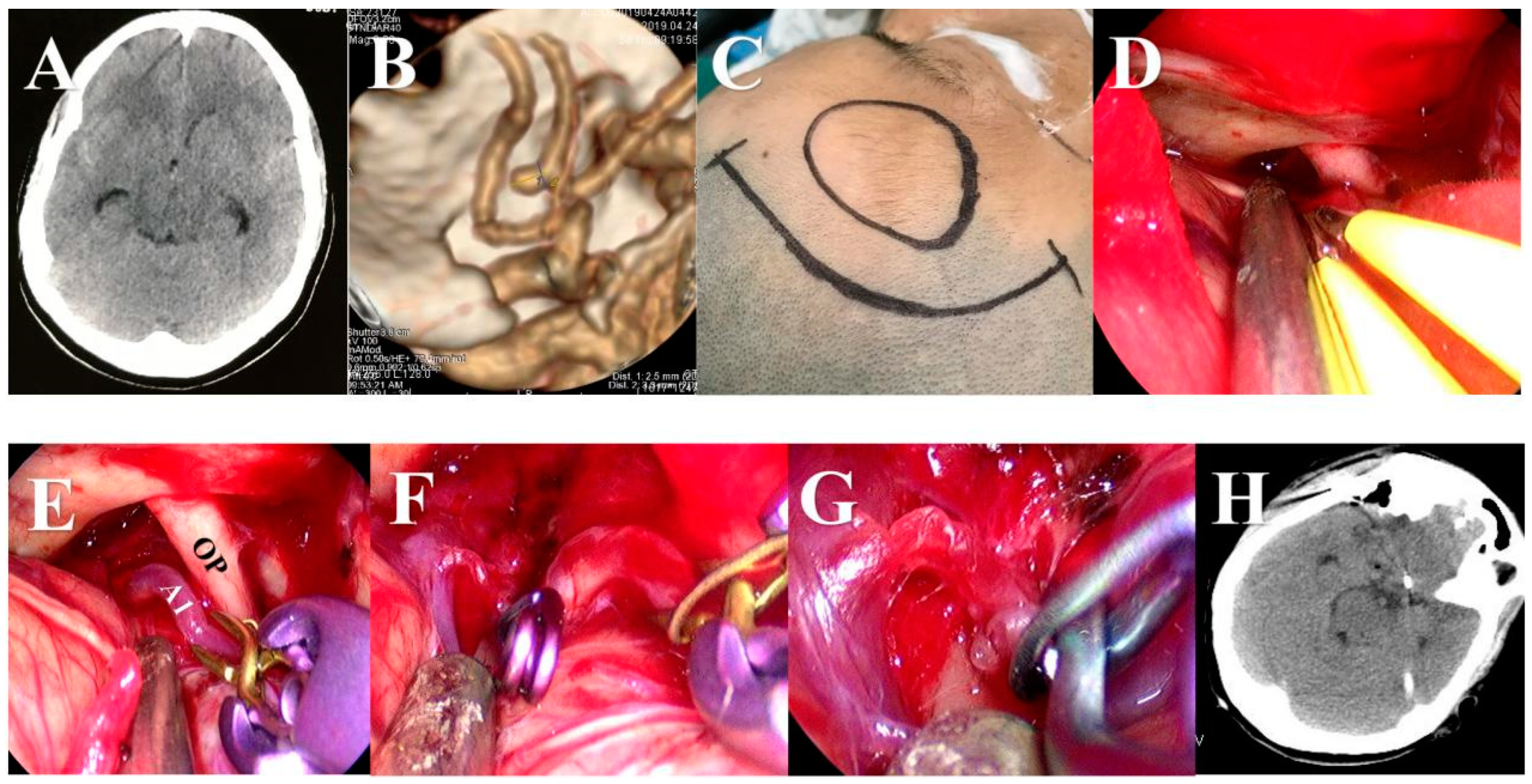
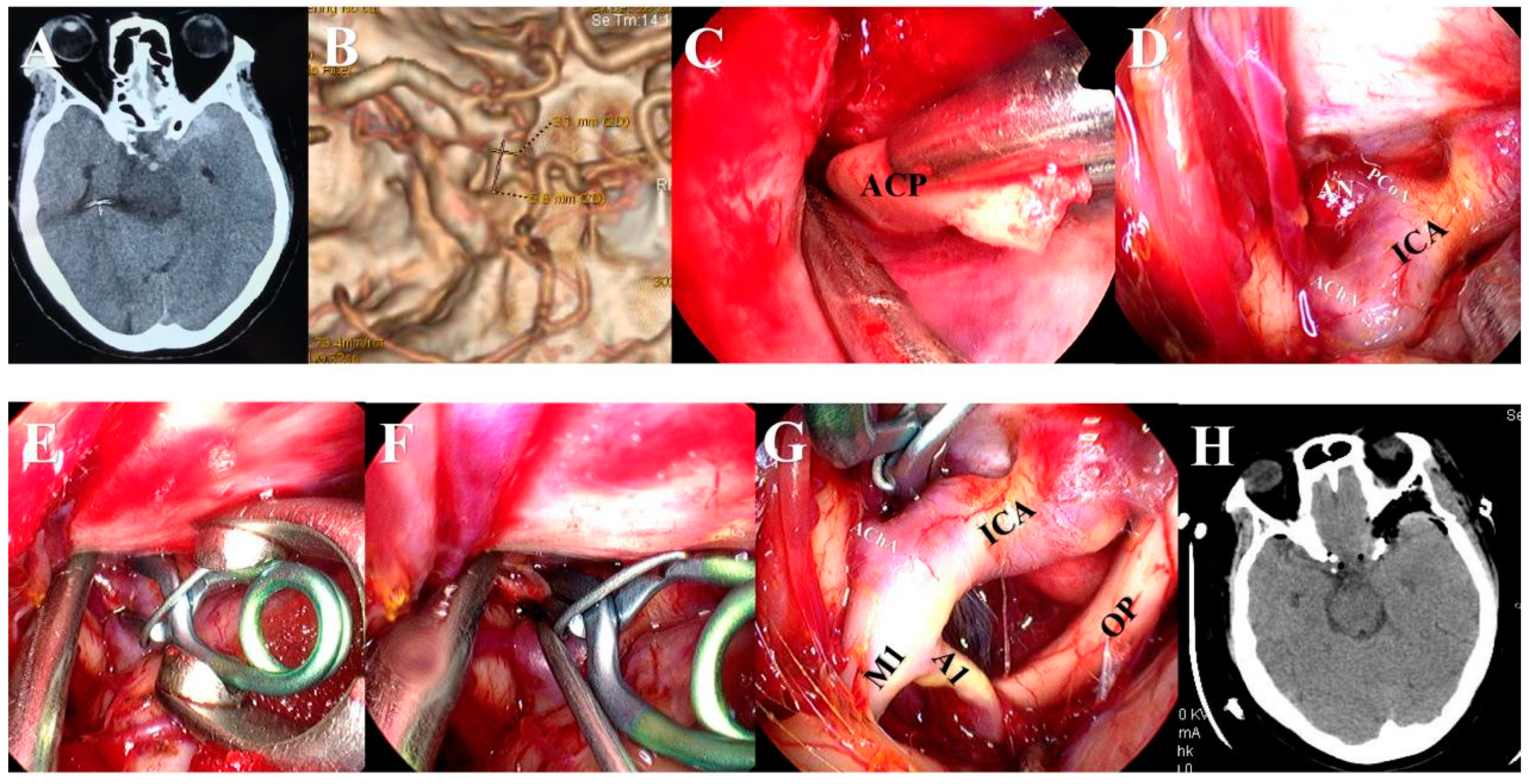
2.3. Surgical Procedures
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. The Surgical Efficacy and Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Q.; Zhang, Y.; Zhu, C.; Liu, W.; Ma, X.; Chen, J.; Mo, S.; Dong, L.; Wang, N.; Wu, J.; et al. Serum IL-1, Pyroptosis and Intracranial Aneurysm Wall Enhancement: Analysis Integrating Radiology, Serum Cytokines and Histology. Front. Cardiovasc. Med. 2022, 9, 818789. [Google Scholar] [CrossRef]
- Park, H.R.; Kim, J.H.; Park, S.; Chang, J.-C.; Park, S.Q. National Trends in the Treatment of Ruptured Cerebral Aneurysms in Korea Using an Age-adjusted Method. J. Korean Med. Sci. 2020, 35, e323. [Google Scholar] [CrossRef]
- Taschner, C.A.; Stracke, C.P.; Dorn, F.; Kadziolka, K.B.; Kreiser, K.; Solymosi, L.; Pham, M.; Buhk, J.H.; Turowski, B.; Reith, W.; et al. Derivo embolization device in the treatment of unruptured intracranial aneurysms: A prospective multicenter study. J. Neurointerv. Surg. 2021, 13, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, X.; Dong, L.; Liu, P.; Jia, L.; Zhang, Y.; Lv, M. Clinical and Angiographic Outcomes After Stent-Assisted Coiling of Cerebral Aneurysms With Laser-Cut and Braided Stents: A Comparative Analysis of the Literatures. Front. Neurol. 2021, 12, 666481. [Google Scholar] [CrossRef]
- Wang, J.; Weng, J.; Li, H.; Jiao, Y.; Fu, W.; Huo, R.; Yan, Z.; Xu, H.; Zhan, J.; Wang, S.; et al. Atorvastatin and growth, rupture of small unruptured intracranial aneurysm: Results of a prospective cohort study. Ther. Adv. Neurol. Disord. 2021, 14, 1756286420987939. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.R.; Ashour, R.; Sullender, C.T.; Dunn, A.K. Continuous blood flow visualization with laser speckle contrast imaging during neurovascular surgery. Neurophotonics 2022, 9, 021908. [Google Scholar] [CrossRef]
- Saqr, K.M.; Tupin, S.; Rashad, S.; Endo, T.; Niizuma, K.; Tominaga, T.; Ohta, M. Physiologic blood flow is turbulent. Sci. Rep. 2020, 10, 15492. [Google Scholar] [CrossRef]
- Zopfs, D.; Lennartz, S.; Pennig, L.; Glauner, A.; Abdullayev, N.; Bremm, J.; Hokamp, N.G.; Persigehl, T.; Kabbasch, C.; Borggrefe, J.; et al. Virtual monoenergetic images and post-processing algorithms effectively reduce CT artifacts from intracranial aneurysm treatment. Sci. Rep. 2020, 10, 6629. [Google Scholar] [CrossRef]
- McKinnon, C.; Voets, N.; Livermore, L.; Obeidat, M.; Plaha, P. Endoscopic Ipsilateral Interhemispheric Approach for Resection of Selected Deep Medial Brain Tumors. World Neurosurg. 2020, 144, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, B.; Mangesius, S.; Seppi, K.; Iglseder, S.; Di Pauli, F.; Ortler, M.; Gizewski, E.; Poewe, W.; Wenning, G.K. Symptomatic hemiparkinsonism due to extensive middle and posterior fossa arachnoid cyst: Case report. BMC Neurol. 2020, 20, 89. [Google Scholar] [CrossRef]
- Kassam, A.B.; Mintz, A.H.; Gardner, P.A.; Horowitz, M.B.; Carrau, R.L.; Snyderman, C.H. The expanded endonasal approach for an endoscopic transnasal clipping and aneurysmorrhaphy of a large vertebral artery aneurysm: Technical case report. Neurosurgery 2006, 59, ONSE162–ONSE165; discussion ONSE162–ONSE165. [Google Scholar] [CrossRef] [PubMed]
- Gardner, P.A.; Vaz-Guimaraes, F.; Jankowitz, B.; Koutourousiou, M.; Fernandez-Miranda, J.C.; Wang, E.W.; Snyderman, C.H. Endoscopic Endonasal Clipping of Intracranial Aneurysms: Surgical Technique and Results. World Neurosurg. 2015, 84, 1380–1393. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Perez, R.; Hardesty, D.A.; Silveira-Bertazzo, G.; Albonette-Felicio, T.; Carrau, R.L.; Prevedello, D.M. Safety and effectiveness of endoscopic endonasal intracranial aneurysm clipping: A systematic review. Neurosurg. Rev. 2020, 44, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhao, K.; Li, Y.; Wan, X.; Li, C.; Niu, H.; Shu, K.; Lei, T. Early Enteral Nutrition Can Reduce Incidence of Postoperative Hydrocephalus in Patients with Severe Hypertensive Intracerebral Hemorrhage. Med. Sci. Monit. 2022, 28, e935850. [Google Scholar] [CrossRef]
- Hu, P.; Yan, T.; Li, Y.; Guo, G.; Gao, X.; Su, Z.; Du, S.; Jin, R.; Tao, J.; Yuan, Y.; et al. Effect of Surgical Clipping versus Endovascular Coiling on the Incidence of Delayed Cerebral Ischemia in Patients with Aneurysmal Subarachnoid Hemorrhage: A Multicenter Observational Cohort Study with Propensity Score Matching. World Neurosurg. 2023, 172, e378–e388. [Google Scholar] [CrossRef]
- Apuzzo, M.L.J.; Heifetz, M.D.; Weiss, M.H.; Kurze, T. Neurosurgical endoscopy using the side-viewing telescope. J. Neurosurg. 1977, 46, 398–400. [Google Scholar] [CrossRef]
- Halves, E.; Bushe, K.A. Transsphenoidal operation on craniopharyngiomas with extrasellar extensions. The advantage of the operating endoscope [proceedings]. Acta Neurochir. Suppl. 1979, 28, 362. [Google Scholar]
- Matula, C.; Tschabitscher, M.; Day, J.D.; Reinprecht, A.; Koos, W.T. Endoscopically assisted microneurosurgery. Acta Neurochir. 1995, 134, 190–195. [Google Scholar] [CrossRef]
- Beer-Furlan, A.; Prevedello, D.; Figueiredo, E.G. Historical Perspective and the Role of Endoscopy in Intracranial Aneurysm Surgery. World Neurosurg. 2016, 88, 681–683. [Google Scholar] [CrossRef]
- Reisch, R.; Fischer, G.; Stadie, A.; Kockro, R.; Cesnulis, E.; Hopf, N. The supraorbital endoscopic approach for aneurysms. World Neurosurg. 2014, 82, S130–S137. [Google Scholar] [CrossRef]
- Di Somma, A.; de Notaris, M.; Ensenat, J.; Alobid, I.; Bernal-Sprekelsen, M.; Cavallo, L.M.; Prats-Galino, A.; Cappabianca, P. The ventral route to intracranial aneurysm: From the origin towards modern transsphenoidal surgery. An historical review and current perspective. Rhinology 2014, 52, 195–207. [Google Scholar] [CrossRef]
- Froelich, S.; Cebula, H.; Debry, C.; Boyer, P. Anterior communicating artery aneurysm clipped via an endoscopic endonasal approach: Technical note. Neurosurg. 2011, 68, 310–316, discussion 315–316. [Google Scholar] [CrossRef]
- Vaz-Guimaraes, F.; Gardnerl, P.A.; Fernandez-Miranda, J.C.; Wang, E.; Snyderman, C.H. Endoscopic endonasal skull base surgery for vascular lesions: A systematic review of the literature. J. Neurosurg. Sci. 2016, 60, 503–513. [Google Scholar]
- Yoshioka, H.; Kinouchi, H. The Roles of Endoscope in Aneurysmal Surgery. Neurol. Med. Chir. 2015, 55, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Huang, C.; Li, Z.; Jiang, X.; Zhao, X.; Wu, D.; Lai, N.; Liu, J.; Zhang, B.; Qin, F.; et al. Hemodynamic and Morphological Parameters of Ruptured Mirror Posterior Communicating Artery Aneurysms. Front. Neurol. 2021, 12, 653589. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.-H.; Su, S.-X.; Zhang, X.; Bi, Y.-M.; Duan, C.-Z.; Huang, W.-M.; Wang, X.-L. U-Shaped Association of Aspect Ratio and Single Intracranial Aneurysm Rupture in Chinese Patients: A Cross-Sectional Study. Front. Neurol. 2021, 12, 731129. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liang, X.; Chen, G.; Yang, P.; Zhang, J.; Liu, H.; Zhao, S.; Li, Y.; Sun, B.; Kang, J. Treatment of Intracranial Pseudoaneurysms With a Novel Covered Stent: A Series of 19 Patients With Midterm Follow-Up. Front. Neurol. 2020, 11, 580877. [Google Scholar] [CrossRef]
- Yu, J.; Lv, X. Flow Diversion for Intracranial Aneurysms Beyond the Circle of Willis. Front. Neurol. 2021, 12, 674966. [Google Scholar] [CrossRef]





| Characteristics | Neuroendoscopic Group (n = 123) | Microscopic Group (n = 106) | Total (n = 229) | p Value |
|---|---|---|---|---|
| Gender, n (%) | 0.045 | |||
| Male | 54 (43.9%) | 60 (56.6%) | 114 (49.8%) | |
| Female | 69 (56.1%) | 46 (43.4%) | 115 (50.2%) | |
| Age, y, mean ± sd | 58.504 ± 7.4837 | 59.5 ± 7.4645 | 58.965 ± 7.47 | 0.316 |
| Locations of aneurysms, n (%) | 0.794 | |||
| MCA (M1-M4) | 40 (32.5%) | 29 (27.4%) | 69 (30.1%) | |
| PCoA | 38 (30.9%) | 34 (32.1%) | 72 (31.4%) | |
| ACoA | 27 (22%) | 22 (20.8%) | 49 (21.4%) | |
| A2-A4 | 10 (8.1%) | 9 (8.5%) | 19 (8.3%) | |
| PICA | 5 (4.1%) | 6 (5.7%) | 11 (4.8%) | |
| Opht | 3 (2.4%) | 6 (5.7%) | 9 (3.9%) | |
| Aneurysm diameter, mean ± sd | 9.15 ± 1.9521 | 9.5167 ± 1.6403 | 9.31 ± 1.81 | 0.129 |
| Hunt–Hess grade, n (%) | 0.435 | |||
| 0 | 13 (10.6%) | 7 (6.6%) | 20 (8.7%) | |
| I | 23 (18.7%) | 25 (23.6%) | 48 (21%) | |
| II | 54 (43.9%) | 49 (46.2%) | 103 (45%) | |
| III | 18 (14.6%) | 18 (17%) | 36 (15.7%) | |
| IV | 15 (12.2%) | 7 (6.6%) | 22 (9.6%) | |
| Number of bleeding instances, n (%) | 0.254 | |||
| 0 | 13 (10.6%) | 8 (7.5%) | 21 (9.2%) | |
| 1 | 87 (70.7%) | 85 (80.2%) | 172 (75.1%) | |
| ≥2 | 23 (18.7%) | 13 (12.3%) | 36 (15.7%) |
| Characteristics | Neuroendoscopic Group (n = 123) | Microscopic Group (n = 106) | Total (n = 229) | p Value |
|---|---|---|---|---|
| Inadequately clipped aneurysms | 0.095 | |||
| No | 123 (100%) | 102 (96.2%) | 225 (98.2%) | |
| Yes | 0 (0%) | 4 (3.8%) | 4 (1.8%) | |
| Parent vessel occlusion | 0.669 | |||
| No | 123 (100%) | 104 (98.1%) | 227 (99.1%) | |
| Yes | 0 (0%) | 2 (1.9%) | 2 (0.9%) | |
| Surgical procedure time (minutes) | 181 (167.5, 192.5) | 154 (138, 169) | 168 ± 25.61 | <0.001 |
| mRS score, n (%) | 0.277 | |||
| 0~1 | 90 (73.2%) | 83 (78.3%) | 173 (75.5%) | |
| 2~3 | 29 (23.6%) | 17 (16%) | 46 (20.1%) | |
| 4~5 | 4 (3.3%) | 6 (5.7%) | 10 (4.4%) | |
| Limb hemiplegia, n (%) | 0.601 | |||
| No | 105 (85.4%) | 93 (87.7%) | 198 (86.5%) | |
| Yes | 18 (14.6%) | 13 (12.3%) | 31 (13.5%) | |
| Hydrocephalus, n (%) | 0.537 | |||
| No | 110 (89.4%) | 92 (86.8%) | 202 (88.2%) | |
| Yes | 13 (10.6%) | 14 (13.2%) | 27 (11.8%) | |
| Vision loss, n (%) | 0.082 | |||
| No | 122 (99.2%) | 100 (94.3%) | 222 (96.9%) | |
| Yes | 1 (0.8%) | 6 (5.7%) | 7 (3.1%) | |
| Intracranial infection, n (%) | 0.300 | |||
| No | 120 (97.6%) | 106 (100%) | 226 (98.7%) | |
| Yes | 3 (2.4%) | 0 (0%) | 3 (1.3%) | |
| Cerebral ischemia, n (%) | 0.034 | |||
| No | 115 (93.5%) | 90 (84.9%) | 205 (89.5%) | |
| Yes | 8 (6.5%) | 16 (15.1%) | 24 (10.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Lei, P.; Hua, Q.; Zhou, L.; Song, P.; Gao, L.; Zhang, S.; Cai, Q. Surgical Clipping of Intracranial Aneurysms Using a Transcranial Neuroendoscopic Approach. Brain Sci. 2023, 13, 1544. https://doi.org/10.3390/brainsci13111544
Li Z, Lei P, Hua Q, Zhou L, Song P, Gao L, Zhang S, Cai Q. Surgical Clipping of Intracranial Aneurysms Using a Transcranial Neuroendoscopic Approach. Brain Sciences. 2023; 13(11):1544. https://doi.org/10.3390/brainsci13111544
Chicago/Turabian StyleLi, Zhiyang, Pan Lei, Qiuwei Hua, Long Zhou, Ping Song, Lun Gao, Silei Zhang, and Qiang Cai. 2023. "Surgical Clipping of Intracranial Aneurysms Using a Transcranial Neuroendoscopic Approach" Brain Sciences 13, no. 11: 1544. https://doi.org/10.3390/brainsci13111544
APA StyleLi, Z., Lei, P., Hua, Q., Zhou, L., Song, P., Gao, L., Zhang, S., & Cai, Q. (2023). Surgical Clipping of Intracranial Aneurysms Using a Transcranial Neuroendoscopic Approach. Brain Sciences, 13(11), 1544. https://doi.org/10.3390/brainsci13111544





