Neural Coupling between Interhemispheric and Frontoparietal Functional Connectivity during Semantic Processing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Tasks
2.2. Experimental Material
2.3. Experimental Procedure
2.4. Data Recording
2.5. Surface Potential Analysis
2.6. CSD Analysis
2.7. Dynamic FC and Neural Coupling Analyses
3. Results
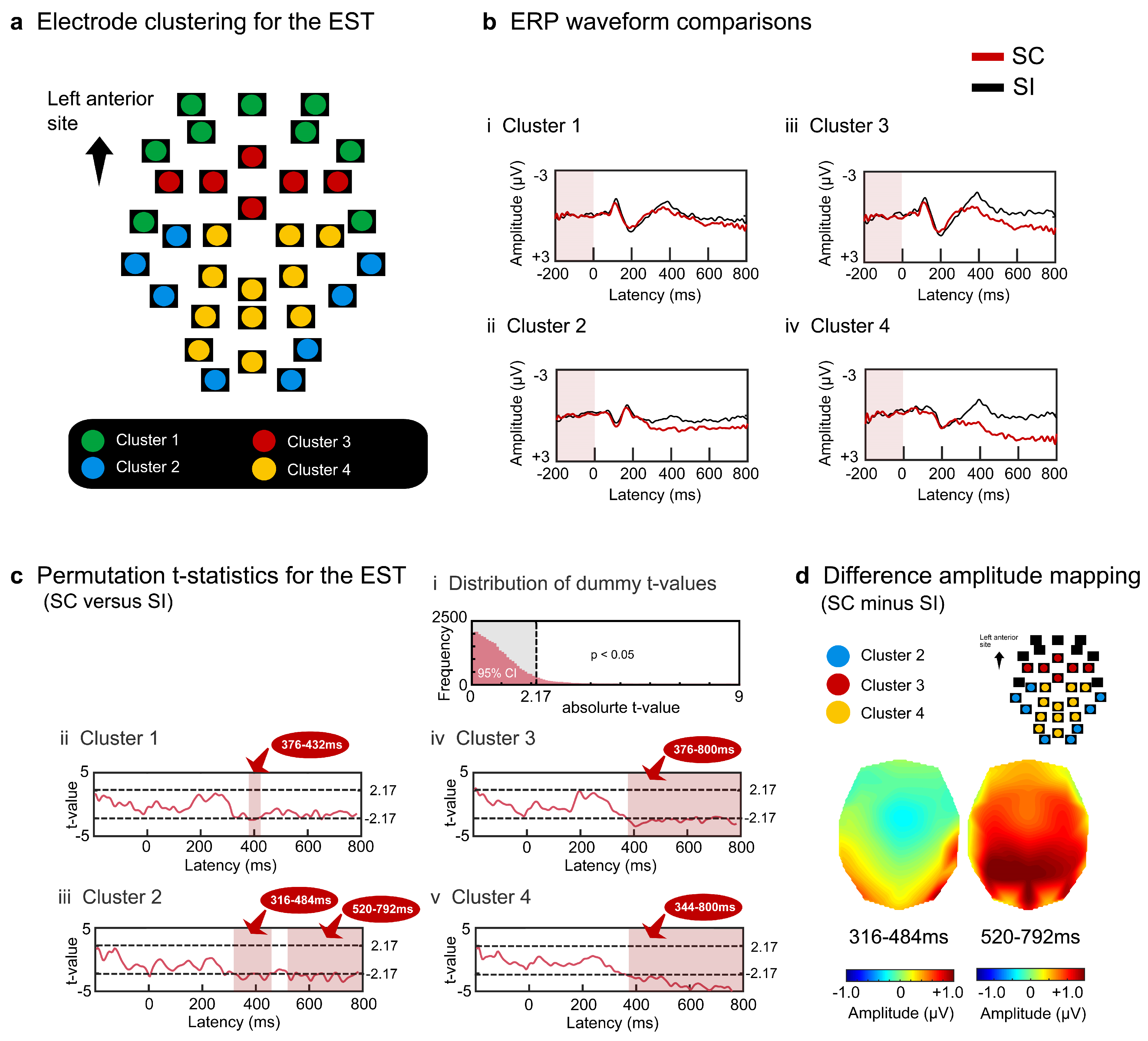
3.1. Results of Surface Potential Analysis
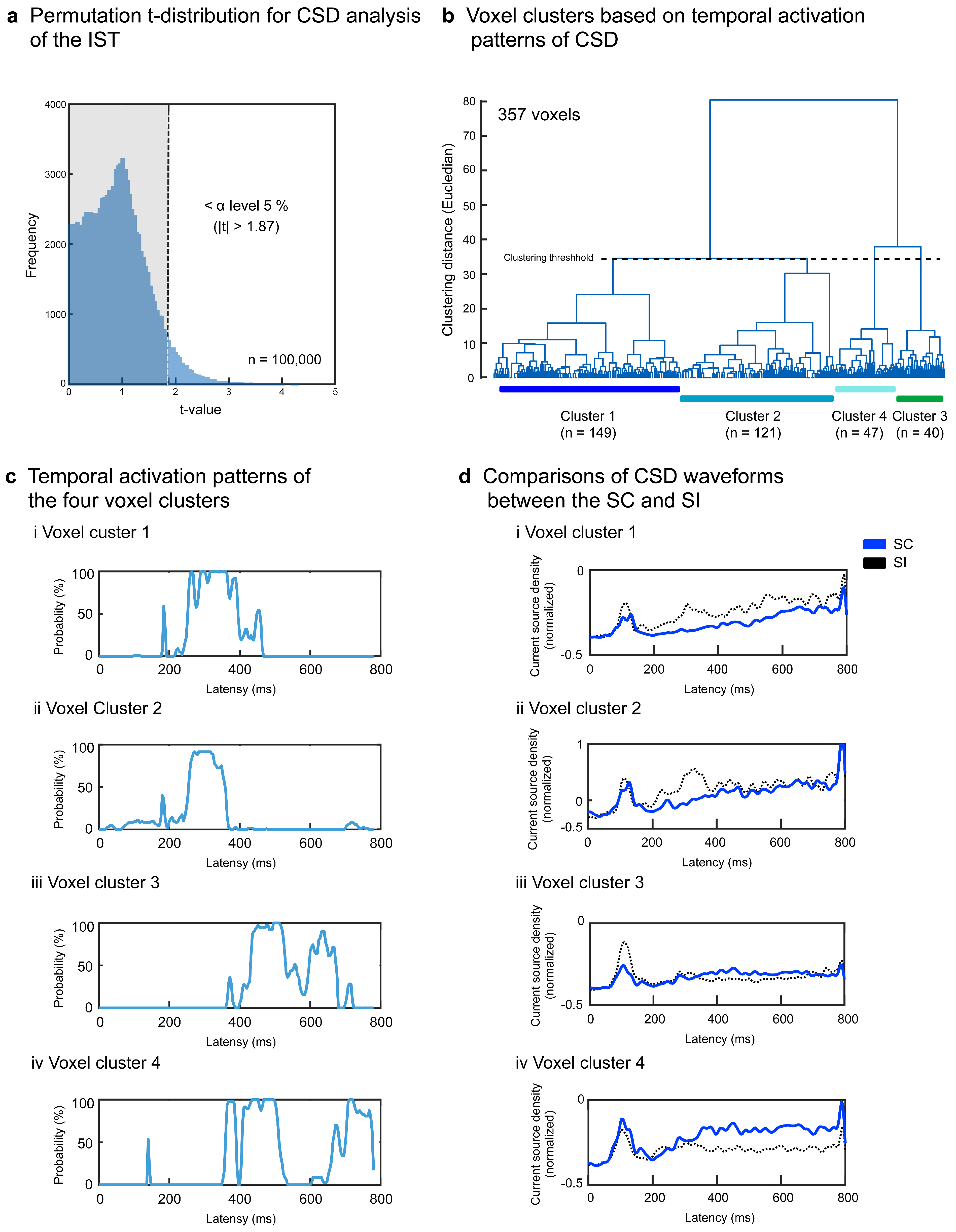
3.2. Results of the Signal Source Analysis
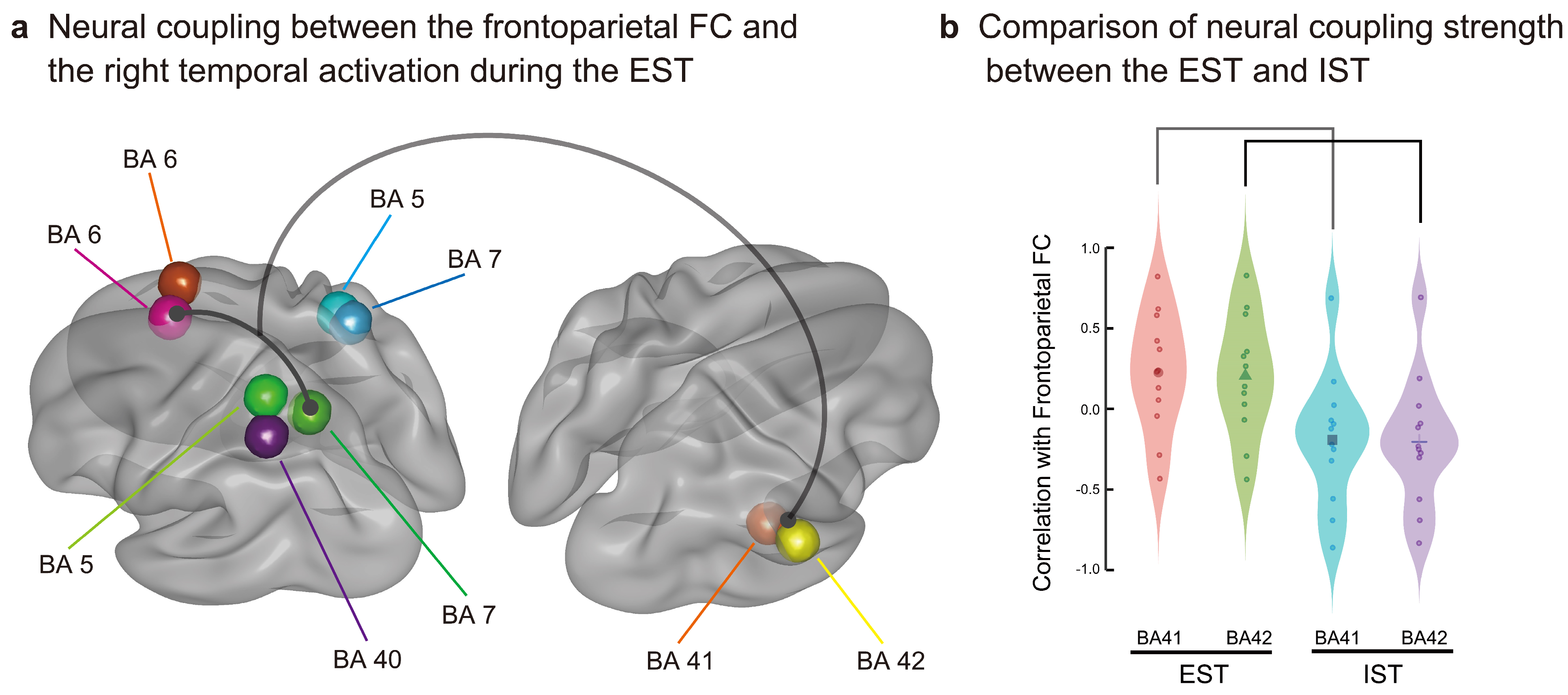
3.3. Results of Dynamic FC and Neural Coupling Analyses
4. Discussion
4.1. Results of Surface Potential Analysis
4.2. Results of CSD Analysis
4.3. Results of Neural Coupling Analysis
4.4. Limitations
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexandrou, A.M.; Saarinen, T.; Mäkelä, S.; Kujala, J.; Salmelin, R. The right hemisphere is highlighted in connected natural speech production and perception. Neuroimage 2017, 152, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Bottini, G.; Corcoran, R.; Sterzi, R.; Paulesu, E.; Schenone, P.; Scarpa, P.; Frackowiak, R.S.; Frith, C.D. The role of the right hemisphere in the interpretation of figurative aspects of language: A positron emission tomography activation study. Brain 1994, 117, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.; Coney, J. Interhemispheric communication is via direct connections. Brain Lang. 1998, 64, 28–52. [Google Scholar] [CrossRef] [PubMed]
- Friederici, A.D.; von Cramon, D.Y.; Kotz, S.A. Role of the corpus callosum in speech comprehension: Interfacing syntax and prosody. Neuron 2007, 53, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Gazzaniga, M.S.; Eliassen, J.C.; Nisenson, L.; Wessinger, C.M.; Fendrich, R.; Baynes, K. Collaboration between the hemispheres of a callosotomy patient: Emerging right hemisphere speech and the left hemisphere interpreter. Brain 1996, 119, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Jung-Beeman, M. Bilateral brain processes for comprehending natural language. Trends Cogn. Sci. 2005, 9, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Northam, G.B.; Liégeois, F.; Tournier, J.D.; Croft, L.J.; Johns, P.N.; Chong, W.K.; Wyatt, J.S.; Baldeweg, T. Interhemispheric temporal lobe connectivity predicts language impairment in adolescents born preterm. Brain 2012, 135, 3781–3798. [Google Scholar] [CrossRef] [PubMed]
- Passarotti, A.M.; Banich, M.T.; Sood, R.K.; Wang, J.M. A generalized role of interhemispheric interaction under attentionally demanding conditions: Evidence from the auditory and tactile modality. Neuropsychologia 2002, 40, 1082–1096. [Google Scholar] [CrossRef]
- Snowden, J.S.; Harris, J.M.; Thompson, J.C.; Kobylecki, C.; Jones, M.; Richardson, A.M.; Neary, D. Semantic dementia and the left and right temporal lobes. Cortex 2018, 107, 188–203. [Google Scholar] [CrossRef]
- Vigneau, M.; Beaucousin, V.; Hervé, P.Y.; Jobard, G.; Petit, L.; Crivello, F.; Mellet, E.; Zago, L.; Mazoyer, B.; Tzourio-Mazoyer, N. What is right-hemisphere contribution to phonological, lexico-semantic, and sentence processing? Insights from a meta-analysis. Neuroimage 2011, 54, 577–593. [Google Scholar] [CrossRef]
- Price, C.J. The anatomy of language: A review of 100 fMRI studies published in 2009. Ann. N. Y. Acad. Sci. 2010, 1191, 62–88. [Google Scholar] [CrossRef] [PubMed]
- Turken, A.U.; Dronkers, N.F. The neural architecture of the language comprehension network: Converging evidence from lesion and connectivity analyses. Front. Syst. Neurosci. 2011, 5, 1. [Google Scholar] [CrossRef]
- Diaz, M.T.; Eppes, A. Factors influencing right hemisphere engagement during metaphor comprehension. Front. Psychol. 2018, 9, 414. [Google Scholar] [CrossRef] [PubMed]
- Coulson, S.; Van Petten, C. A special role for the right hemisphere in metaphor comprehension? ERP evidence from hemifield presentation. Brain Res. 2007, 1146, 128–145. [Google Scholar] [CrossRef]
- Sotillo, M.; Carretié, L.; Hinojosa, J.A.; Tapia, M.; Mercado, F.; López-Martín, S.; Albert, J. Neural activity associated with metaphor comprehension: Spatial analysis. Neurosci. Lett. 2005, 373, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Zempleni, M.Z.; Haverkort, M.; Renken, R.A.; Stowe, L. Evidence for bilateral involvement in idiom comprehension: An fMRI study. Neuroimage 2007, 34, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Prat, C.S.; Mason, R.A.; Just, M.A. An fMRI investigation of analogical mapping in metaphor comprehension: The influence of context and individual cognitive capacities on processing demands. J. Exp. Psychol. Learn. Mem. Cogn. 2012, 38, 282–294. [Google Scholar] [CrossRef]
- Xu, J.; Kemeny, S.; Park, G.; Frattali, C.; Braun, A. Language in context: Emergent features of word, sentence, and narrative comprehension. Neuroimage 2005, 25, 1002–1015. [Google Scholar] [CrossRef]
- Prat, C.S.; Just, M.A. Brain bases of individual differences in cognition. Psychol. Sci. Agenda 2008, 22, 5. [Google Scholar]
- Weissman, D.H.; Banich, M.T. The cerebral hemispheres cooperate to perform complex but not simple tasks. Neuropsychology 2000, 14, 41–59. [Google Scholar] [CrossRef]
- Banich, M.T. The missing link: The role of interhemispheric interaction in attentional processing. Brain Cogn. 1998, 36, 128–157. [Google Scholar] [CrossRef]
- Dosenbach, N.U.; Fair, D.A.; Cohen, A.L.; Schlaggar, B.L.; Petersen, S.E. A dual-networks architecture of top-down control. Trends Cogn. Sci. 2008, 12, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Steinberg, J.L.; Hasan, K.M.; Narayana, P.A.; Kramer, L.A.; Moeller, F.G. Working memory load modulation of parieto-frontal connections: Evidence from dynamic causal modeling. Hum. Brain Mapp. 2012, 33, 1850–1867. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, J.M.; Repovs, G.; Harms, M.P.; Carter, C.S.; Gold, J.M.; MacDonald, A.W., 3rd; Daniel Ragland, J.; Silverstein, S.M.; Godwin, D.; Barch, D.M. Fronto-parietal and cingulo-opercular network integrity and cognition in health and schizophrenia. Neuropsychologia 2015, 73, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Dehaene, S.; Sergent, C.; Changeux, J.P. A neuronal network model linking subjective reports and objective physiological data during conscious perception. Proc. Natl. Acad. Sci. USA 2003, 100, 8520–8525. [Google Scholar] [CrossRef] [PubMed]
- Dehaene, S.; Changeux, J.P.; Naccache, L.; Sackur, J.; Sergent, C. Conscious, preconscious, and subliminal processing: A testable taxonomy. Trends Cogn. Sci. 2006, 10, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Dehaene, S.; Naccache, L.; Cohen, L.; Bihan, D.L.; Mangin, J.F.; Poline, J.B.; Rivière, D. Cerebral mechanisms of word masking and unconscious repetition priming. Nat. Neurosci. 2001, 4, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Auksztulewicz, R.; Spitzer, B.; Blankenburg, F. Recurrent neural processing and somatosensory awareness. J. Neurosci. 2012, 32, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Baars, B.J. A Cognitive Theory of Consciousness; Cambridge University Press: Cambridge, NY, USA, 1998. [Google Scholar]
- Aertsen, A.M.; Gerstein, G.L.; Habib, M.K.; Palm, G. Dynamics of neuronal firing correlation: Modulation of “effective connectivity”. J. Neurophysiol. 1989, 61, 900–917. [Google Scholar] [CrossRef]
- Cabral, J.; Kringelbach, M.L.; Deco, G. Functional connectivity dynamically evolves on multiple time-scales over a static structural connectome: Models and mechanisms. Neuroimage 2017, 160, 84–96. [Google Scholar] [CrossRef]
- Friston, K.J.; Frith, C.D.; Liddle, P.F.; Frackowiak, R.S. Functional connectivity: The principal-component analysis of large (PET) data sets. J. Cereb. Blood Flow Metab. 1993, 13, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.A.; Damaraju, E.; Plis, S.M.; Erhardt, E.B.; Eichele, T.; Calhoun, V.D. Tracking whole-brain connectivity dynamics in the resting state. Cereb. Cortex 2014, 24, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.C.; Battaglia, D.; Spiegler, A.; Deco, G.; Jirsa, V.K. Functional connectivity dynamics: Modeling the switching behavior of the resting state. Neuroimage 2015, 105, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, R.M.; Womelsdorf, T.; Allen, E.A.; Bandettini, P.A.; Calhoun, V.D.; Corbetta, M.; Della Penna, S.; Duyn, J.H.; Glover, G.H.; Gonzalez-Castillo, J.; et al. Dynamic functional connectivity: Promise, issues, and interpretations. Neuroimage 2013, 80, 360–378. [Google Scholar] [CrossRef] [PubMed]
- Preti, M.G.; Bolton, T.A.; Van De Ville, D. The dynamic functional connectome: State-of-the-art and perspectives. Neuroimage 2017, 160, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, R.M.; Womelsdorf, T.; Gati, J.S.; Everling, S.; Menon, R.S. Resting-state networks show dynamic functional connectivity in awake humans and anesthetized macaques. Hum. Brain Mapp. 2013, 34, 2154–2177. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.M.; Loftus, E.F. A spreading-activation theory of semantic processing. Psychol. Rev. 1975, 82, 407–428. [Google Scholar] [CrossRef]
- Foster, P.S.; Wakefield, C.; Pryjmak, S.; Roosa, K.M.; Branch, K.K.; Drago, V.; Harrison, D.W.; Ruff, R. Spreading activation in nonverbal memory networks. Brain Inform. 2017, 4, 187–199. [Google Scholar] [CrossRef]
- Neely, J.H. Semantic priming and retrieval from lexical memory: Evidence for facilitatory and inhibitory processes. Mem. Cognit. 1976, 4, 648–654. [Google Scholar] [CrossRef]
- Maxfield, L. Attention and semantic priming: A review of prime task effects. Conscious. Cogn. 1997, 6, 204–218. [Google Scholar] [CrossRef]
- Valdés, B.; Catena, A.; Marí-Beffa, P. Automatic and controlled semantic processing: A masked prime-task effect. Conscious. Cogn. 2005, 14, 278–295. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Marqui, R.D.; Esslen, M.; Kochi, K.; Lehmann, D. Functional imaging with low-resolution brain electromagnetic tomography (LORETA): A review. Methods Find. Exp. Clin. Pharmacol. 2002, 24 (Suppl. C), 91–95. [Google Scholar] [PubMed]
- Soshi, T.; Nakajima, H.; Hagiwara, H. Grammatical markers switch roles and elicit different electrophysiological responses under shallow and deep semantic requirements. Heliyon 2016, 2, e00180. [Google Scholar] [CrossRef]
- Jiang, X.; Bian, G.B.; Tian, Z. Removal of artifacts from EEG signals: A review. Sensors 2019, 19, 987. [Google Scholar] [CrossRef] [PubMed]
- Tonin, A.; Jaramillo-Gonzalez, A.; Rana, A.; Khalili-Ardali, M.; Birbaumer, N.; Chaudhary, U. Auditory electrooculogram-based communication system for ALS patients in transition from locked-in to complete locked-in state. Sci. Rep. 2020, 10, 8452. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, I.T. Principal Component Analysis, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Camargo, A.; Azuaje, F.; Wang, H.; Zheng, H. Permutation-based statistical tests for multiple hypotheses. Source Code Biol. Med. 2008, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Dudoit, S.; Shaffer, J.P.; Boldrick, J.C. Multiple hypothesis testing in microarray experiments. Statist. Sci. 2003, 18, 71–103. [Google Scholar] [CrossRef]
- Nichols, T.E.; Holmes, A.P. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum. Brain Mapp. 2002, 15, 1–25. [Google Scholar] [CrossRef]
- Becker, C.A. Semantic context effects in visual word recognition: An analysis of semantic strategies. Mem. Cognit. 1980, 8, 493–512. [Google Scholar] [CrossRef]
- Kuperberg, G.R.; Sitnikova, T.; Lakshmanan, B.M. Neuroanatomical distinctions within the semantic system during sentence comprehension: Evidence from functional magnetic resonance imaging. Neuroimage 2008, 40, 367–388. [Google Scholar] [CrossRef]
- Brown, C.; Hagoort, P. The processing nature of the N400: Evidence from masked priming. J. Cogn. Neurosci. 1993, 5, 34–44. [Google Scholar] [CrossRef]
- Hagoort, P.; Hald, L.; Bastiaansen, M.; Petersson, K.M. Integration of word meaning and world knowledge in language comprehension. Science 2004, 304, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Chiou, R.; Humphreys, G.F.; Jung, J.; Lambon Ralph, M.A. Controlled semantic cognition relies upon dynamic and flexible interactions between the executive ‘semantic control’ and hub-and-spoke ‘semantic representation’ systems. Cortex 2018, 103, 100–116. [Google Scholar] [CrossRef] [PubMed]
- Lambon Ralph, M.; Jefferies, E.; Patterson, K.; Rogers, T.T. The neural and computational bases of semantic cognition. Nat. Rev. Neurosci. 2017, 18, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Anwander, A.; Tittgemeyer, M.; von Cramon, D.Y.; Friederici, A.D.; Knösche, T.R. Connectivity-based parcellation of Broca’s area. Cereb. Cortex 2007, 17, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Shekari, E.; Nozari, N. A narrative review of the anatomy and function of the white matter tracts in language production and comprehension. Front. Hum. Neurosci. 2023, 17, 1139292. [Google Scholar] [CrossRef] [PubMed]
- Teige, C.; Cornelissen, P.L.; Mollo, G.; Gonzalez Alam, T.R.D.J.; McCarty, K.; Smallwood, J.; Jefferies, E. Dissociations in semantic cognition: Oscillatory evidence for opposing effects of semantic control and type of semantic relation in anterior and posterior temporal cortex. Cortex 2019, 120, 308–325. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.; Nestor, P.J.; Rogers, T. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat. Rev. Neurosci. 2007, 8, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Dapretto, M.; Bookheimer, S.Y. Form and content: Dissociating syntax and semantics in sentence comprehension. Neuron 1999, 24, 427–432. [Google Scholar] [CrossRef]
- Ihara, A.S.; Mimura, T.; Soshi, T.; Yorifuji, S.; Hirata, M.; Goto, T.; Yoshinime, T.; Umehara, H.; Fujimaki, N. Facilitated lexical ambiguity processing by transcranial direct current stimulation over the left inferior frontal cortex. J. Cogn. Neurosci. 2015, 27, 26–34. [Google Scholar] [CrossRef]
- Thompson-Schill, S.L.; D’Esposito, M.; Aguirre, G.K.; Farah, M.J. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proc. Natl. Acad. Sci. USA 1997, 94, 14792–14797. [Google Scholar] [CrossRef] [PubMed]
- Yvert, G.; Perrone-Bertolotti, M.; Baciu, M.; David, O. Dynamic causal modeling of spatiotemporal integration of phonological and semantic processes: An electroencephalographic study. J. Neurosci. 2012, 32, 4297–4306. [Google Scholar] [CrossRef] [PubMed]
- Grady, C.L.; Van Meter, J.W.; Maisog, J.M.; Pietrini, P.; Krasuski, J.; Rauschecker, J.P. Attention-related modulation of activity in primary and secondary auditory cortex. Neuroreport 1997, 8, 2511–2516. [Google Scholar] [CrossRef] [PubMed]
- Hickok, G. Computational neuroanatomy of speech production. Nat. Rev. Neurosci. 2012, 13, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, R.; Nyberg, L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J. Cogn. Neurosci. 2000, 12, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Dronkers, N.F.; Wilkins, D.P.; Van Valin, R.D., Jr.; Redfern, B.B.; Jaeger, J.J. Lesion analysis of the brain areas involved in language comprehension. Cognition 2004, 92, 145–177. [Google Scholar] [CrossRef] [PubMed]
- Friederici, A.D.; Meyer, M.; von Cramon, D.Y. Auditory language comprehension: An event-related fMRI study on the processing of syntactic and lexical information. Brain Lang. 2000, 75, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.H.; Gaskell, M.G. A complementary systems account of word learning: Neural and behavioural evidence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 3773–3800. [Google Scholar] [CrossRef]
- Hofer, S.; Frahm, J. Topography of the human corpus callosum revisited: Comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage 2006, 32, 989–994. [Google Scholar] [CrossRef]
- Raichle, M.E. The restless brain: How intrinsic activity organizes brain function. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140172. [Google Scholar] [CrossRef]
- Vossel, S.; Geng, J.J.; Fink, G.R. Dorsal and ventral attention systems: Distinct neural circuits but collaborative roles. Neuroscientist 2014, 20, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Alho, K.; Vorobyev, V.A.; Medvedev, S.V.; Pakhomov, S.V.; Roudas, M.S.; Tervaniemi, M.; van Zuijen, T.; Näätänen, R. Hemispheric lateralization of cerebral blood-flow changes during selective listening to dichotically presented continuous speech. Brain Res. Cogn. Brain Res. 2003, 17, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Alho, K.; Rinne, T.; Herron, T.J.; Woods, D.L. Stimulus-dependent activations and attention-related modulations in the auditory cortex: A meta-analysis of fMRI studies. Hear Res. 2014, 307, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Wildgruber, D.; Ackermann, H.; Grodd, W. Differential contributions of motor cortex, basal ganglia, and cerebellum to speech motor control: Effects of syllable repetition rate evaluated by fMRI. Neuroimage 2001, 13, 101–109. [Google Scholar] [CrossRef]
- Song, J.; Davey, C.; Poulsen, C.; Luu, P.; Turovets, S.; Anderson, E.; Li, K.; Tucker, D. EEG source localization: Sensor density and head surface coverage. J. Neurosci. Methods 2015, 256, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Yu, Y. Robust evaluation and comparison of EEG source localization algorithms for accurate reconstruction of deep cortical activity. Mathematics 2023, 11, 2450. [Google Scholar] [CrossRef]




| Cluster | Cortical Area | BA | Numbers of Voxels | MNI Coordinates | |||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| M | SD | M | SD | M | SD | ||||
| 1 | R. precentral/middle frontal gyrus | 6 | 7 | 45.7 | 6.1 | −5.0 | 4.1 | 44.3 | 3.5 |
| R. inferior temporal/fusiform gyrus | 20 | 25 | 38.2 | 9.0 | −11.6 | 15.9 | −35.0 | 9.5 | |
| R. middle temporal gyrus | 21 | 37 | 51.1 | 7.6 | −0.3 | 6.8 | −26.6 | 8.7 | |
| R. parahippocampal gyrus | 36 | 8 | 32.5 | 3.8 | −23.8 | 9.2 | −27.5 | 7.6 | |
| R. fusiform gyrus | 37 | 14 | 45.4 | 7.7 | −51.4 | 6.6 | −17.9 | 6.1 | |
| L. inferior parietal gyrus | 40 | 21 | −42.4 | 5.2 | −47.9 | 4.4 | 51.7 | 4.0 | |
| 2 | R. inferior temporal/fusiform gyrus | 20 | 82 | 50.0 | 7.8 | −24.0 | 13.7 | −27.2 | 7.0 |
| R. middle temporal gyrus | 21 | 56 | 62.3 | 5.0 | −29.7 | 9.6 | −9.1 | 5.9 | |
| R. middle/superior temporal gyrus | 22 | 30 | 59.0 | 7.2 | −34.0 | 7.1 | 3.5 | 5.3 | |
| R. inferior temporal/fusiform gyrus | 37 | 25 | 52.0 | 4.8 | −47.4 | 5.2 | −19.6 | 4.8 | |
| L. inferior parietal gyrus | 40 | 8 | −41.9 | 5.9 | −40.0 | 3.8 | 45.6 | 4.2 | |
| R. inferior parietal/postcentral gyrus | 40 | 8 | 48.1 | 10.3 | −36.3 | 9.9 | 30.6 | 12.1 | |
| R. superior temporal gyrus | 41 | 11 | 48.6 | 5.0 | −30.9 | 3.8 | 10.0 | 3.9 | |
| R. superior temporal gyrus | 42 | 10 | 62.5 | 4.9 | −29.0 | 3.2 | 11.5 | 4.1 | |
| 3 | R. precentral gyrus | 6 | 6 | 46.7 | 5.2 | −8.3 | 2.6 | 39.2 | 3.8 |
| R. insula/superior temporal gyrus | 13 | 9 | 43.3 | 2.5 | −16.1 | 8.6 | 3.9 | 8.6 | |
| L. inferior temporal gyrus | 20 | 9 | −59.4 | 3.9 | −20.0 | 4.3 | −25.6 | 5.3 | |
| L. middle temporal gyrus | 21 | 19 | −59.2 | 6.5 | −22.1 | 5.4 | −13.2 | 4.5 | |
| R. middle temporal gyrus | 21 | 11 | 56.4 | 9.2 | −9.5 | 6.1 | −7.7 | 4.1 | |
| R. superior temporal gyrus | 22 | 13 | 56.5 | 8.5 | −11.2 | 4.2 | 0.4 | 3.8 | |
| R. superior temporal gyrus | 41 | 11 | 46.8 | 5.6 | −25.0 | 5.0 | 10.0 | 3.2 | |
| R. superior temporal gyrus | 42 | 7 | 61.4 | 3.8 | −15.0 | 4.1 | 10.0 | 0.0 | |
| 4 | L. precentral gyrus | 6 | 16 | −50.0 | 7.5 | −5.3 | 3.9 | 35.6 | 3.1 |
| L. insula | 13 | 10 | −41.0 | 3.2 | −18.0 | 4.8 | 6.0 | 3.2 | |
| R. inferior parietal/supramarginal gyrus | 40 | 31 | 59.7 | 3.9 | −44.0 | 4.7 | 34.0 | 8.2 | |
| Cluster | Cortical Area | BA | Numbers of Voxels | MNI Coordinates | |||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| M | SD | M | SD | M | SD | ||||
| 1 | L. middle temporal gyrus | 21 | 50 | −57.0 | 7.8 | −15.6 | 16.7 | −15.6 | 13.2 |
| L. middle/superior temporal gyrus | 22 | 22 | −59.1 | 5.0 | −26.8 | 9.2 | 2.0 | 3.3 | |
| L. superior temporal gyrus | 38 | 52 | −38.3 | 7.9 | 13.5 | 4.6 | −28.3 | 7.5 | |
| L. superior temporal gyrus | 41 | 8 | −53.1 | 2.6 | −25.0 | 3.8 | 8.1 | 2.59 | |
| 2 | R. postcentral gyrus | 3 | 12 | 36.7 | 4.9 | −28.8 | 4.3 | 55.4 | 5.0 |
| L. precentral gyrus | 4 | 18 | −34.3 | 7.3 | −27.1 | 7.6 | 62.1 | 7.6 | |
| R. precentral gyrus | 4 | 11 | 31.4 | 3.2 | −26.8 | 4.0 | 56.8 | 6.8 | |
| R. precentral/middle frontal gyrus | 6 | 17 | 27.1 | 3.1 | −14.4 | 5.0 | 60.9 | 7.3 | |
| L. inferior parietal/supramarginal gyrus | 40 | 24 | −53.3 | 8.6 | −43.1 | 3.6 | 37.9 | 8.2 | |
| L. inferior frontal gyrus | 47 | 13 | −31.5 | 6.6 | 17.3 | 3.9 | −19.6 | 3.2 | |
| 3 | R. insula | 13 | 25 | 37.8 | 5.0 | −23.4 | 5.9 | 15.4 | 4.3 |
| R. superior temporal gyrus | 41 | 13 | 42.7 | 3.3 | −29.6 | 5.6 | 9.2 | 3.4 | |
| 4 | R. inferior temporal gyrus | 20 | 17 | 54.7 | 4.1 | −44.7 | 6.7 | −19.7 | 5.7 |
| R. inferior temporal/fusiform gyrus | 37 | 22 | 50.7 | 5.8 | −46.1 | 4.9 | −18.6 | 4.9 | |
| Cortical Area | Cortical Area Name | BA | Numbers of Voxels | MNI Coordinates | |||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| M | SD | M | SD | M | SD | ||||
| Frontal | L. superior frontal gyrus | 6 | 28 | −10.5 | 5.8 | 8.9 | 10.7 | 66.6 | 3.6 |
| R. superior frontal gyrus | 6 | 16 | 7.5 | 3.2 | 14.1 | 9.3 | 65.0 | 3.7 | |
| Parietal | L. postcentral gyrus | 5 | 13 | −29.2 | 6.4 | −46.9 | 2.5 | 65.8 | 3.4 |
| R. postcentral gyrus | 5 | 19 | 23.7 | 11.0 | −47.1 | 2.5 | 66.3 | 3.7 | |
| L. superior parietal gyrus/precuneus | 7 | 46 | −24.6 | 10.0 | −61.7 | 6.6 | 59.7 | 7.6 | |
| R. superior parietal/postcentral gyrus | 7 | 16 | 21.3 | 10.1 | −55.0 | 3.2 | 65.3 | 4.6 | |
| L. inferior parietal gyrus | 40 | 14 | −40.7 | 4.3 | −51.1 | 4.0 | 56.1 | 2.9 | |
| Right Temporal Area | BA | Numbers of Voxels | MNI Coordinates | |||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| M | SD | M | SD | M | SD | |||
| Fusiform gyrus | 20 | 30 | 47.3 | 6.9 | −25.7 | 11.1 | −27.2 | 3.9 |
| Inferior temporal gyrus | 20 | 43 | 52.7 | 7.1 | −23.1 | 15.1 | −27.7 | 8.3 |
| Middle temporal gyrus | 21 | 52 | 62.9 | 4.5 | −30.5 | 9.5 | −9.1 | 5.8 |
| Middle temporal gyrus | 22 | 11 | 56.4 | 6.4 | −36.4 | 3.9 | 1.4 | 2.3 |
| Superior temporal gyrus | 22 | 19 | 60.5 | 7.4 | −32.6 | 8.2 | 4.7 | 6.1 |
| Fusiform gyrus | 37 | 14 | 50.0 | 3.9 | −48.6 | 4.6 | −20.7 | 4.7 |
| Inferior temporal gyrus | 37 | 11 | 54.5 | 4.7 | −45.9 | 5.8 | −18.2 | 4.6 |
| Superior temporal gyrus | 41 | 9 | 48.3 | 5.0 | −31.7 | 3.5 | 10.0 | 4.3 |
| Superior temporal gyrus | 42 | 10 | 62.5 | 4.9 | −29.0 | 3.2 | 11.5 | 4.1 |
| Parietal Area | Time Window (ms) | Task | Right Temporal Area × Task | ||||||
|---|---|---|---|---|---|---|---|---|---|
| L. BA 6 | R. BA 6 | L. BA 6 | R. BA 6 | ||||||
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | ||
| L. BA 5 | 400–500 | 0.014 | 0.907 | 1.333 | 0.261 | 0.744 | 0.653 | 0.498 | 0.857 |
| 500–600 | 1.658 | 0.212 | 0.660 | 0.425 | 0.075 | 1.000 | 0.319 | 0.958 | |
| 600–700 | 0.122 | 0.730 | 0.244 | 0.625 | 2.966 ** | 0.004 | 4.913 *** | <0.001 | |
| R. BA 5 | 400–500 | 0.103 | 0.751 | 0.038 | 0.847 | 0.471 | 0.876 | 0.741 | 0.656 |
| 500–600 | 1.108 | 0.304 | 0.474 | 0.498 | 0.069 | 1.000 | 0.105 | 0.999 | |
| 600–700 | 0.556 | 0.463 | 0.005 | 0.944 | 1.572 | 0.136 | 1.409 | 0.196 | |
| L. BA 7 | 400–500 | 0.000 | 1.000 | 0.292 | 0.594 | 0.561 | 0.810 | 0.665 | 0.723 |
| 500–600 | 0.735 | 0.401 | 2.425 | 0.134 | 1.083 | 0.377 | 0.424 | 0.906 | |
| 600–700 | 1.263 | 0.273 | 0.682 | 0.418 | 4.775 *** | <0.001 | 4.586 *** | <0.001 | |
| R. BA 7 | 400–500 | 0.167 | 0.687 | 0.903 | 0.352 | 0.459 | 0.884 | 0.719 | 0.676 |
| 500–600 | 1.494 | 0.235 | 0.622 | 0.438 | 0.149 | 0.996 | 0.097 | 0.999 | |
| 600–700 | 0.019 | 0.891 | 0.252 | 0.620 | 1.300 | 0.247 | 3.529 ** | 0.001 | |
| L. BA 40 | 400–500 | 0.787 | 0.385 | 0.000 | 1.000 | 0.619 | 0.762 | 0.442 | 0.894 |
| 500–600 | 0.222 | 0.642 | 0.069 | 0.795 | 0.810 | 0.596 | 0.685 | 0.705 | |
| 600–700 | 0.393 | 0.536 | 1.194 | 0.286 | 4.061 *** | <0.001 | 3.588 ** | 0.001 | |
| Right Temporal Area | Task | |||||
|---|---|---|---|---|---|---|
| L. BA 6–L. BA 5 | R. BA 6–L. BA 5 | L. BA 6–L. BA 5 | ||||
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | |
| Fusiform gyrus (BA 20) | 0.005 | 0.945 | 0.054 | 0.819 | 0.600 | 0.448 |
| Inferior temporal gyrus (BA 20) | 0.025 | 0.876 | 0.11 | 0.744 | 0.888 | 0.358 |
| Middle temporal gyrus (BA 21) | 0.502 | 0.487 | 0.667 | 0.424 | 2.376 | 0.139 |
| Middle temporal gyrus (BA 22) | 0.115 | 0.738 | 0.024 | 0.879 | 0.076 | 0.786 |
| Superior temporal gyrus (BA 22) | 0.206 | 0.655 | 0.208 | 0.654 | 0.014 | 0.907 |
| Fusiform gyrus (BA 37) | 0.041 | 0.842 | 0.07 | 0.794 | 0.032 | 0.860 |
| Inferior temporal gyrus (BA 37) | 0.513 | 0.482 | 0.59 | 0.451 | 2.489 | 0.130 |
| Superior temporal gyrus (BA 41) | 2.063 | 0.167 | 3.368 | 0.080 | 6.363 | 0.018 * |
| Superior temporal gyrus (BA 42) | 1.594 | 0.222 | 2.524 | 0.127 | 6.112 | 0.020 * |
| Right temporal area | Task | |||||
| R. BA 6–R. BA 7 | L. BA 6–L. BA 40 | R. BA 6–L. BA 40 | ||||
| F-value | p-value | F-value | p-value | F-value | p-value | |
| Fusiform gyrus (BA 20) | 0.514 | 0.482 | 0.133 | 0.720 | 0.841 | 0.370 |
| Inferior temporal gyrus (BA 20) | 0.476 | 0.498 | 0.229 | 0.638 | 1.088 | 0.310 |
| Middle temporal gyrus (BA 21) | 0.007 | 0.934 | 0.918 | 0.350 | 2.037 | 0.169 |
| Middle temporal gyrus (BA 22) | 1.502 | 0.235 | 0.008 | 0.930 | 0.236 | 0.633 |
| Superior temporal gyrus (BA 22) | 1.132 | 0.301 | 0.185 | 0.672 | 0.003 | 0.957 |
| Fusiform gyrus (BA 37) | 1.509 | 0.234 | 0.051 | 0.824 | 0.066 | 0.800 |
| Inferior temporal gyrus (BA 37) | 0.021 | 0.886 | 1.118 | 0.304 | 2.160 | 0.157 |
| Superior temporal gyrus (BA 41) | 0.510 | 0.484 | 2.414 | 0.135 | 3.724 | 0.066 |
| Superior temporal gyrus (BA 42) | 0.322 | 0.577 | 2.419 | 0.135 | 3.490 | 0.075 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soshi, T. Neural Coupling between Interhemispheric and Frontoparietal Functional Connectivity during Semantic Processing. Brain Sci. 2023, 13, 1601. https://doi.org/10.3390/brainsci13111601
Soshi T. Neural Coupling between Interhemispheric and Frontoparietal Functional Connectivity during Semantic Processing. Brain Sciences. 2023; 13(11):1601. https://doi.org/10.3390/brainsci13111601
Chicago/Turabian StyleSoshi, Takahiro. 2023. "Neural Coupling between Interhemispheric and Frontoparietal Functional Connectivity during Semantic Processing" Brain Sciences 13, no. 11: 1601. https://doi.org/10.3390/brainsci13111601






