Alzheimer’s Disease CSF Biomarkers as Possible Indicators of Tap-Test Response in Idiopathic Normal Pressure Hydrocephalus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ethical Issues
2.3. Tap-Test Evaluation
2.4. CSF Sampling and Biomarkers’ Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rekate, H.L. A contemporary definition and classification of hydrocephalus. Semin. Pediatr. Neurol. 2009, 16, 9–15. [Google Scholar] [CrossRef]
- Leinonen, V.; Vanninen, R.; Rauramaa, T. Cerebrospinal fluid circulation and hydrocephalus. Handb. Clin. Neurol. 2017, 145, 39–50. [Google Scholar] [CrossRef]
- Ransohoff, J.; Shulman, K.; Fishman, R.A. Hydrocephalus: A review of etiology and treatment. J. Pediatr. 1960, 56, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.M.; Nitrini, R.; Roman, G.C. Normal-pressure hydrocephalus: A critical review. Dement. Neuropsychol. 2019, 13, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Skalicky, P.; Mladek, A.; Vlasak, A.; De Lacy, P.; Benes, V.; Bradac, O. Normal pressure hydrocephalus-an overview of pathophysiological mechanisms and diagnostic procedures. Neurosurg. Rev. 2020, 43, 1451–1464. [Google Scholar] [CrossRef]
- Adams, R.D.; Fisher, C.M.; Hakim, S.; Ojemann, R.G.; Sweet, W.H. Symptomatic Occult Hydrocephalus with “Normal” Cerebrospinal-Fluid Pressure.A Treatable Syndrome. N. Engl. J. Med. 1965, 273, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Hakim, S.; Venegas, J.G.; Burton, J.D. The physics of the cranial cavity, hydrocephalus and normal pressure hydrocephalus: Mechanical interpretation and mathematical model. Surg. Neurol. 1976, 5, 187–210. [Google Scholar] [PubMed]
- Capone, P.M.; Bertelson, J.A.; Ajtai, B. Neuroimaging of Normal Pressure Hydrocephalus and Hydrocephalus. Neurol. Clin. 2020, 38, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Relkin, N.; Marmarou, A.; Klinge, P.; Bergsneider, M.; Black, P.M. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery 2005, 57, S4–S16. [Google Scholar] [CrossRef]
- Marmarou, A.; Black, P.; Bergsneider, M.; Klinge, P.; Relkin, N.; International NPH Consultant Group. Guidelines for management of idiopathic normal pressure hydrocephalus: Progress to date. Acta Neurochir. Suppl. 2005, 95, 237–240. [Google Scholar] [CrossRef]
- Mori, E.; Ishikawa, M.; Kato, T.; Kazui, H.; Miyake, H.; Miyajima, M.; Nakajima, M.; Hashimoto, M.; Kuriyama, N.; Tokuda, T.; et al. Guidelines for management of idiopathic normal pressure hydrocephalus: Second edition. Neurol. Med.-Chir. 2012, 52, 775–809. [Google Scholar] [CrossRef]
- Zaccaria, V.; Bacigalupo, I.; Gervasi, G.; Canevelli, M.; Corbo, M.; Vanacore, N.; Lacorte, E. A systematic review on the epidemiology of normal pressure hydrocephalus. Acta Neurol. Scand. 2020, 141, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, K.; Meguro, K.; Mori, E. Prevalence of idiopathic normal-pressure hydrocephalus in the elderly population of a Japanese rural community. Neurol. Med.-Chir. 2008, 48, 197–199; discussion 199–200. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.; Rosell, M.; Kockum, K.; Lilja-Lund, O.; Soderstrom, L.; Laurell, K. Prevalence of idiopathic normal pressure hydrocephalus: A prospective, population-based study. PLoS ONE 2019, 14, e0217705. [Google Scholar] [CrossRef] [PubMed]
- Kubo, Y.; Kazui, H.; Yoshida, T.; Kito, Y.; Kimura, N.; Tokunaga, H.; Ogino, A.; Miyake, H.; Ishikawa, M.; Takeda, M. Validation of grading scale for evaluating symptoms of idiopathic normal-pressure hydrocephalus. Dement. Geriatr. Cogn. Disord. 2008, 25, 37–45. [Google Scholar] [CrossRef]
- Williams, M.A.; Relkin, N.R. Diagnosis and management of idiopathic normal-pressure hydrocephalus. Neurol. Clin. Pract. 2013, 3, 375–385. [Google Scholar] [CrossRef]
- Toma, A.K.; Papadopoulos, M.C.; Stapleton, S.; Kitchen, N.D.; Watkins, L.D. Systematic review of the outcome of shunt surgery in idiopathic normal-pressure hydrocephalus. Acta Neurochir. 2013, 155, 1977–1980. [Google Scholar] [CrossRef]
- Shaw, R.; Everingham, E.; Mahant, N.; Jacobson, E.; Owler, B. Clinical outcomes in the surgical treatment of idiopathic normal pressure hydrocephalus. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2016, 29, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Mihalj, M.; Dolic, K.; Kolic, K.; Ledenko, V. CSF tap test—Obsolete or appropriate test for predicting shunt responsiveness? A systemic review. J. Neurol. Sci. 2016, 362, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Siraj, S. An overview of normal pressure hydrocephalus and its importance: How much do we really know? J. Am. Med. Dir. Assoc. 2011, 12, 19–21. [Google Scholar] [CrossRef]
- Savolainen, S.; Laakso, M.P.; Paljarvi, L.; Alafuzoff, I.; Hurskainen, H.; Partanen, K.; Soininen, H.; Vapalahti, M. MR imaging of the hippocampus in normal pressure hydrocephalus: Correlations with cortical Alzheimer’s disease confirmed by pathologic analysis. Am. J. Neuroradiol. 2000, 21, 409–414. [Google Scholar] [PubMed]
- Golomb, J.; Wisoff, J.; Miller, D.C.; Boksay, I.; Kluger, A.; Weiner, H.; Salton, J.; Graves, W. Alzheimer’s disease comorbidity in normal pressure hydrocephalus: Prevalence and shunt response. J. Neurol. Neurosurg. Psychiatry 2000, 68, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Yamada, S.; Miyajima, M.; Ishii, K.; Kuriyama, N.; Kazui, H.; Kanemoto, H.; Suehiro, T.; Yoshiyama, K.; Kameda, M.; et al. Guidelines for Management of Idiopathic Normal Pressure Hydrocephalus (Third Edition): Endorsed by the Japanese Society of Normal Pressure Hydrocephalus. Neurol. Med.-Chir. 2021, 61, 63–97. [Google Scholar] [CrossRef] [PubMed]
- Pyrgelis, E.S.; Paraskevas, G.P.; Constantinides, V.C.; Boufidou, F.; Velonakis, G.; Stefanis, L.; Kapaki, E. Callosal Angle Sub-Score of the Radscale in Patients with Idiopathic Normal Pressure Hydrocephalus Is Associated with Positive Tap Test Response. J. Clin. Med. 2022, 11, 2898. [Google Scholar] [CrossRef]
- Schniepp, R.; Trabold, R.; Romagna, A.; Akrami, F.; Hesselbarth, K.; Wuehr, M.; Peraud, A.; Brandt, T.; Dieterich, M.; Jahn, K. Walking assessment after lumbar puncture in normal-pressure hydrocephalus: A delayed improvement over 3 days. J. Neurosurg. 2017, 126, 148–157. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Dubois, B.; Slachevsky, A.; Litvan, I.; Pillon, B. The FAB: A Frontal Assessment Battery at bedside. Neurology 2000, 55, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Touchon, J.; Portet, F.; Ousset, P.J.; Vellas, B.; Michel, B. “The 5 words”: A simple and sensitive test for the diagnosis of Alzheimer’s disease. Presse Med. 2002, 31, 1696–1699. [Google Scholar] [PubMed]
- Royall, D.R.; Cordes, J.A.; Polk, M. CLOX: An executive clock drawing task. J. Neurol. Neurosurg. Psychiatry 1998, 64, 588–594. [Google Scholar] [CrossRef] [PubMed]
- del Campo, M.; Mollenhauer, B.; Bertolotto, A.; Engelborghs, S.; Hampel, H.; Simonsen, A.H.; Kapaki, E.; Kruse, N.; Le Bastard, N.; Lehmann, S.; et al. Recommendations to standardize preanalytical confounding factors in Alzheimer’s and Parkinson’s disease cerebrospinal fluid biomarkers: An update. Biomark. Med. 2012, 6, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Paraskevas, G.P.; Kasselimis, D.; Kourtidou, E.; Constantinides, V.; Bougea, A.; Potagas, C.; Evdokimidis, I.; Kapaki, E. Cerebrospinal Fluid Biomarkers as a Diagnostic Tool of the Underlying Pathology of Primary Progressive Aphasia. J. Alzheimer’s Dis. 2017, 55, 1453–1461. [Google Scholar] [CrossRef]
- Molinuevo, J.L.; Blennow, K.; Dubois, B.; Engelborghs, S.; Lewczuk, P.; Perret-Liaudet, A.; Teunissen, C.E.; Parnetti, L. The clinical use of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: A consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2014, 10, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, A.H.; Herukka, S.K.; Andreasen, N.; Baldeiras, I.; Bjerke, M.; Blennow, K.; Engelborghs, S.; Frisoni, G.B.; Gabryelewicz, T.; Galluzzi, S.; et al. Recommendations for CSF AD biomarkers in the diagnostic evaluation of dementia. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2017, 13, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Ko, P.W.; Jin, M.; Suk, K.; Lee, H.W. Idiopathic normal-pressure hydrocephalus, cerebrospinal fluid biomarkers, and the cerebrospinal fluid tap test. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2014, 21, 1398–1403. [Google Scholar] [CrossRef]
- Santangelo, R.; Cecchetti, G.; Bernasconi, M.P.; Cardamone, R.; Barbieri, A.; Pinto, P.; Passerini, G.; Scomazzoni, F.; Comi, G.; Magnani, G. Cerebrospinal Fluid Amyloid-beta 42, Total Tau and Phosphorylated Tau are Low in Patients with Normal Pressure Hydrocephalus: Analogies and Differences with Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 60, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Kanemoto, H.; Mori, E.; Tanaka, T.; Suehiro, T.; Yoshiyama, K.; Suzuki, Y.; Kakeda, K.; Wada, T.; Hosomi, K.; Kishima, H.; et al. Cerebrospinal fluid amyloid beta and response of cognition to a tap test in idiopathic normal pressure hydrocephalus: A case–control study. Int. Psychogeriatr. 2021, 35, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; de Leon, M.J.; Zetterberg, H. Alzheimer’s disease. Lancet 2006, 368, 387–403. [Google Scholar] [CrossRef]
- Jeppsson, A.; Zetterberg, H.; Blennow, K.; Wikkelso, C. Idiopathic normal-pressure hydrocephalus: Pathophysiology and diagnosis by CSF biomarkers. Neurology 2013, 80, 1385–1392. [Google Scholar] [CrossRef]
- Kapaki, E.; Paraskevas, G.P.; Papageorgiou, S.G.; Bonakis, A.; Kalfakis, N.; Zalonis, I.; Vassilopoulos, D. Diagnostic value of CSF biomarker profile in frontotemporal lobar degeneration. Alzheimer Dis. Assoc. Disord. 2008, 22, 47–53. [Google Scholar] [CrossRef]
- Paraskevas, G.P.; Kapaki, E.; Papageorgiou, S.G.; Kalfakis, N.; Andreadou, E.; Zalonis, I.; Vassilopoulos, D. CSF biomarker profile and diagnostic value in vascular dementia. Eur. J. Neurol. 2009, 16, 205–211. [Google Scholar] [CrossRef]
- Paraskevas, G.P.; Bougea, A.; Constantinides, V.C.; Bourbouli, M.; Petropoulou, O.; Kapaki, E. In vivo Prevalence of Alzheimer Biomarkers in Dementia with Lewy Bodies. Dement. Geriatr. Cogn. Disord. 2019, 47, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, V.C.; Paraskevas, G.P.; Boufidou, F.; Bourbouli, M.; Pyrgelis, E.-S.; Stefanis, L.; Kapaki, E. CSF Aβ42 and Aβ42/Aβ40 Ratio in Alzheimer’s Disease and Frontotemporal Dementias. Diagnostics 2023, 13, 783. [Google Scholar] [CrossRef]
- Akiba, C.; Nakajima, M.; Miyajima, M.; Ogino, I.; Motoi, Y.; Kawamura, K.; Adachi, S.; Kondo, A.; Sugano, H.; Tokuda, T.; et al. Change of Amyloid-beta 1-42 Toxic Conformer Ratio after Cerebrospinal Fluid Diversion Predicts Long-Term Cognitive Outcome in Patients with Idiopathic Normal Pressure Hydrocephalus. J. Alzheimer’s Dis. 2018, 63, 989–1002. [Google Scholar] [CrossRef]
- Kudo, T.; Mima, T.; Hashimoto, R.; Nakao, K.; Morihara, T.; Tanimukai, H.; Tsujio, I.; Koike, Y.; Tagami, S.; Mori, H.; et al. Tau protein is a potential biological marker for normal pressure hydrocephalus. Psychiatry Clin. Neurosci. 2000, 54, 199–202. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Feldman, H.H.; Frisoni, G.B.; Hampel, H.; Jagust, W.J.; Johnson, K.A.; Knopman, D.S.; et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016, 87, 539–547. [Google Scholar] [CrossRef]
- Agren-Wilsson, A.; Lekman, A.; Sjoberg, W.; Rosengren, L.; Blennow, K.; Bergenheim, A.T.; Malm, J. CSF biomarkers in the evaluation of idiopathic normal pressure hydrocephalus. Acta Neurol. Scand. 2007, 116, 333–339. [Google Scholar] [CrossRef]
- Kapaki, E.N.; Paraskevas, G.P.; Tzerakis, N.G.; Sfagos, C.; Seretis, A.; Kararizou, E.; Vassilopoulos, D. Cerebrospinal fluid tau, phospho-tau181 and beta-amyloid1-42 in idiopathic normal pressure hydrocephalus: A discrimination from Alzheimer’s disease. Eur. J. Neurol. 2007, 14, 168–173. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Ray, B.; Reyes, P.F.; Lahiri, D.K. Biochemical studies in Normal Pressure Hydrocephalus (NPH) patients: Change in CSF levels of amyloid precursor protein (APP), amyloid-beta (Abeta) peptide and phospho-tau. J. Psychiatr. Res. 2011, 45, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Lee, E.B.; Xie, S.X.; Law, A.; Jackson, E.M.; Arnold, S.E.; Clark, C.M.; Shaw, L.M.; Grady, M.S.; Trojanowski, J.Q.; et al. Phosphorylated tau/amyloid beta 1-42 ratio in ventricular cerebrospinal fluid reflects outcome in idiopathic normal pressure hydrocephalus. Fluids Barriers CNS 2012, 9, 7. [Google Scholar] [CrossRef]
- Lim, T.S.; Choi, J.Y.; Park, S.A.; Youn, Y.C.; Lee, H.Y.; Kim, B.G.; Joo, I.S.; Huh, K.; Moon, S.Y. Evaluation of coexistence of Alzheimer’s disease in idiopathic normal pressure hydrocephalus using ELISA analyses for CSF biomarkers. BMC Neurol. 2014, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Muller-Schmitz, K.; Krasavina-Loka, N.; Yardimci, T.; Lipka, T.; Kolman, A.G.J.; Robbers, S.; Menge, T.; Kujovic, M.; Seitz, R.J. Normal Pressure Hydrocephalus Associated with Alzheimer’s Disease. Ann. Neurol. 2020, 88, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Thavarajasingam, S.G.; El-Khatib, M.; Vemulapalli, K.V.; Iradukunda, H.A.S.; Laleye, J.; Russo, S.; Eichhorn, C.; Eide, P.K. Cerebrospinal fluid and venous biomarkers of shunt-responsive idiopathic normal pressure hydrocephalus: A systematic review and meta-analysis. Acta Neurochir. 2022, 164, 1719–1746. [Google Scholar] [CrossRef] [PubMed]
- Solana, E.; Poca, M.A.; Sahuquillo, J.; Benejam, B.; Junqué, C.; Dronavalli, M. Cognitive and motor improvement after retesting in normal-pressure hydrocephalus: A real change or merely a learning effect? J. Neurosurg. 2010, 112, 399–409. [Google Scholar] [CrossRef] [PubMed]


| Variable | Tap-Test Responders N = 27 | Tap-Test Non-Responders N = 26 | p |
|---|---|---|---|
| Gender (F/M) | 14/13 | 8/18 | 0.119 † |
| Age | 75 (69–77) | 74.5 (70.75–77.25) | 0.891 ‡ |
| iNPH Grading scale | 6 (5–7) | 6 (4–7) | 0.839 ‡ |
| Disease duration (months) | 24 (14–48) | 24 (12–48) | 0.903 ‡ |
| MMSE before LP | 23 (17–26) | 23.5 (20.5–28.25) | 0.062 ‡ |
| FAB before LP | 9 (8–13) | 12 (9.75–15) | 0.184 ‡ |
| 10 m timed walk test: steps before LP | 27 (20–45) | 19.5 (16–27.75) | 0.04 ‡,* |
| 10 m timed walk test: time before LP | 17 (11–36) | 10.75 (9–15) | 0.02 ‡,* |
| N = 27 | Neuropsychological | Gait | ||||||
|---|---|---|---|---|---|---|---|---|
| MMSE | FAB | 5WT Immediate | 5WT Delayed | CLOX-1 | CLOX-2 | 10 m Timed Walk Test: Steps | 10 m Timed Walk Test: Time | |
| Before LP | 23 (17–26) | 9 (8–13) | 5 (5–5) | 5 (4–5) | 7 (4–11) | 10 (8–13) | 27 (20–45) | 17 (11–36) |
| 48 h after LP | 25 (20–28) | 12 (9–14) | 5 (5–5) | 5 (4–5) | 8 (6–11) | 12 (8–14) | 22 (17–35) | 14 (9–25) |
| Median % change | 0.103 | 0.182 | 0.000 | 0.000 | 0.000 | 0.071 | 0.231 | 0.235 |
| p | <0.001 † | <0.001 † | NS † | NS † | 0.012 † | 0.011 † | 0.001 † | <0.001 † |
| N = 26 | Neuropsychological | Gait | ||||||
|---|---|---|---|---|---|---|---|---|
| MMSE | FAB | 5WT Immediate | 5WT Delayed | CLOX-1 | CLOX-2 | 10 m Timed Walk Test: Steps | 10 m Timed Walk Test: Time | |
| Before LP | 23.5 (20.5–28.25) | 12 (9.75–15) | 5 (4.75–5) | 4 (2–5) | 9.5 (4.75–13) | 12 (8–13.25) | 19.5 (16–27.75) | 10.75 (9–15) |
| 48 h after LP | 24.5 (19.5–29.25) | 13 (10.75–15.5) | 5 (4.75–5) | 4.5 (1.75–5) | 10 (4–12) | 11 (7.5–13) | 19 (15.75–29) | 11 (8.38–18) |
| Median % change | 0.017 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.024 | 0.038 |
| p | NS † | 0.029 † | NS † | NS † | NS † | NS † | NS † | NS † |
| CSF Biomarker | Tap-Test Responders Ν = 27 | Tap-Test Non-Responders Ν = 26 | p |
|---|---|---|---|
| total Tau | 194 (157–272.3) | 272.2 (151.2–548.3) | 0.0409 †* |
| phospho-Tau | 26 (17.7–38) | 42.5 (21.97–81.5) | 0.0184 †* |
| Aβ42 | 389 (304–609.5) | 406.2 (317.1–660.8) | 0.5871 † |
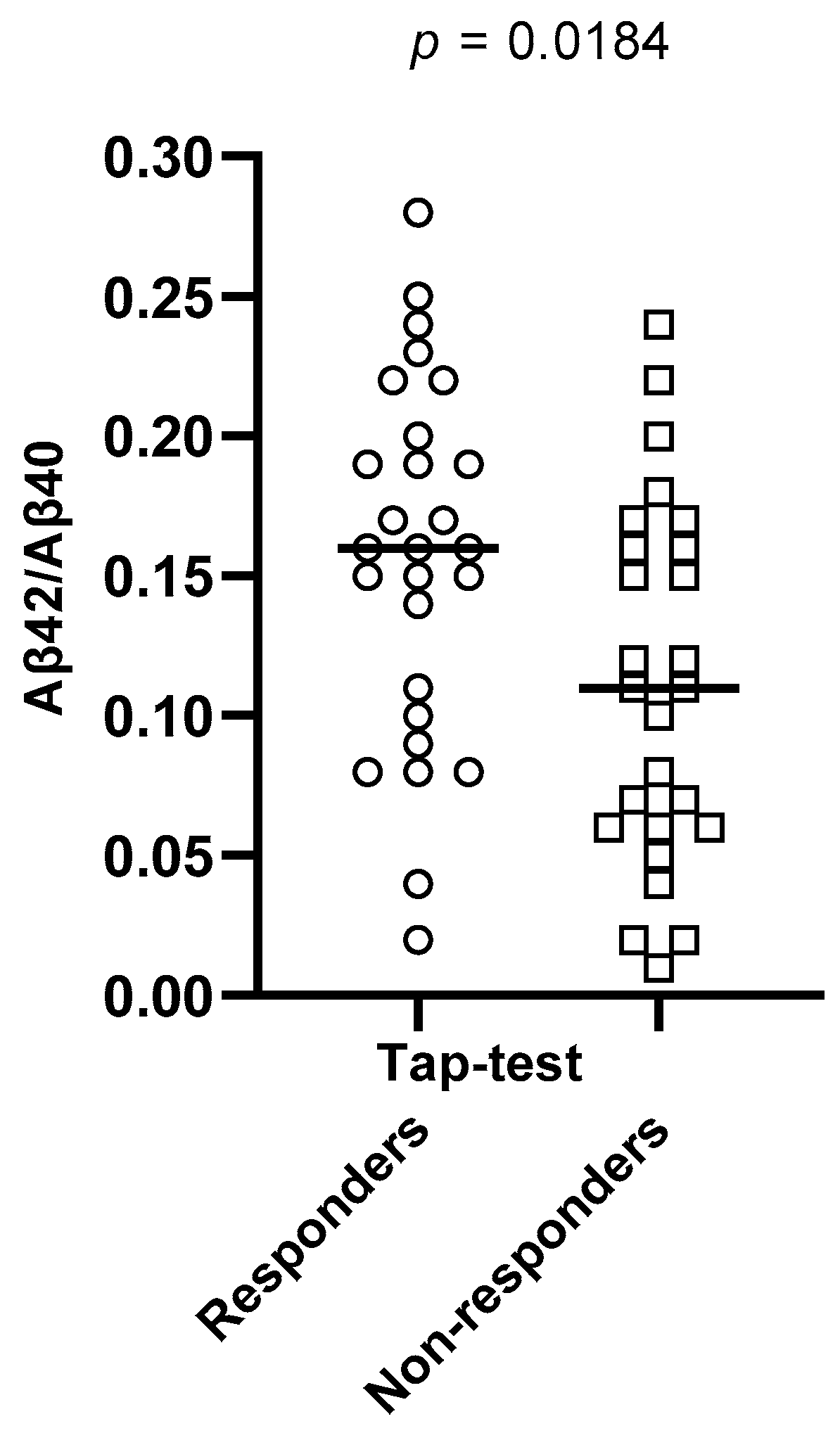
| Aβ42/Aβ40 ratio | 0.16 (0.1–0.2) | 0.11 (0.06–0.163) | 0.0184 †* |
| phospho-Tau/Aβ42 ratio | 0.049 (0.037–0.106) | 0.135 (0.036–0.211) | 0.2116 † |
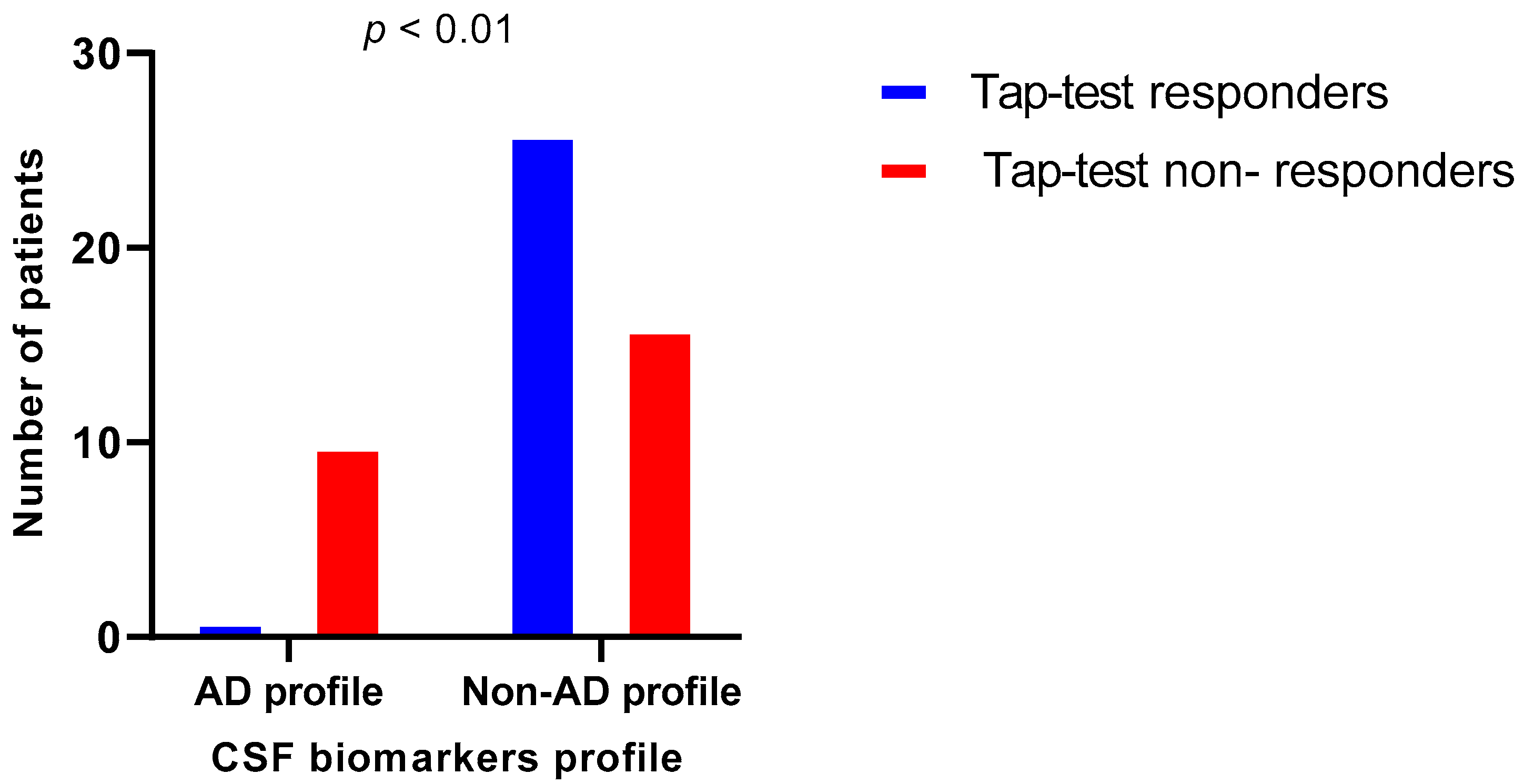
| Variable | iNPH Patients with AD Profile N = 11 | iNPH Patients with Non-AD Profile N = 42 | p |
|---|---|---|---|
| Gender (F/M) | 5/6 | 17/25 | 0.765 † |
| Age | 76 (66–82) | 74.5 (70–77) | 0.668 ‡ |
| iNPH Grading scale | 6 (4–7) | 6 (5–7) | 0.984 ‡ |
| Disease duration (months) | 24 (15–74.5) | 24 (13–48) | 0.960 ‡ |
| MMSE before LP | 23 (11–29) | 24 (18.75–27) | 0.252 ‡ |
| FAB before LP | 11 (5–14) | 11.5 (9–13) | 0.640 ‡ |
| 10 m timed walk test: steps before LP | 22 (15–31) | 25 (18.5–33) | 0.199 ‡ |
| 10 m timed walk test: time before LP | 14 (8–15) | 14.25 (10–24) | 0.640 ‡ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyrgelis, E.-S.; Paraskevas, G.P.; Constantinides, V.C.; Boufidou, F.; Papaioannou, M.; Stefanis, L.; Kapaki, E. Alzheimer’s Disease CSF Biomarkers as Possible Indicators of Tap-Test Response in Idiopathic Normal Pressure Hydrocephalus. Brain Sci. 2023, 13, 1593. https://doi.org/10.3390/brainsci13111593
Pyrgelis E-S, Paraskevas GP, Constantinides VC, Boufidou F, Papaioannou M, Stefanis L, Kapaki E. Alzheimer’s Disease CSF Biomarkers as Possible Indicators of Tap-Test Response in Idiopathic Normal Pressure Hydrocephalus. Brain Sciences. 2023; 13(11):1593. https://doi.org/10.3390/brainsci13111593
Chicago/Turabian StylePyrgelis, Efstratios-Stylianos, George P. Paraskevas, Vasilios C. Constantinides, Fotini Boufidou, Myrto Papaioannou, Leonidas Stefanis, and Elisabeth Kapaki. 2023. "Alzheimer’s Disease CSF Biomarkers as Possible Indicators of Tap-Test Response in Idiopathic Normal Pressure Hydrocephalus" Brain Sciences 13, no. 11: 1593. https://doi.org/10.3390/brainsci13111593
APA StylePyrgelis, E.-S., Paraskevas, G. P., Constantinides, V. C., Boufidou, F., Papaioannou, M., Stefanis, L., & Kapaki, E. (2023). Alzheimer’s Disease CSF Biomarkers as Possible Indicators of Tap-Test Response in Idiopathic Normal Pressure Hydrocephalus. Brain Sciences, 13(11), 1593. https://doi.org/10.3390/brainsci13111593









