Predictive Biomarkers of Treatment Response in Major Depressive Disorder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Resting-State EEG Acquisition
2.2. Participants and rTMS Therapy
2.3. EEG Preprocessing
2.4. EEG Analysis
2.5. Predictive Modeling
right_exponent + exponent_asymmetry:rTMS_protocol
beta_power + gamma_power + exponent
power + beta_band-power + gamma_band-power
2.6. Statistical Analyses
3. Results
3.1. Group Differences
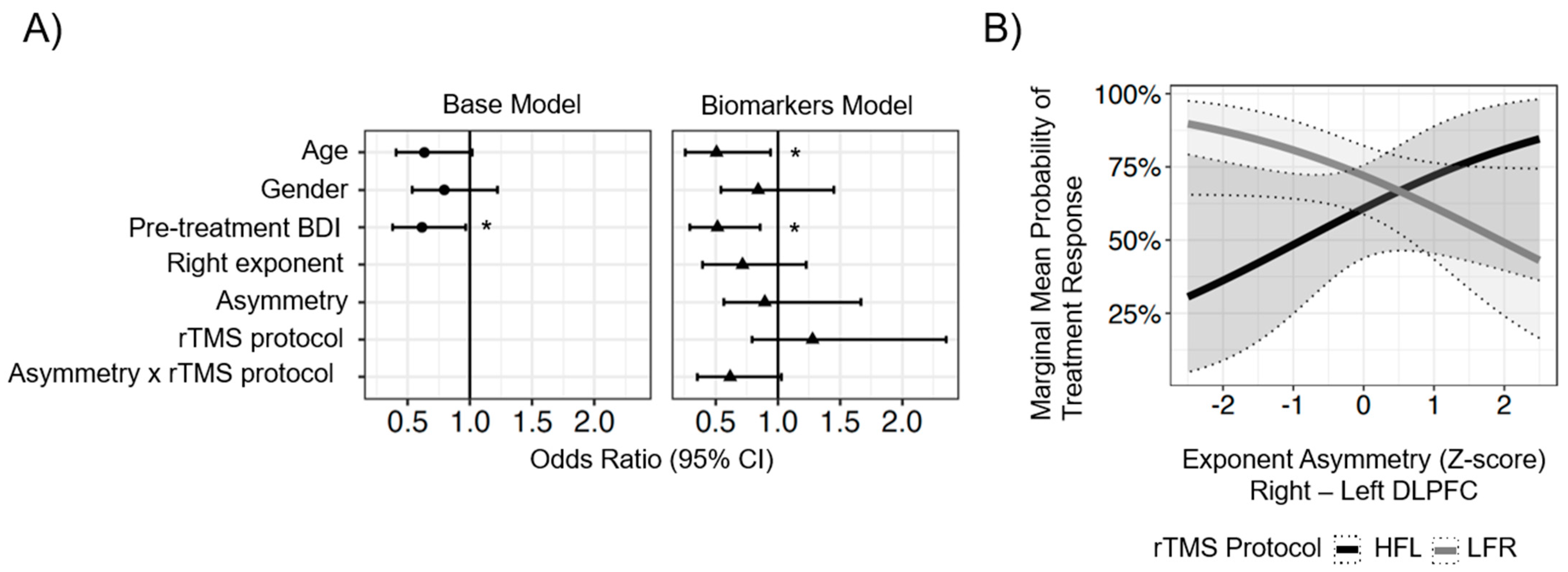
3.2. Lateralized ROI Model Results
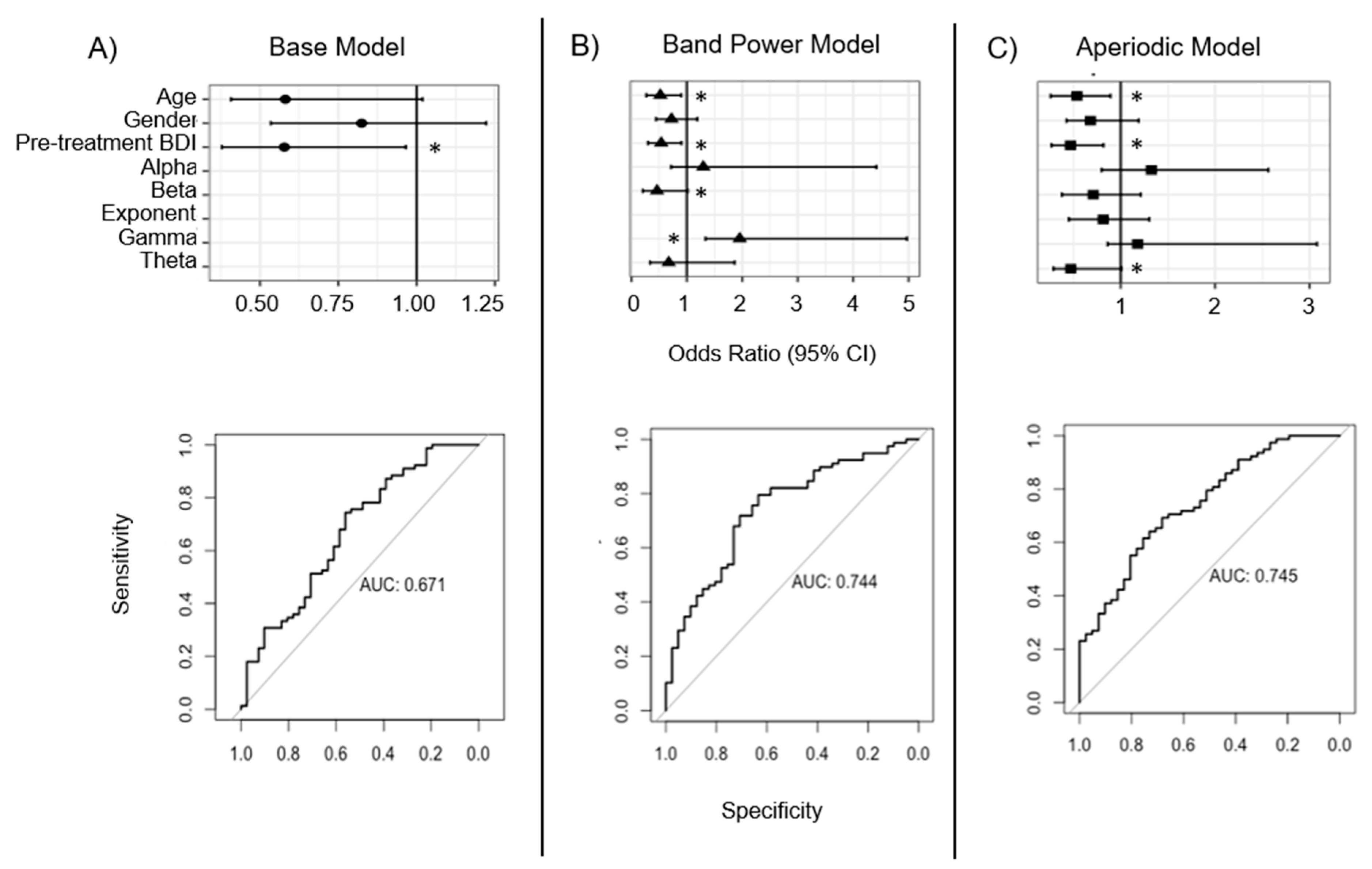
3.3. Whole-Head Model Results
4. Discussion
4.1. Age and Baseline BDI as Predictors of Treatment Response
4.2. Lateralized ROI Model Findings
4.3. Whole-Head Model Findings
4.4. Limitations and Further Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Depressive Disorder (Depression). 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 6 September 2023).
- Monroe, S.M.; Harkness, K.L. Recurrence in major depression: A conceptual analysis. Psychol. Rev. 2011, 118, 655–674. [Google Scholar] [CrossRef]
- Conway, C.R.; George, M.S.; Sackeim, H.A. Toward an Evidence-Based, Operational Definition of Treatment-Resistant Depression: When Enough Is Enough. JAMA Psychiatry 2017, 74, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Luscher, B.; Shen, Q.; Sahir, N. The GABAergic deficit hypothesis of major depressive disorder. Mol. Psychiatry 2011, 16, 383–406. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.D.; Buckner, R.L.; White, M.P.; Greicius, M.D.; Pascual-Leone, A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol. Psychiatry 2012, 72, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Grimm, S.; Beck, J.; Schuepbach, D.; Hell, D.; Boesiger, P.; Bermpohl, F.; Niehaus, L.; Boeker, H.; Northoff, G. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: An fMRI study in severe major depressive disorder. Biol. Psychiatry 2008, 63, 369–376. [Google Scholar] [CrossRef]
- Fitzgerald, P.B.; Brown, T.L.; Marston, N.A.; Daskalakis, Z.J.; De Castella, A.; Kulkarni, J. Transcranial magnetic stimulation in the treatment of depression: A double-blind, placebo-controlled trial. Arch. Gen. Psychiatry 2003, 60, 1002–1008. [Google Scholar] [CrossRef]
- Voineskos, D.; Blumberger, D.M.; Zomorrodi, R.; Rogasch, N.C.; Farzan, F.; Foussias, G.; Rajji, T.K.; Daskalakis, Z.J. Altered Transcranial Magnetic Stimulation-Electroencephalographic Markers of Inhibition and Excitation in the Dorsolateral Prefrontal Cortex in Major Depressive Disorder. Biol. Psychiatry 2019, 85, 477–486. [Google Scholar] [CrossRef]
- Wassermann, E.; Epstein, C.; Ziemann, U.; Walsh, V.; Paus, T.; Lisanby, S. (Eds.) The Oxford Handbook of Transcranial Stimulation; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Jaworska, N.; de la Salle, S.; Ibrahim, M.H.; Blier, P.; Knott, V. Leveraging Machine Learning Approaches for Predicting Antidepressant Treatment Response Using Electroencephalography (EEG) and Clinical Data. Front. Psychiatry 2018, 9, 768. [Google Scholar] [CrossRef]
- Fitzgerald, P.B.; Hoy, K.E.; Anderson, R.J.; Daskalakis, Z.J. A study of the pattern of response to rTMS treatment in depression. Depress. Anxiety 2016, 33, 746–753. [Google Scholar] [CrossRef]
- Fregni, F.; Marcolin, M.A.; Myczkowski, M.; Amiaz, R.; Hasey, G.; Rumi, D.O.; Rosa, M.; Rigonatti, S.P.; Camprodon, J.; Walpoth, M.; et al. Predictors of antidepressant response in clinical trials of transcranial magnetic stimulation. Int. J. Neuropsychopharmacol. 2006, 9, 641–654. [Google Scholar] [CrossRef]
- Drysdale, A.T.; Grosenick, L.; Downar, J.; Dunlop, K.; Mansouri, F.; Meng, Y.; Fetcho, R.N.; Zebley, B.; Oathes, D.J.; Etkin, A.; et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017, 23, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Rose, N. Biomarkers in psychiatry. Nature 2009, 460, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Kalia, M.; Costa ESilva, J. Biomarkers of psychiatric diseases: Current status and future prospects. Metabolism 2015, 64 (Suppl. S1), S11–S15. [Google Scholar] [CrossRef]
- Lozupone, M.; La Montagna, M.; D’Urso, F.; Daniele, A.; Greco, A.; Seripa, D.; Logroscino, G.; Bellomo, A.; Panza, F. The Role of Biomarkers in Psychiatry. Adv. Exp. Med. Biol. 2019, 1118, 135–162. [Google Scholar] [PubMed]
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef]
- Ippolito, G.; Bertaccini, R.; Tarasi, L.; Di Gregorio, F.; Trajkovic, J.; Battaglia, S.; Romei, V. The Role of Alpha Oscillations among the Main Neuropsychiatric Disorders in the Adult and Developing Human Brain: Evidence from the Last 10 Years of Research. Biomedicines 2022, 10, 3189. [Google Scholar] [CrossRef]
- Fitzgerald, P.J.; Watson, B.O. Gamma oscillations as a biomarker for major depression: An emerging topic. Transl. Psychiatry 2018, 8, 177. [Google Scholar] [CrossRef]
- Akar, S.A.; Kara, S.; Agambayev, S.; Bilgic, V. Nonlinear analysis of EEG in major depression with fractal dimensions. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; IEEE: New York, NY, USA, 2015; pp. 7410–7413. [Google Scholar]
- Lee, P.S.; Chen, Y.S.; Hsieh, J.C.; Su, T.P.; Chen, L.F. Distinct neuronal oscillatory responses between patients with bipolar and unipolar disorders: A magnetoencephalographic study. J. Affect. Disord. 2010, 123, 270–275. [Google Scholar] [CrossRef]
- Widge, A.S.; Bilge, M.T.; Montana, R.; Chang, W.; Rodriguez, C.I.; Deckersbach, T.; Carpenter, L.L.; Kalin, N.H.; Nemeroff, C.B. Electroencephalographic Biomarkers for Treatment Response Prediction in Major Depressive Illness: A Meta-Analysis. Am. J. Psychiatry 2019, 176, 44–56. [Google Scholar] [CrossRef]
- Olbrich, S.; Arns, M. EEG biomarkers in major depressive disorder: Discriminative power and prediction of treatment response. Int. Rev. Psychiatry 2013, 25, 604–618. [Google Scholar] [CrossRef]
- Pizzagalli, D.A.; Webb, C.A.; Dillon, D.G.; Tenke, C.E.; Kayser, J.; Goer, F.; Fava, M.; McGrath, P.; Weissman, M.; Parsey, R.; et al. Pretreatment Rostral Anterior Cingulate Cortex Theta Activity in Relation to Symptom Improvement in Depression: A Randomized Clinical Trial. JAMA Psychiatry 2018, 75, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Bailey, N.W.; Hoy, K.E.; Rogasch, N.C.; Thomson, R.H.; McQueen, S.; Elliot, D.; Sullivan, C.M.; Fulcher, B.D.; Daskalakis, Z.J.; Fitzgerald, P.B. Responders to rTMS for depression show increased fronto-midline theta and theta connectivity compared to non-responders. Brain Stimul. 2018, 11, 190–203. [Google Scholar] [CrossRef]
- Arns, M.; Drinkenburg, W.H.; Fitzgerald, P.B.; Kenemans, J.L. Neurophysiological predictors of non-response to rTMS in depression. Brain Stimul. 2012, 5, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Voetterl, H.; Miron, J.P.; Mansouri, F.; Fox, L.; Hyde, M.; Blumberger, D.M.; Daskalakis, Z.J.; Vila-Rodriguez, F.; Sack, S.T.; Downar, J. Investigating EEG biomarkers of clinical response to low frequency rTMS in depression. J. Affect. Disord. Rep. 2021, 6, 100250. [Google Scholar] [CrossRef]
- Klooster, D.; Voetterl, H.; Baeken, C.; Arns, M. Evaluating Robustness of Brain Stimulation Biomarkers for depression: A Systematic Review of MRI and EEG Studies. Biol. Psychiatry 2023, in press. [CrossRef]
- van Dijk, H.; van Wingen, G.; Denys, D.; Olbrich, S.; van Ruth, R.; Arns, M. The two decades brainclinics research archive for insights in neurophysiology (TDBRAIN) database. Sci. Data 2022, 9, 333. [Google Scholar] [CrossRef]
- He, B.J. Scale-free brain activity: Past, present, and future. Trends Cogn. Sci. 2014, 18, 480–487. [Google Scholar] [CrossRef]
- Buzsáki, G.; Logothetis, N.; Singer, W. Scaling brain size, keeping timing: Evolutionary preservation of brain rhythms. Neuron 2013, 80, 751–764. [Google Scholar] [CrossRef]
- Gao, R.; Peterson, E.J.; Voytek, B. Inferring synaptic excitation/inhibition balance from field potentials. Neuroimage 2017, 158, 70–78. [Google Scholar] [CrossRef]
- Donoghue, T.; Haller, M.; Peterson, E.J.; Varma, P.; Sebastian, P.; Gao, R.; Noto, T.; Lara, A.H.; Wallis, J.D.; Knight, R.T.; et al. Parameterizing neural power spectra into periodic and aperiodic components. Nat. Neurosci. 2020, 23, 1655–1665. [Google Scholar] [CrossRef]
- Buzsáki, G.; Anastassiou, C.A.; Koch, C. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 2012, 13, 407–420. [Google Scholar] [CrossRef]
- Ostlund, B.D.; Alperin, B.R.; Drew, T.; Karalunas, S.L. Behavioral and cognitive correlates of the aperiodic (1/f-like) exponent of the EEG power spectrum in adolescents with and without ADHD. Dev. Cogn. Neurosci. 2021, 48, 100931. [Google Scholar] [CrossRef]
- Voytek, B.; Kramer, M.A.; Case, J.; Lepage, K.Q.; Tempesta, Z.R.; Knight, R.T.; Gazzaley, A. Age-Related Changes in 1/f Neural Electrophysiological Noise. J. Neurosci. 2015, 35, 13257–13265. [Google Scholar] [CrossRef] [PubMed]
- Donse, L.; Padberg, F.; Sack, A.T.; Rush, A.J.; Arns, M. Simultaneous rTMS and psychotherapy in major depressive disorder: Clinical outcomes and predictors from a large naturalistic study. Brain Stimul. 2018, 11, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Dozois, D.J.; Dobson, K.S.; Ahnberg, J.L. A psychometric evaluation of the Beck Depression Inventory–II. Psychol. Assess. 1998, 10, 83. [Google Scholar] [CrossRef]
- Gramfort, A.; Luessi, M.; Larson, E.; Engemann, D.A.; Strohmeier, D.; Brodbeck, C.; Goj, R.; Jas, M.; Brooks, T.; Parkkonen, L.; et al. MEG and EEG data analysis with MNE-Python. Front. Neurosci. 2013, 7, 267. [Google Scholar] [CrossRef]
- Li, A.; Feitelberg, J.; Saini, A.P.; Höchenberger, R.; Scheltienne, M. MNE-ICALabel: Automatically annotating ICA components with ICLabel in Python. J. Open Source Softw. 2022, 7, 4484. [Google Scholar] [CrossRef]
- Hohaia, W.; Saurels, B.W.; Johnston, A.; Yarrow, K.; Arnold, D.H. Occipital alpha-band brain waves when the eyes are closed are shaped by ongoing visual processes. Sci. Rep. 2022, 12, 1194. [Google Scholar] [CrossRef]
- Rostami, R.; Kazemi, R.; Nitsche, M.A.; Gholipour, F.; Salehinejad, M.A. Clinical and demographic predictors of response to rTMS treatment in unipolar and bipolar depressive disorders. Clin. Neurophysiol. 2017, 128, 1961–1970. [Google Scholar] [CrossRef]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Balsamo, M.; Saggino, A. Determining a diagnostic cut-off on the Teate Depression Inventory. Neuropsychiatr. Dis. Treat. 2014, 10, 987–995. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Harrell, F.E.; Borsboom, G.J.; Eijkemans, M.J.; Vergouwe, Y.; Habbema, J.D. Internal validation of predictive models: Efficiency of some procedures for logistic regression analysis. J. Clin. Epidemiol. 2001, 54, 774–781. [Google Scholar] [CrossRef]
- Nagelkerke, N.J.D. A note on a general definition of the coefficient of determination. Biometrika 1991, 78, 691–692. [Google Scholar] [CrossRef]
- DiCiccio, T.J.; Efron, B. Bootstrap confidence intervals. Stat. Sci. 1996, 11, 189–228. [Google Scholar] [CrossRef]
- Kar, S.K. Predictors of Response to Repetitive Transcranial Magnetic Stimulation in Depression: A Review of Recent Updates. Clin. Psychopharmacol. Neurosci. 2019, 17, 25–33. [Google Scholar] [CrossRef]
- Pallanti, S.; Cantisani, A.; Grassi, G.; Antonini, S.; Cecchelli, C.; Burian, J.; Cauli, G.; Quercioli, L. rTMS age-dependent response in treatment-resistant depressed subjects: A mini-review. CNS Spectr. 2012, 17, 24–30. [Google Scholar] [CrossRef]
- Aguirre, I.; Carretero, B.; Ibarra, O.; Kuhalainen, J.; Martínez, J.; Ferrer, A.; Salva, J.; Roca, M.; Gili, M.; Montoya, P.; et al. Age predicts low-frequency transcranial magnetic stimulation efficacy in major depression. J. Affect. Disord. 2011, 130, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Grammer, G.G.; Kuhle, A.R.; Clark, C.C.; Dretsch, M.N.; Williams, K.A.; Cole, J.T. Severity of Depression Predicts Remission Rates Using Transcranial Magnetic Stimulation. Front. Psychiatry 2015, 6, 114. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.N.; Barnes, C.A. Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 2006, 7, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.; Farzan, F.; Pascual-Leone, A. Assessing brain plasticity across the lifespan with transcranial magnetic stimulation: Why, how, and what is the ultimate goal? Front. Neurosci. 2013, 7, 42. [Google Scholar] [CrossRef]
- Goldsworthy, M.R.; Rogasch, N.C.; Ballinger, S.; Graetz, L.; Van Dam, J.M.; Harris, R.; Yu, S.; Pitcher, J.B.; Baune, B.T.; Ridding, M.C. Age-related decline of neuroplasticity to intermittent theta burst stimulation of the lateral prefrontal cortex and its relationship with late-life memory performance. Clin. Neurophysiol. 2020, 131, 2181–2191. [Google Scholar] [CrossRef]
- Appelbaum, L.G.; Shenasa, M.A.; Stolz, L.; Daskalakis, Z. Synaptic plasticity and mental health: Methods, challenges and opportunities. Neuropsychopharmacology 2022, 48, 113–120. [Google Scholar] [CrossRef]
- Cappon, D.; den Boer, T.; Jordan, C.; Yu, W.; Metzger, E.; Pascual-Leone, A. Transcranial magnetic stimulation (TMS) for geriatric depression. Ageing Res. Rev. 2022, 74, 101531. [Google Scholar] [CrossRef]
- Scahill, R.I.; Frost, C.; Jenkins, R.; Whitwell, J.L.; Rossor, M.N.; Fox, N.C. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch. Neurol. 2003, 60, 989–994. [Google Scholar] [CrossRef]
- Wagner, T.; Eden, U.; Fregni, F.; Valero-Cabre, A.; Ramos-Estebanez, C.; Pronio-Stelluto, V.; Grodzinsky, A.; Zahn, M.; Pascual-Leone, A. Transcranial magnetic stimulation and brain atrophy: A computer-based human brain model study. Exp. Brain Res. 2008, 186, 539–550. [Google Scholar] [CrossRef]
- Pizzagalli, D.A. Depression, stress, and anhedonia: Toward a synthesis and integrated model. Annu. Rev. Clin. Psychol. 2014, 10, 393–423. [Google Scholar] [CrossRef]
- McMakin, D.L.; Olino, T.M.; Porta, G.; Dietz, L.J.; Emslie, G.; Clarke, G.; Wagner, K.D.; Asarnow, J.R.; Ryan, N.D.; Birmaher, B.; et al. Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment-resistant depression. J. Am. Acad. Child. Adolesc. Psychiatry 2012, 51, 404–411. [Google Scholar] [CrossRef]
- Downar, J.; Geraci, J.; Salomons, T.V.; Dunlop, K.; Wheeler, S.; McAndrews, M.P.; Bakker, N.; Blumberger, D.M.; Daskalakis, Z.J.; Kennedy, S.H.; et al. Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biol. Psychiatry 2014, 76, 176–185. [Google Scholar] [CrossRef]
- Mazza, F.; Guet-McCreight, A.; Valiante, T.A.; Griffiths, J.D.; Hay, E. In-silico EEG biomarkers of reduced inhibition in human cortical microcircuits in depression. PLoS Comput. Biol. 2023, 19, e1010986. [Google Scholar] [CrossRef]
| MDD Responder | MDD Non-Responder | Test Statistics | Healthy Controls | |
|---|---|---|---|---|
| Number of participants | 78 | 41 | 36 | |
| Average age (years) | 41.4 (1.4) | 46.3 (2.3) | t72 = 1.85, p = 0.07 | 32.2 (2.3) |
| Number male | 37 | 22 | = 0.20, p = 0.65 | 0.42 |
| Pre-treatment BDI | 30.1 (1.0) | 33.6 (1.8) | t66 = 1.67, p = 0.10 | N/A |
| Post-treatment BDI | 7.2 (0.6) | 28.4 (1.7) | t51 = 11.4, p < 0.001 | N/A |
| rTMS protocol (% HFL/LFR/Bi) | 36/64/3 | 41/59/2 | = 0.07, p = 0.79 | N/A |
| Base Model | Band Power Model | Aperiodic Model | |
|---|---|---|---|
| ROC AUC | 67.1% (58.5, 78.9) | 74.4% (68.7, 85.5) | 74.5% (68.1, 85.4) |
| Optimism-corrected AUC | 63.6% (−3.5%) | 68.0% (−6.4%) | 67.4% (−7.1%) |
| Nagelkerke R2 | 11.9% | 22.0% | 25.5% |
| Delta-AUC (vs. Base) | Z = 1.77, p = 0.078 | Z = 1.76, p = 0.079 | |
| Delta-AUC (vs. BP) | Z = 0.05, p = 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stolz, L.A.; Kohn, J.N.; Smith, S.E.; Benster, L.L.; Appelbaum, L.G. Predictive Biomarkers of Treatment Response in Major Depressive Disorder. Brain Sci. 2023, 13, 1570. https://doi.org/10.3390/brainsci13111570
Stolz LA, Kohn JN, Smith SE, Benster LL, Appelbaum LG. Predictive Biomarkers of Treatment Response in Major Depressive Disorder. Brain Sciences. 2023; 13(11):1570. https://doi.org/10.3390/brainsci13111570
Chicago/Turabian StyleStolz, Louise A., Jordan N. Kohn, Sydney E. Smith, Lindsay L. Benster, and Lawrence G. Appelbaum. 2023. "Predictive Biomarkers of Treatment Response in Major Depressive Disorder" Brain Sciences 13, no. 11: 1570. https://doi.org/10.3390/brainsci13111570
APA StyleStolz, L. A., Kohn, J. N., Smith, S. E., Benster, L. L., & Appelbaum, L. G. (2023). Predictive Biomarkers of Treatment Response in Major Depressive Disorder. Brain Sciences, 13(11), 1570. https://doi.org/10.3390/brainsci13111570






