Neural Mechanisms of Neuro-Rehabilitation Using Transcranial Direct Current Stimulation (tDCS) over the Front-Polar Area
Abstract
:1. Introduction
2. tDCS Effects of the Front-Polar Area (FPA) in Rehabilitation
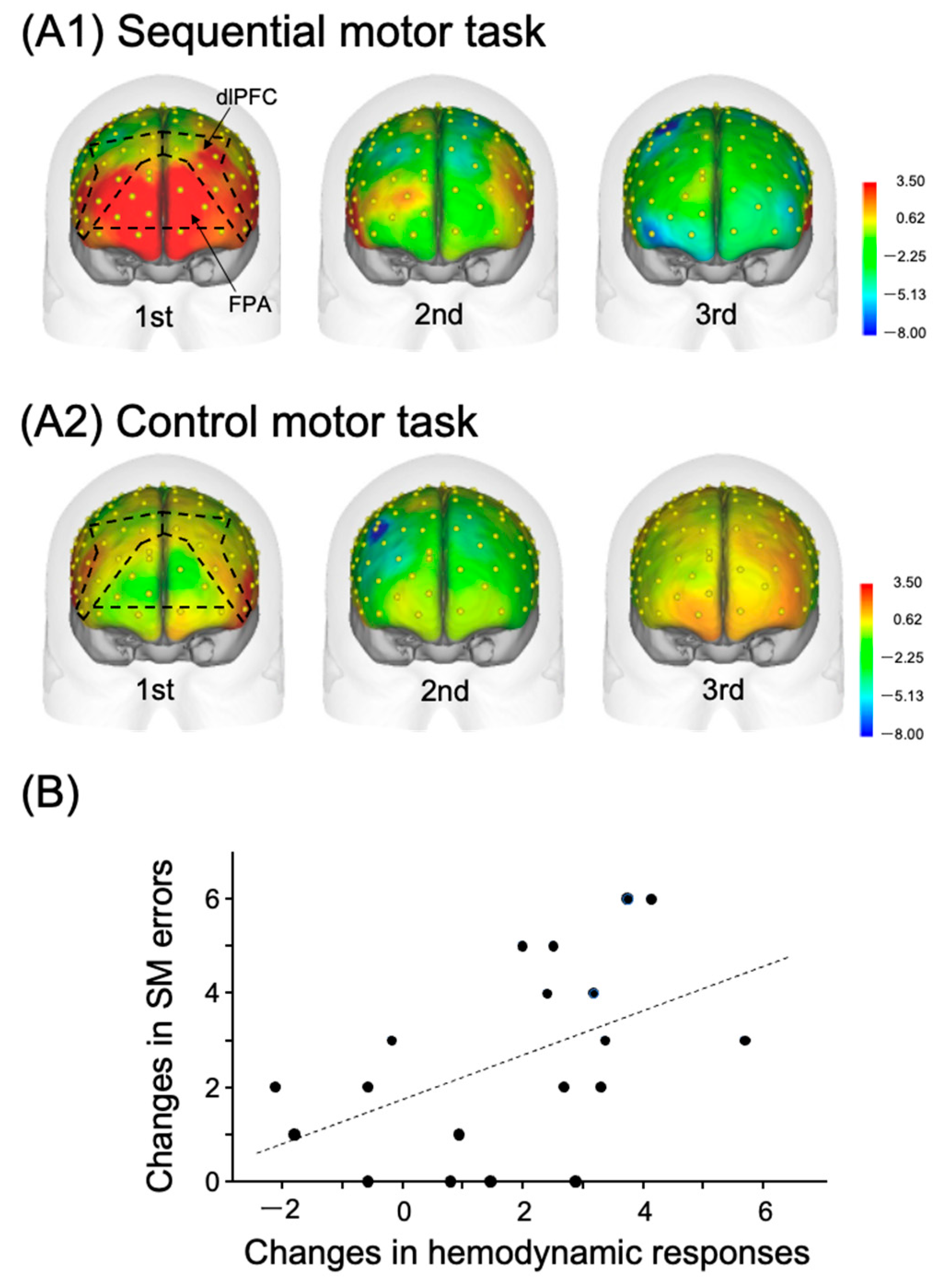
2.1. A Role of the FPA in Motor Learning and Rehabilitation
2.2. Modulatory Effects of the FPA on the Motor-Related Regions
3. Parkinson’s Disease (PD)
3.1. Effects of tDCS over the M1, Dorsolateral PFC (dlPFC), and Cerebellum
3.2. Effects of tDCS over the FPA in PD Patients
4. Neural Mechanisms of Effects of FPA tDCS
5. Future Issues on tDCS over the FPA
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antal, A.; Alekseichuk, I.; Bikson, M.; Brockmöller, J.; Brunoni, A.; Chen, R.; Cohen, L.; Dowthwaite, G.; Ellrich, J.; Flöel, A.; et al. Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clin. Neurophysiol. 2017, 128, 1774–1809. [Google Scholar] [CrossRef]
- Bikson, M.; Grossman, P.; Thomas, C.; Zannou, A.L.; Jiang, J.; Adnan, T.; Mourdoukoutas, A.P.; Kronberg, G.; Truong, D.; Boggio, P.; et al. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimul. 2016, 9, 641–661. [Google Scholar] [CrossRef]
- Flöel, A. TDCS-Enhanced Motor and Cognitive Function in Neurological Diseases. Neuroimage 2014, 85, 934–947. [Google Scholar] [CrossRef]
- Khedr, E.M.; Shawky, O.A.; El-Hammady, D.H.; Rothwell, J.C.; Darwish, E.S.; Mostafa, O.M.; Tohamy, A.M. Effect of Anodal versus Cathodal Transcranial Direct Current Stimulation on Stroke Rehabilitation: A Pilot Randomized Controlled Trial. Neurorehabilit. Neural Repair 2013, 27, 592–601. [Google Scholar] [CrossRef]
- Meinzer, M.; Darkow, R.; Lindenberg, R.; Flöel, A. Electrical stimulation of the motor cortex enhances treatment outcome in post-stroke aphasia. Brain 2016, 139, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Kaski, D.; Dominguez, R.; Allum, J.; Islam, A.; Bronstein, A. Combining physical training with transcranial direct current stimulation to improve gait in Parkinson’s disease: A pilot randomized controlled study. Clin. Rehabil. 2014, 28, 1115–1124. [Google Scholar] [CrossRef]
- Bikson, M.; Paulus, W.; Esmaeilpour, Z.; Kronberg, G.; Michael, A.; Nitsche, M.A. Chapter 3 Mechanisms of Acute and after Effects of Transcranial Direct Current Stimulation. In Practical Guide to Transcranial Direct Current Stimulation; Knotkova, H., Nitsche, M., Bikson, M., Woods, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 81–113. [Google Scholar]
- Nitsche, M.A.; Fricke, K.; Henschke, U.; Schlitterlau, A.; Liebetanz, D.; Lang, N.; Henning, S.; Tergau, F.; Paulus, W. Pharmacological Modulation of Cortical Excitability Shifts Induced by Transcranial Direct Current Stimulation in Humans. J. Physiol. 2003, 553, 293–301. [Google Scholar] [CrossRef]
- Liebetanz, D.; Nitsche, M.A.; Tergau, F.; Paulus, W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain 2002, 125, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Bogaard, A.R.; Lajoie, G.; Boyd, H.; Morse, A.; Zanos, S.; Fetz, E.E. Cortical Network Mechanisms of Anodal and Cathodal Transcranial Direct Current Stimulation in Awake Primates. BioRxiv 2019, 516260. [Google Scholar] [CrossRef]
- Fritsch, B.; Reis, J.; Martinowich, K.; Schambra, H.M.; Ji, Y.; Cohen, L.G.; Lu, B. Direct Current Stimulation Promotes BDNF-Dependent Synaptic Plasticity: Potential Implications for Motor Learning. Neuron 2010, 66, 198–204. [Google Scholar] [CrossRef]
- Barbati, S.A.; Cocco, S.; Longo, V.; Spinelli, M.; Gironi, K.; Mattera, A.; Paciello, F.; Colussi, C.; Podda, M.V.; Grassi, C. Enhancing Plasticity Mechanisms in the Mouse Motor Cortex by Anodal Transcranial Direct-Current Stimulation: The Contribution of Nitric Oxide Signaling. Cereb. Cortex 2020, 30, 2972–2985. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Liebetanz, D.; Lang, N.; Antal, A.; Tergau, F.; Paulus, W. Safety Criteria for Transcranial Direct Current Stim-ulation (tDCS) in Humans. Clin. Neurophysiol. 2003, 114, 2220–2223. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Carneiro, M.I.S.; Bolognini, N.; Fregni, F. Safety Review of Transcranial Direct Current Stimulation in Stroke. Neuromodulation 2017, 20, 215–222. [Google Scholar] [CrossRef]
- Liew, S.-L.; Santarnecchi, E.; Buch, E.R.; Cohen, L.G. Non-invasive brain stimulation in neurorehabilitation: Local and distant effects for motor recovery. Front. Hum. Neurosci. 2014, 8, 378. [Google Scholar] [CrossRef]
- Kobayashi, S.; Iwama, Y.; Nishimaru, H.; Matsumoto, J.; Setogawa, T.; Ono, T.; Nishijo, H. Examination of the Prefrontal Cortex Hemodynamic Responses to the Fist-Edge-Palm Task in Naïve Subjects Using Functional Near-Infrared Spectroscopy. Front. Hum. Neurosci. 2021, 15, 617626. [Google Scholar] [CrossRef]
- Ishikuro, K.; Urakawa, S.; Takamoto, K.; Ishikawa, A.; Ono, T.; Nishijo, H. Cerebral functional imaging using near-infrared spectroscopy during repeated performances of motor rehabilitation tasks tested on healthy subjects. Front. Hum. Neurosci. 2014, 8, 292. [Google Scholar] [CrossRef] [PubMed]
- Ishikuro, K.; Dougu, N.; Nukui, T.; Yamamoto, M.; Nakatsuji, Y.; Kuroda, S.; Matsushita, I.; Nishimaru, H.; Araujo, M.F.P.; Nishijo, H. Effects of Transcranial Direct Current Stimulation (tDCS) over the Frontal Polar Area on Motor and Executive Functions in Parkinson’s Disease; A Pilot Study. Front. Aging Neurosci. 2018, 10, 231. [Google Scholar] [CrossRef]
- Ishikuro, K.; Hattori, N.; Imanishi, R.; Furuya, K.; Nakata, T.; Dougu, N.; Yamamoto, M.; Konishi, H.; Nukui, T.; Hayashi, T.; et al. A Parkinson’s disease patient displaying increased neuromelanin-sensitive areas in the substantia nigra after rehabilitation with tDCS: A case report. Neurocase 2021, 27, 407–414. [Google Scholar] [CrossRef]
- Ota, Y.; Takamoto, K.; Urakawa, S.; Nishimaru, H.; Matsumoto, J.; Takamura, Y.; Mihara, M.; Ono, T.; Nishijo, H. Motor Imagery Training with Neurofeedback from the Frontal Pole Facilitated Sensorimotor Cortical Activity and Improved Hand Dexterity. Front. Neurosci. 2020, 14, 34. [Google Scholar] [CrossRef]
- Jenkins, I.; Brooks, D.; Nixon, P.; Frackowiak, R.; Passingham, R. Motor sequence learning: A study with positron emission tomography. J. Neurosci. 1994, 14, 3775–3790. [Google Scholar] [CrossRef]
- Floyer-Lea, A.; Matthews, P.M. Changing Brain Networks for Visuomotor Control with Increased Movement Automaticity. J. Neurophysiol. 2004, 92, 2405–2412. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Watanabe, K.; Ogawa, H.; Matsushita, K.; Imada, N.; Taki, S.; Iwamoto, Y.; Imura, T.; Araki, H.; Araki, O.; et al. Involvement of the Rostromedial Prefrontal Cortex in Human-Robot Interaction: fNIRS Evidence from a Robot-Assisted Motor Task. Front. Neurorobot. 2022, 16, 795079. [Google Scholar] [CrossRef] [PubMed]
- Ohbayashi, M. The Roles of the Cortical Motor Areas in Sequential Movements. Front. Behav. Neurosci. 2021, 15, 640659. [Google Scholar] [CrossRef]
- Hasan, A.; Galea, J.M.; Casula, E.P.; Falkai, P.; Bestmann, S.; Rothwell, J.C. Muscle and Timing-specific Functional Connectivity between the Dorsolateral Prefrontal Cortex and the Primary Motor Cortex. J. Cogn. Neurosci. 2013, 25, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Orr, J.M.; Smolker, H.R.; Banich, M.T. Organization of the Human Frontal Pole Revealed by Large-Scale DTI-Based Connectivity: Implications for Control of Behavior. PLoS ONE 2015, 10, e0124797. [Google Scholar] [CrossRef]
- Jung, M.; Ryu, S.; Kang, M.; Javadi, A.-H.; Loprinzi, P.D. Evaluation of the transient hypofrontality theory in the context of exercise: A systematic review with meta-analysis. Q. J. Exp. Psychol. 2022, 75, 1193–1214. [Google Scholar] [CrossRef]
- Polanía, R.; Paulus, W.; Antal, A.; Nitsche, M.A. Introducing graph theory to track for neuroplastic alterations in the resting human brain: A transcranial direct current stimulation study. NeuroImage 2011, 54, 2287–2296. [Google Scholar] [CrossRef]
- Polanía, R.; Nitsche, M.A.; Paulus, W. Modulating functional connectivity patterns and topological functional organization of the human brain with transcranial direct current stimulation. Hum. Brain Mapp. 2011, 32, 1236–1249. [Google Scholar] [CrossRef]
- Polanía, R.; Paulus, W.; Nitsche, M.A. Modulating cortico-striatal and thalamo-cortical functional connectivity with transcranial direct current stimulation. Hum. Brain Mapp. 2012, 33, 2499–2508. [Google Scholar] [CrossRef] [PubMed]
- Bonini, F.; Burle, B.; Liégeois-Chauvel, C.; Régis, J.; Chauvel, P.; Vidal, F. Action Monitoring and Medial Frontal Cortex: Leading Role of Supplementary Motor Area. Science 2014, 343, 888–891. [Google Scholar] [CrossRef] [PubMed]
- Pellicano, C.; Benincasa, D.; Pisani, V.; Buttarelli, F.R.; Giovannelli, M.; Pontieri, F.E. Prodromal non-motor symptoms of Parkinson’s disease. Neuropsychiatr. Dis. Treat. 2007, 3, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Alexander, G.E.; DeLong, M.R.; Strick, P.L. Parallel Organization of Functionally Segregated Circuits Linking Basal Ganglia and Cortex. Annu. Rev. Neurosci. 1986, 9, 357–381. [Google Scholar] [CrossRef] [PubMed]
- DeLong, M.R. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990, 13, 281–285. [Google Scholar] [CrossRef] [PubMed]
- DeLong, M.; Wichmann, T. Update on models of basal ganglia function and dysfunction. Park. Relat. Disord. 2009, 15, S237–S240. [Google Scholar] [CrossRef]
- Tessitore, A.; Esposito, F.; Vitale, C.; Santangelo, G.; Amboni, M.; Russo, A.; Corbo, D.; Cirillo, G.; Barone, P.; Tedeschi, G. Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology 2012, 79, 2226–2232. [Google Scholar] [CrossRef] [PubMed]
- Seer, C.; Adab, H.Z.; Sidlauskaite, J.; Dhollander, T.; Chalavi, S.; Gooijers, J.; Sunaert, S.; Swinnen, S.P. Bridging cognition and action: Executive functioning mediates the relationship between white matter fiber density and complex motor abilities in older adults. Aging 2022, 14, 7263–7281. [Google Scholar] [CrossRef]
- Rodríguez-Aranda, C.; Mittner, M.; Vasylenko, O. Association Between Executive Functions, Working Memory, and Manual Dexterity in Young and Healthy Older Adults: An Exploratory Study. Percept. Mot. Ski. 2016, 122, 165–192. [Google Scholar] [CrossRef]
- Carment, L.; Abdellatif, A.; Lafuente-Lafuente, C.; Pariel, S.; Maier, M.A.; Belmin, J.; Lindberg, P.G. Manual Dexterity and Aging: A Pilot Study Disentangling Sensorimotor from Cognitive Decline. Front. Neurol. 2018, 9, 910. [Google Scholar] [CrossRef]
- Fregni, F.; El-Hagrassy, M.M.; Pacheco-Barrios, K.; Carvalho, S.; Leite, J.; Simis, M.; Brunelin, J.; Nakamura-Palacios, E.M.; Marangolo, P.; Venkatasubramanian, G.; et al. Evidence-Based Guidelines and Secondary Meta-Analysis for the Use of Transcranial Direct Current Stimulation in Neurological and Psychiatric Disorders. Int. J. Neuropsychopharmacol. 2021, 24, 256–313. [Google Scholar] [CrossRef]
- Elsner, B.; Kugler, J.; Pohl, M.; Mehrholz, J. Transcranial direct current stimulation (tDCS) for idiopathic Parkinson’s disease. Cochrane Database Syst. Rev. 2016, 2016, CD010916. [Google Scholar] [CrossRef]
- Simpson, M.W.; Mak, M. The effect of transcranial direct current stimulation on upper limb motor performance in Parkinson’s disease: A systematic review. J. Neurol. 2020, 267, 3479–3488. [Google Scholar] [CrossRef]
- Fregni, F.; Boggio, P.S.; Santos, M.C.; Lima, M.; Vieira, A.L.; Rigonatti, S.P.; Silva, M.T.A.; Barbosa, E.R.; Nitsche, M.A.; Pascual-Leone, A. Noninvasive cortical stimulation with transcranial direct current stimulation in Parkinson’s disease. Mov. Disord. 2006, 21, 1693–1702. [Google Scholar] [CrossRef]
- Benninger, D.H.; Lomarev, M.; Lopez, G.; Wassermann, E.M.; Li, X.; Considine, E.; Hallett, M. Transcranial direct current stimulation for the treatment of Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2010, 81, 1105–1111. [Google Scholar] [CrossRef]
- Boggio, P.S.; Ferrucci, R.; Rigonatti, S.P.; Covre, P.; Nitsche, M.; Pascual-Leone, A.; Fregni, F. Effects of transcranial direct current stimulation on working memory in patients with Parkinson’s disease. J. Neurol. Sci. 2006, 249, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Doruk, D.; Gray, Z.; Bravo, G.L.; Pascual-Leone, A.; Fregni, F. Effects of tDCS on executive function in Parkinson’s disease. Neurosci. Lett. 2014, 582, 27–31. [Google Scholar] [CrossRef]
- Wu, T.; Hallett, M. The cerebellum in Parkinson’s disease. Brain 2013, 136, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.; McColl, R.; Barnard, H.; Ringe, W.K.; Fleckenstein, J.; Cullum, C.M. Magnetic resonance imaging of cerebellar–prefrontal and cerebellar–parietal functional connectivity. NeuroImage 2005, 28, 39–48. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, J.X.; Beckmann, C.F.; Tomassini, V.; Ramnani, N.; Johansen-Berg, H. Distinct and Overlapping Functional Zones in the Cerebellum Defined by Resting State Functional Connectivity. Cereb. Cortex 2010, 20, 953–965. [Google Scholar] [CrossRef]
- de Albuquerque, L.L.; Pantovic, M.; Clingo, M.G.; Fischer, K.M.; Jalene, S.; Landers, M.R.; Mari, Z.; Poston, B. Long-Term Application of Cerebellar Transcranial Direct Current Stimulation Does Not Improve Motor Learning in Parkinson’s Disease. Cerebellum 2022, 21, 333–349. [Google Scholar] [CrossRef] [PubMed]
- de Albuquerque, L.L.; Pantovic, M.; Clingo, M.; Fischer, K.; Jalene, S.; Landers, M.; Mari, Z.; Poston, B. A Single Application of Cerebellar Transcranial Direct Current Stimulation Fails to Enhance Motor Skill Acquisition in Parkinson’s Disease: A Pilot Study. Biomedicines 2023, 11, 2219. [Google Scholar] [CrossRef]
- Workman, C.D.; Fietsam, A.C.; Uc, E.Y.; Rudroff, T. Cerebellar Transcranial Direct Current Stimulation in People with Parkinson’s Disease: A Pilot Study. Brain Sci. 2020, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Kalivas, P.W. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res. Rev. 1993, 18, 75–113. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.B.; Sesack, S.R. Projections from the Rat Prefrontal Cortex to the Ventral Tegmental Area: Target Specificity in the Synaptic Associations with Mesoaccumbens and Mesocortical Neurons. J. Neurosci. 2000, 20, 3864–3873. [Google Scholar] [CrossRef]
- Omelchenko, N.; Sesack, S. Glutamate synaptic inputs to ventral tegmental area neurons in the rat derive primarily from subcortical sources. Neuroscience 2007, 146, 1259–1274. [Google Scholar] [CrossRef]
- Han, X.; Jing, M.Y.; Zhao, T.Y.; Wu, N.; Song, R.; Li, J. Role of Dopamine Projections from Ventral Tegmental Area to Nu-cleus Accumbens and Medial Prefrontal Cortex in Reinforcement Behaviors Assessed Using Optogenetic Manipulation. Metab. Brain Dis. 2017, 32, 1491–1502. [Google Scholar] [CrossRef]
- Gao, M.; Liu, C.-L.; Yang, S.; Jin, G.-Z.; Bunney, B.S.; Shi, W.-X. Functional Coupling between the Prefrontal Cortex and Dopamine Neurons in the Ventral Tegmental Area. J. Neurosci. 2007, 27, 5414–5421. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, M.; Xu, D.; Shi, W.-X.; Gutkin, B.S.; Steffensen, S.C.; Lukas, R.J.; Wu, J. Impact of Prefrontal Cortex in Nicotine-Induced Excitation of Ventral Tegmental Area Dopamine Neurons in Anesthetized Rats. J. Neurosci. 2012, 32, 12366–12375. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wei, Y.; Hu, R.; Wang, Y.; Li, K.; Li, X. Transcranial Direct Current Stimulation Ameliorates Behavioral Deficits and Reduces Oxidative Stress in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Mouse Model of Parkinson’s Disease. Neuromodulation 2015, 18, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Okuzumi, A.; Hatano, T.; Kamagata, K.; Hori, M.; Mori, A.; Oji, Y.; Taniguchi, D.; Daida, K.; Shimo, Y.; Yanagisawa, N.; et al. Neuromelanin or DaT- SPECT: Which is the better marker for discriminating advanced Parkinson’s disease? Eur. J. Neurol. 2019, 26, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Biondetti, E.; Santin, M.D.; Valabrègue, R.; Mangone, G.; Gaurav, R.; Pyatigorskaya, N.; Hutchison, M.; Yahia-Cherif, L.; Villain, N.; Habert, M.-O.; et al. The spatiotemporal changes in dopamine, neuromelanin and iron characterizing Parkinson’s disease. Brain 2021, 144, 3114–3125. [Google Scholar] [CrossRef]
- Isaias, I.U.; Trujillo, P.; Summers, P.; Marotta, G.; Mainardi, L.; Pezzoli, G.; Zecca, L.; Costa, A. Neuromelanin Imaging and Dopaminergic Loss in Parkinson’s Disease. Front. Aging Neurosci. 2016, 8, 196. [Google Scholar] [CrossRef] [PubMed]
- Safai, A.; Prasad, S.; Chougule, T.; Saini, J.; Pal, P.K.; Ingalhalikar, M. Microstructural abnormalities of substantia nigra in Parkinson’s disease: A neuromelanin sensitive MRI atlas based study. Hum. Brain Mapp. 2019, 41, 1323–1333. [Google Scholar] [CrossRef]
- Aumann, T.; Horne, M. Activity-dependent regulation of the dopamine phenotype in substantia nigra neurons. J. Neurochem. 2012, 121, 497–515. [Google Scholar] [CrossRef]
- Michel, P.P.; Alvarez-Fischer, D.; Guerreiro, S.; Hild, A.; Hartmann, A.; Hirsch, E.C. Role of activity-dependent mechanisms in the control of dopaminergic neuron survival. J. Neurochem. 2007, 101, 289–297. [Google Scholar] [CrossRef]
- Puig, M.V.; Antzoulatos, E.G.; Miller, E.K. Prefrontal dopamine in associative learning and memory. Neuroscience 2014, 282, 217–229. [Google Scholar] [CrossRef]
- Ott, T.; Nieder, A. Dopamine and Cognitive Control in Prefrontal Cortex. Trends Cogn. Sci. 2019, 23, 213–234. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Wang, D.; Wang, L.; Li, X.; Chen, R.; Liu, Y.; Zhang, J. Connectivity from ipsilateral and contralateral dorsolateral prefrontal cortex to the active primary motor cortex during approaching-avoiding behavior. Cortex 2022, 157, 155–166. [Google Scholar] [CrossRef] [PubMed]
- de la Vega, A.; Yarkoni, T.; Wager, T.D.; Banich, M.T. Large-scale Meta-analysis Suggests Low Regional Modularity in Lateral Frontal Cortex. Cereb. Cortex 2018, 28, 3414–3428. [Google Scholar] [CrossRef]
- Burgess, P.W.; Dumontheil, I.; Gilbert, S.J. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends Cogn. Sci. 2007, 11, 290–298. [Google Scholar] [CrossRef]
- Burgess, P.W.; Gilbert, S.J.; Dumontheil, I. Function and localization within rostral prefrontal cortex (area 10). Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 887–899. [Google Scholar] [CrossRef]
- Li, X.D.; Huang, Y.Q.; Li, L.M.; Wang, Y.F. A Nested Case-Control Study on Child Sensory Integrative Dysfunction. Zhonghua Liu Xing Bing Xue Za Zhi 2003, 24, 374–376. [Google Scholar]
- Molina-Luna, K.; Pekanovic, A.; Röhrich, S.; Hertler, B.; Schubring-Giese, M.; Rioult-Pedotti, M.-S.; Luft, A.R. Dopamine in Motor Cortex Is Necessary for Skill Learning and Synaptic Plasticity. PLoS ONE 2009, 4, e7082. [Google Scholar] [CrossRef] [PubMed]
- Hosp, J.A.; Pekanovic, A.; Rioult-Pedotti, M.S.; Luft, A.R. Dopaminergic Projections from Midbrain to Primary Motor Cortex Mediate Motor Skill Learning. J. Neurosci. 2011, 31, 2481–2487. [Google Scholar] [CrossRef]
- Hosoda, C.; Tsujimoto, S.; Tatekawa, M.; Honda, M.; Osu, R.; Hanakawa, T. Plastic frontal pole cortex structure related to individual persistence for goal achievement. Commun. Biol. 2020, 3, 194. [Google Scholar] [CrossRef] [PubMed]
- Soutschek, A.; Kang, P.; Ruff, C.C.; Hare, T.A.; Tobler, P.N. Brain Stimulation Over the Frontopolar Cortex Enhances Motivation to Exert Effort for Reward. Biol. Psychiatry 2018, 84, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Carmody, T.P.; Senner, J.W.; Malinow, M.R.; Matarazzo, J.D. Physical exercise rehabilitation: Long-term dropout rate in cardiac patients. J. Behav. Med. 1980, 3, 163–168. [Google Scholar] [CrossRef]
- Dennett, R.; Madsen, L.T.; Connolly, L.; Hosking, J.; Dalgas, U.; Freeman, J. Adherence and drop-out in randomized controlled trials of exercise interventions in people with multiple sclerosis: A systematic review and meta-analyses. Mult. Scler. Relat. Disord. 2020, 43, 102169. [Google Scholar] [CrossRef]
- Natta, L.; Guido, F.; Algieri, L.; Mastronardi, V.M.; Rizzi, F.; Scarpa, E.; Qualtieri, A.; Todaro, M.T.; Sallustio, V.; De Vittorio, M. Conformable AlN Piezoelectric Sensors as a Non-invasive Approach for Swallowing Disorder Assessment. ACS Sens. 2021, 6, 1761–1769. [Google Scholar] [CrossRef]
- Schneider, J.S. Behavioral persistence deficit in Parkinson’s disease patients. Eur. J. Neurol. 2007, 14, 300–304. [Google Scholar] [CrossRef]
- Grahek, I.; Shenhav, A.; Musslick, S.; Krebs, R.M.; Koster, E.H. Motivation and cognitive control in depression. Neurosci. Biobehav. Rev. 2019, 102, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Katayama, T.; Takahashi, K.; Yahara, O.; Sawada, J.; Ishida, K.-I.; Asanome, A.; Endo, H.; Saito, T.; Hasebe, N.; Kishibe, M.; et al. NOTCH2NLC mutation-positive neuronal intranuclear inclusion disease with retinal dystrophy: A case report and literature review. Medicine 2023, 102, e33789. [Google Scholar] [CrossRef]
- Cousins, M.S.; Salamone, J.D. Nucleus accumbens dopamine depletions in rats affect relative response allocation in a novel cost/benefit procedure. Pharmacol. Biochem. Behav. 1994, 49, 85–91. [Google Scholar] [CrossRef]
- Salamone, J.D.; Cousins, M.S.; McCullough, L.D.; Carriero, D.L.; Berkowitz, R.J. Nucleus accumbens dopamine release increases during instrumental lever pressing for food but not free food consumption. Pharmacol. Biochem. Behav. 1994, 49, 25–31. [Google Scholar] [CrossRef]
- Sasayama, D.; Hori, H.; Teraishi, T.; Hattori, K.; Ota, M.; Matsuo, J.; Kawamoto, Y.; Kinoshita, Y.; Hashikura, M.; Amano, N.; et al. More severe impairment of manual dexterity in bipolar disorder compared to unipolar major depression. J. Affect. Disord. 2012, 136, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Onoe, H.; Onoe, K.; Morichika, Y.; Tsukada, H.; Isa, T. Neural Substrates for the Motivational Regulation of Motor Recovery after Spinal-Cord Injury. PLoS ONE 2011, 6, e24854. [Google Scholar] [CrossRef] [PubMed]
- Lerner, O.; Friedman, J.; Frenkel-Toledo, S. The effect of high-definition transcranial direct current stimulation intensity on motor performance in healthy adults: A randomized controlled trial. J. Neuroeng. Rehabil. 2021, 18, 103. [Google Scholar] [CrossRef]
- Kuo, H.-I.; Bikson, M.; Datta, A.; Minhas, P.; Paulus, W.; Kuo, M.-F.; Nitsche, M.A. Comparing Cortical Plasticity Induced by Conventional and High-Definition 4 × 1 Ring tDCS: A Neurophysiological Study. Brain Stimul. 2013, 6, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Leech, K.A.; Roemmich, R.T.; Gordon, J.; Reisman, D.S.; Cherry-Allen, K.M. Updates in Motor Learning: Implications for Physical Therapist Practice and Education. Phys. Ther. 2021, 102, pzab250. [Google Scholar] [CrossRef] [PubMed]
- Therrien, A.S.; Wong, A.L. Mechanisms of Human Motor Learning Do Not Function Independently. Front. Hum. Neurosci. 2022, 15, 785992. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishikuro, K.; Hattori, N.; Otomune, H.; Furuya, K.; Nakada, T.; Miyahara, K.; Shibata, T.; Noguchi, K.; Kuroda, S.; Nakatsuji, Y.; et al. Neural Mechanisms of Neuro-Rehabilitation Using Transcranial Direct Current Stimulation (tDCS) over the Front-Polar Area. Brain Sci. 2023, 13, 1604. https://doi.org/10.3390/brainsci13111604
Ishikuro K, Hattori N, Otomune H, Furuya K, Nakada T, Miyahara K, Shibata T, Noguchi K, Kuroda S, Nakatsuji Y, et al. Neural Mechanisms of Neuro-Rehabilitation Using Transcranial Direct Current Stimulation (tDCS) over the Front-Polar Area. Brain Sciences. 2023; 13(11):1604. https://doi.org/10.3390/brainsci13111604
Chicago/Turabian StyleIshikuro, Koji, Noriaki Hattori, Hironori Otomune, Kohta Furuya, Takeshi Nakada, Kenichiro Miyahara, Takashi Shibata, Kyo Noguchi, Satoshi Kuroda, Yuji Nakatsuji, and et al. 2023. "Neural Mechanisms of Neuro-Rehabilitation Using Transcranial Direct Current Stimulation (tDCS) over the Front-Polar Area" Brain Sciences 13, no. 11: 1604. https://doi.org/10.3390/brainsci13111604
APA StyleIshikuro, K., Hattori, N., Otomune, H., Furuya, K., Nakada, T., Miyahara, K., Shibata, T., Noguchi, K., Kuroda, S., Nakatsuji, Y., & Nishijo, H. (2023). Neural Mechanisms of Neuro-Rehabilitation Using Transcranial Direct Current Stimulation (tDCS) over the Front-Polar Area. Brain Sciences, 13(11), 1604. https://doi.org/10.3390/brainsci13111604




