Correlation of Diffusion Tensor Tractography with Restless Legs Syndrome Severity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Diffusion Tensor Imaging MRI Acquisition
2.3. Connectometry Analysis with Statistical Analysis
3. Results
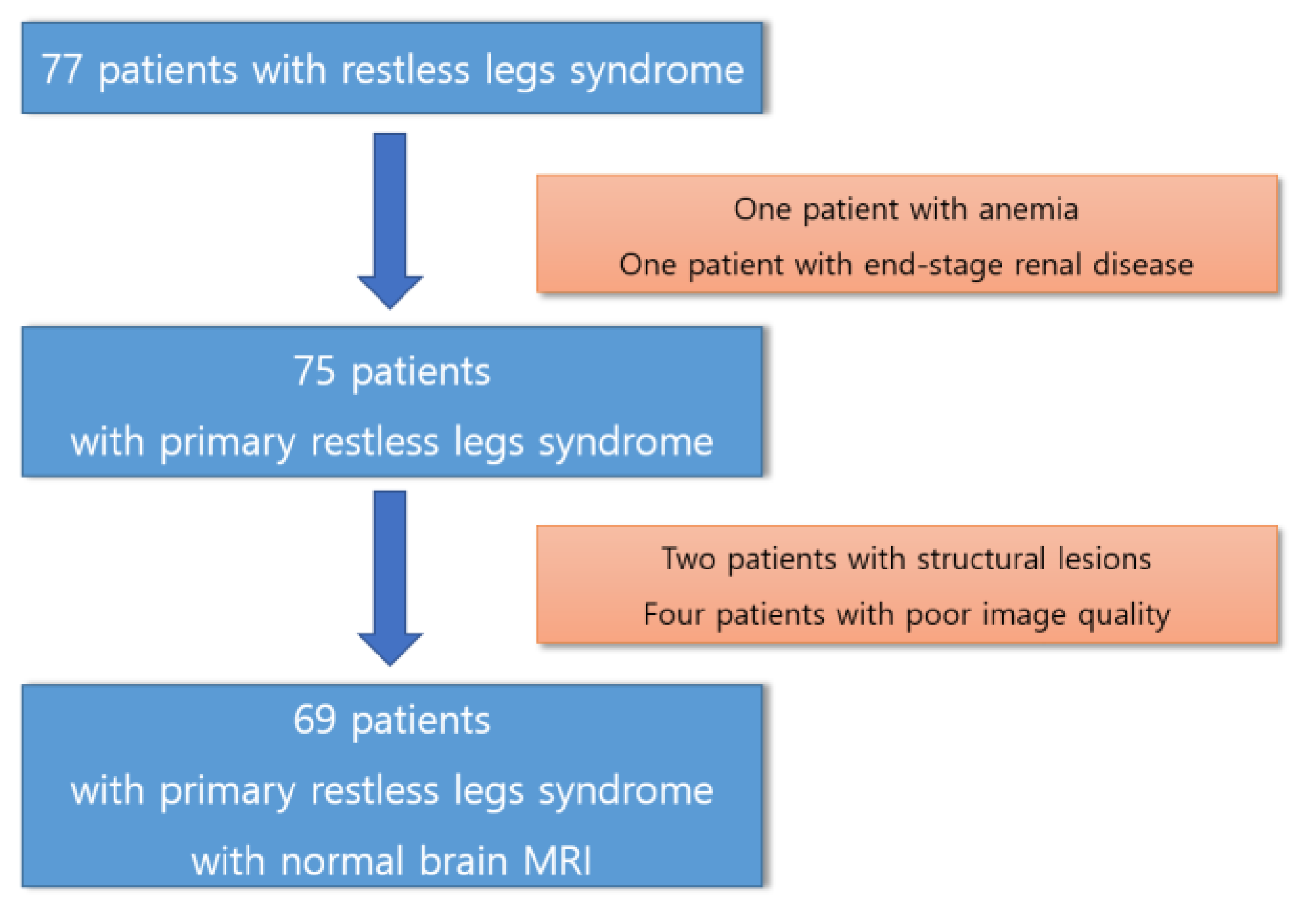
3.1. Participants
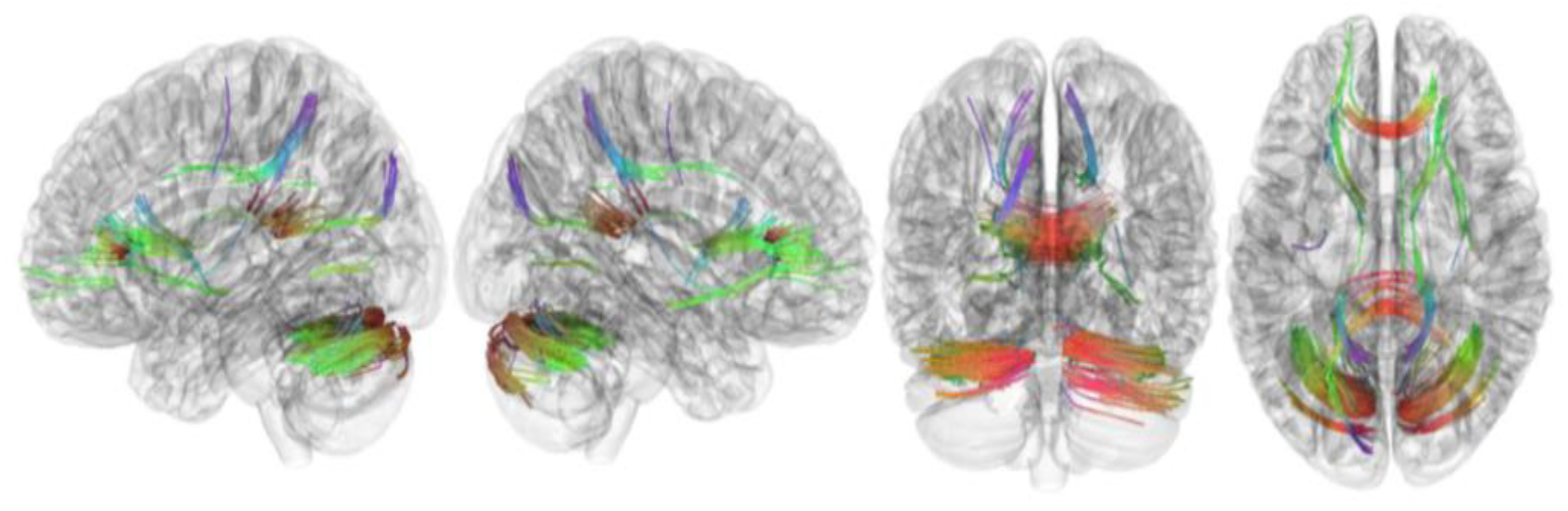
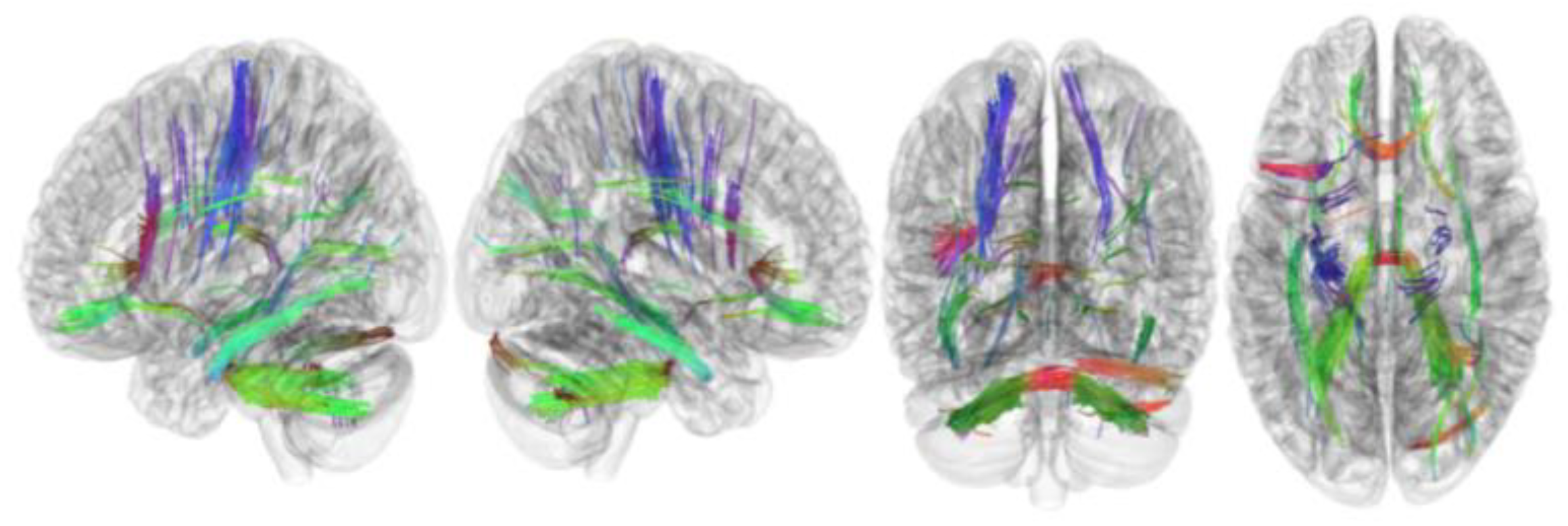
3.2. Tracks with FA Correlated with RLS Severity
3.3. Tracks with QA Correlated with RLS Severity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, R.P.; Picchietti, D.L.; Garcia-Borreguero, D.; Ondo, W.G.; Walters, A.S.; Winkelman, J.W.; Zucconi, M.; Ferri, R.; Trenkwalder, C.; Lee, H.B.; et al. Restless legs syndrome/Willis–Ekbom disease diagnostic criteria: Updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria—History, rationale, description, and significance. Sleep Med. 2014, 15, 860–873. [Google Scholar] [CrossRef]
- Walters, A.S.; Frauscher, B.; Allen, R.; Benes, H.; Chaudhuri, K.R.; Garcia-Borreguero, D.; Lee, H.B.; Picchietti, D.L.; Trenkwalder, C.; Martinez-Martin, P.; et al. Review of Quality of Life Instruments for the Restless Legs Syndrome/Willis-Ekbom Disease (RLS/WED): Critique and Recommendations. Sleep Med. 2014, 10, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Song, M.L.; Oldham, M.A.; Park, K.M.; Lee, E.-S.; Lee, H.B.; Cho, Y.W. Comparison of impact of insomnia on depression and quality of life in restless legs syndrome/Willis–Ekbom disease and primary insomnia patients. Sleep Med. 2015, 16, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Kim, K.T.; Kang, K.W.; Park, J.A.; Seo, J.-G.; Kim, J.; Chang, H.; Kim, E.Y.; Cho, Y.W. Alterations of Functional Connectivity in Patients with Restless Legs Syndrome. J. Clin. Neurol. 2022, 18, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Kim, K.T.; Lee, D.A.; Cho, Y.W. Structural brain connectivity in patients with restless legs syndrome: A diffusion tensor imaging study. Sleep 2022, 45, zsac099. [Google Scholar] [CrossRef] [PubMed]
- Turk-Browne, N.B. Functional Interactions as Big Data in the Human Brain. Science 2013, 342, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Sporns, O. The human connectome: Origins and challenges. NeuroImage 2013, 80, 53–61. [Google Scholar] [CrossRef]
- Akil, H.; Martone, M.E.; Van Essen, D.C. Challenges and Opportunities in Mining Neuroscience Data. Science 2011, 331, 708–712. [Google Scholar] [CrossRef]
- Seung, H.S. Neuroscience: Towards functional connectomics. Nature 2011, 471, 171–172. [Google Scholar] [CrossRef]
- Reveley, C.; Seth, A.K.; Pierpaoli, C.; Silva, A.C.; Yu, D.; Saunders, R.C.; Leopold, D.A.; Ye, F.Q. Superficial white matter fiber systems impede detection of long-range cortical connections in diffusion MR tractography. Proc. Natl. Acad. Sci. USA 2015, 112, E2820–E2828. [Google Scholar] [CrossRef]
- Thomas, C.; Ye, F.Q.; Irfanoglu, M.O.; Modi, P.; Saleem, K.S.; Leopold, D.A.; Pierpaoli, C. Anatomical accuracy of brain connections derived from diffusion MRI tractography is inherently limited. Proc. Natl. Acad. Sci. USA 2014, 111, 16574–16579. [Google Scholar] [CrossRef] [PubMed]
- Yeh, F.-C.; Badre, D.; Verstynen, T. Connectometry: A statistical approach harnessing the analytical potential of the local connectome. NeuroImage 2016, 125, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Nichols, T.E.; Holmes, A.P. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum. Brain Mapp. 2002, 15, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-J.; Yeh, F.-C.; Huang, S.-H.; Huang, C.-X.; Zhang, H.; Liu, J. Differential Tractography and Correlation Tractography Findings on Patients with Mild Traumatic Brain Injury: A Pilot Study. Front. Hum. Neurosci. 2022, 16, 751902. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.W.; Jelescu, I.O.; Ades-Aron, B.; Novikov, D.S.; Friedman, K.; Babb, J.S.; Osorio, R.S.; Galvin, J.E.; Shepherd, T.M.; Fieremans, E. Diffusion MRI biomarkers of white matter microstructure vary nonmonotonically with increasing cerebral amyloid deposition. Neurobiol. Aging 2020, 89, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Jahanshahi, A.; Salehi, M.A.; Darvishi, R.; Seyedmirzaei, H.; Luna, L.P. White matter microstructural changes in internet addiction disorder: A systematic review of diffusion tensor imaging studies. Addict. Behav. 2023, 143, 107690. [Google Scholar] [CrossRef] [PubMed]
- Yeh, F.-C.; Wedeen, V.J.; Tseng, W.-Y.I. Generalized q-sampling imaging. IEEE Trans. Med. Imaging 2010, 29, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yang, X.; Yuan, Z.; Qiu, J.; Lu, W. A comparison between diffusion tensor imaging and generalized q-sampling imaging in the age prediction of healthy adults via machine learning approaches. J. Neural Eng. 2022, 19, 016013. [Google Scholar] [CrossRef]
- Cho, Y.W.; Song, M.L.; Morinc, C.M. Validation of a Korean Version of the Insomnia Severity Index. J. Clin. Neurol. 2014, 10, 210–215. [Google Scholar] [CrossRef]
- Sohn, S.I.; Kim, D.H.; Lee, M.Y.; Cho, Y.W. The reliability and validity of the Korean version of the Pittsburgh Sleep Quality Index. Sleep Breath. 2012, 16, 803–812. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Walters, A.S.; LeBrocq, C.; Dhar, A.; Hening, W.; Rosen, R.; Allen, R.P.; Trenkwalder, C.; International Restless Legs Syndrome Study Group. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003, 4, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Abetz, L.; Allen, R.; Follet, A.; Washburn, T.; Early, C.; Kirsch, J.; Knight, H. Evaluating the quality of life of patients with restless legs syndrome. Clin. Ther. 2004, 26, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Yeh, F.-C.; Vettel, J.M.; Singh, A.; Poczos, B.; Grafton, S.T.; Erickson, K.I.; Tseng, W.-Y.I.; Verstynen, T.D. Quantifying Differences and Similarities in Whole-Brain White Matter Architecture Using Local Connectome Fingerprints. PLOS Comput. Biol. 2016, 12, e1005203. [Google Scholar] [CrossRef] [PubMed]
- Yeh, F.-C.; Panesar, S.; Barrios, J.; Fernandes, D.; Abhinav, K.; Meola, A.; Fernandez-Miranda, J.C. Automatic Removal of False Connections in Diffusion MRI Tractography Using Topology-Informed Pruning (TIP). Neurotherapeutics 2019, 16, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Yeh, F.-C.; Verstynen, T.D.; Wang, Y.; Fernández-Miranda, J.C.; Tseng, W.-Y.I. Deterministic Diffusion Fiber Tracking Improved by Quantitative Anisotropy. PLoS ONE 2013, 8, e80713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Lu, T.; Qiu, B.; Tang, Y.; Ou, S.; Tie, X.; Sun, C.; Xu, K.; Wang, Y. Differences between Generalized Q-Sampling Imaging and Diffusion Tensor Imaging in the Preoperative Visualization of the Nerve Fiber Tracts within Peritumoral Edema in Brain. Neurosurgery 2013, 73, 1044–1053; discussion 1053. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ribas, E.C.; Zhang, Z.; Wu, X.; Wang, X.; Liu, X.; Liang, J.; Chen, G.; Li, M. Tractography of the ansa lenticularis in the human brain. Clin. Anat. 2022, 35, 269–279. [Google Scholar] [CrossRef]
- Li, M.; Yeh, F.; Zeng, Q.; Wu, X.; Wang, X.; Zhu, Z.; Liu, X.; Liang, J.; Chen, G.; Zhang, H.; et al. The trajectory of the medial longitudinal fasciculus in the human brain: A diffusion imaging-based tractography study. Hum. Brain Mapp. 2021, 42, 6070–6086. [Google Scholar] [CrossRef]
- Jin, Z.; Bao, Y.; Wang, Y.; Li, Z.; Zheng, X.; Long, S.; Wang, Y. Differences between generalized Q-sampling imaging and diffusion tensor imaging in visualization of crossing neural fibers in the brain. Surg. Radiol. Anat. 2019, 41, 1019–1028. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Sun, H.; Yang, J.; Ma, Y.; Chen, K.; Su, J.; Yu, X.; Yang, F.; Zhang, Z.; et al. Cerebellum drives functional dysfunctions in restless leg syndrome. Sleep Med. 2023, 110, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Sigirli, D.; Gunes, A.; Ozdemir, S.T.; Ercan, I.; Durmus, Y.; Gursel, B.E. Statistical shape analysis of corpus callosum in restless leg syndrome. Neurol. Res. 2020, 42, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Paik, J.S.; Lee, H.J.; Chang, H.W.; Moon, H.-J.; Allen, R.P.; Earley, C.J.; Cho, Y.W. Altered white matter integrity in primary restless legs syndrome patients: Diffusion tensor imaging study. Neurol. Res. 2014, 36, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Park, H.R.; Kim, H.R.; Oh, S.; Seong, J.-K.; Joo, E.Y. White matter tract-specific alterations in patients with primary restless legs syndrome. Sci. Rep. 2021, 11, 16116. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Xie, S.; Wang, X.; Xi, X.; Liu, C. White matter microstructure alterations in idiopathic restless legs syndrome: A study combining crossing fiber-based and tensor-based approaches. Front. Neurosci. 2023, 17, 1240929. [Google Scholar] [CrossRef]
- Descoteaux, M.; Deriche, R.; Knosche, T.R.; Anwander, A. Deterministic and probabilistic tractography based on complex fibre orientation distributions. IEEE Trans. Med. Imaging 2008, 28, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Ouyang, X.; Liu, Z.; Chen, X.; Hu, X.; Lee, E.; Chen, E.Y.H.; Pu, W.; Shan, B.; Rohrbaugh, R.M. Associations among Suicidal Ideation, White Matter Integrity and Cognitive Deficit in First-Episode Schizophrenia. Front. Psychiatry 2018, 9, 391. [Google Scholar] [CrossRef]

| Patients with Restless Legs Syndrome (N = 69) | |
|---|---|
| Age, years | 57.0 ± 6.6 |
| Male, n (%) | 20 (28.9) |
| Age of onset, years | 47 (41.7–54.0) |
| Symptom duration, months | 120 (39–162) |
| IRLS | 27.1 ± 6.5 |
| Disease-specific quality of life | 8.7 ± 3.3 |
| PSQI | 12 (9.0–14.2) |
| ISI | 16 (11–23) |
| HAS | 7 (4–9) |
| HDS | 8 (5–11) |
| FA | |
|---|---|
| Tracks with positive correlation with RLS severity | Cerebellum, corpus callosum forceps minor, corpus callosum forceps major, corpus callosum body, and cingulum frontoparietal track |
| Tracks with negative correlation with RLS severity | Middle cerebellar peduncle, inferior longitudinal fasciculus, corticospinal tract, corpus callosum forceps minor, cerebellum, frontal aslant tract, dentato-rubrothalamic tract, inferior longitudinal fasciculus, corticostriatal tract superior, and cingulum parahippocampoparietal tract |
| QA | |
| Tracks with positive or negative correlation with RLS severity | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.M.; Kim, K.T.; Lee, D.A.; Cho, Y.W. Correlation of Diffusion Tensor Tractography with Restless Legs Syndrome Severity. Brain Sci. 2023, 13, 1560. https://doi.org/10.3390/brainsci13111560
Park KM, Kim KT, Lee DA, Cho YW. Correlation of Diffusion Tensor Tractography with Restless Legs Syndrome Severity. Brain Sciences. 2023; 13(11):1560. https://doi.org/10.3390/brainsci13111560
Chicago/Turabian StylePark, Kang Min, Keun Tae Kim, Dong Ah Lee, and Yong Won Cho. 2023. "Correlation of Diffusion Tensor Tractography with Restless Legs Syndrome Severity" Brain Sciences 13, no. 11: 1560. https://doi.org/10.3390/brainsci13111560
APA StylePark, K. M., Kim, K. T., Lee, D. A., & Cho, Y. W. (2023). Correlation of Diffusion Tensor Tractography with Restless Legs Syndrome Severity. Brain Sciences, 13(11), 1560. https://doi.org/10.3390/brainsci13111560







