Bridging the Divide: Brain and Behavior in Developmental Language Disorder
Abstract
1. Introduction
Purpose
2. Structural Neuroimaging Findings in DLD
2.1. Structural Brain Differences
2.1.1. Global Brain Volume
2.1.2. Total Gray Matter Volume
2.2. Regional Brain Differences
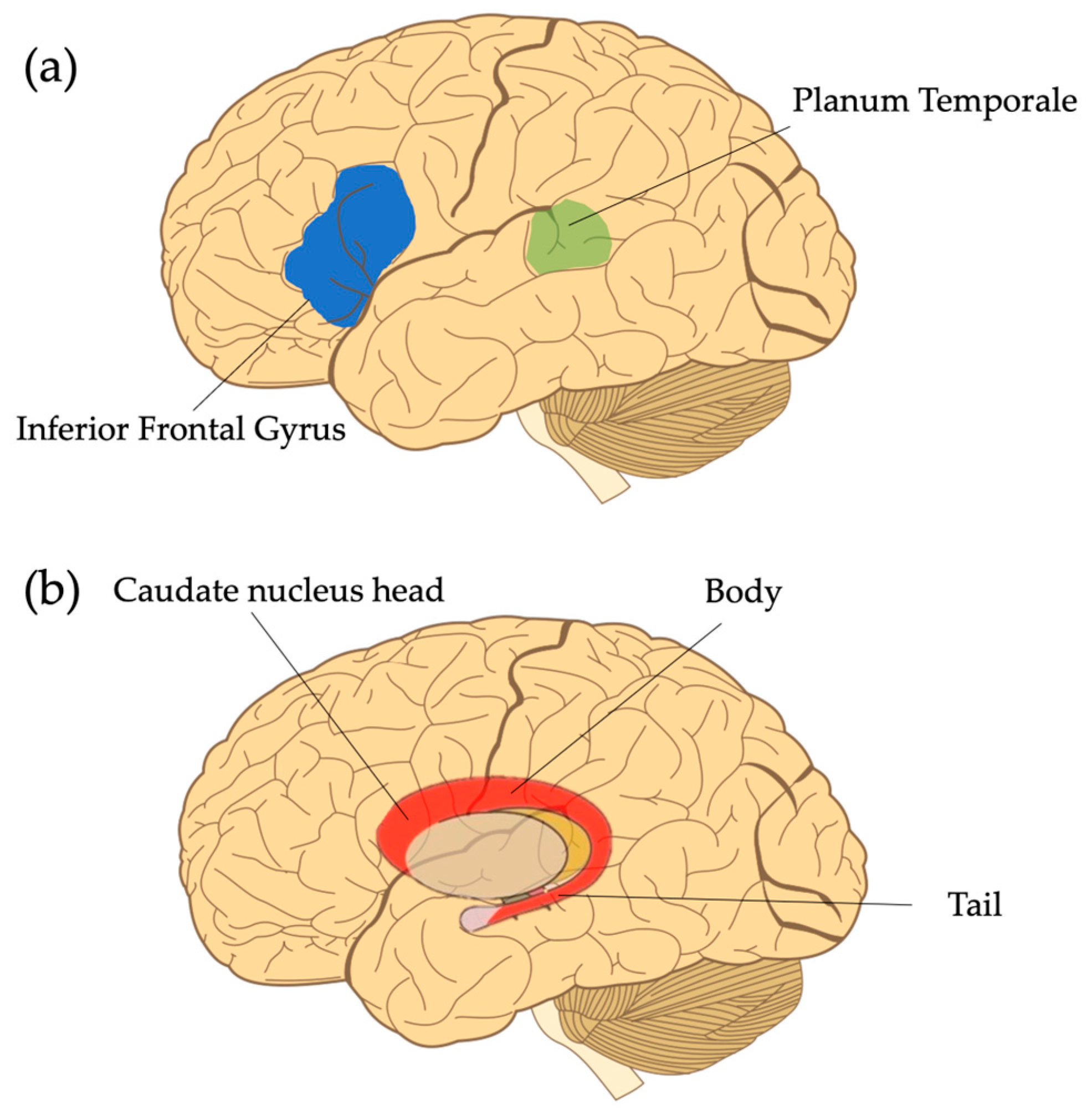
2.2.1. Planum Temporale
2.2.2. Inferior Frontal Gyrus
2.2.3. Caudate Nucleus
2.3. White Matter Pathways
2.3.1. White Matter Volume
2.3.2. White Matter Diffusivity in Child Language-Impaired Populations
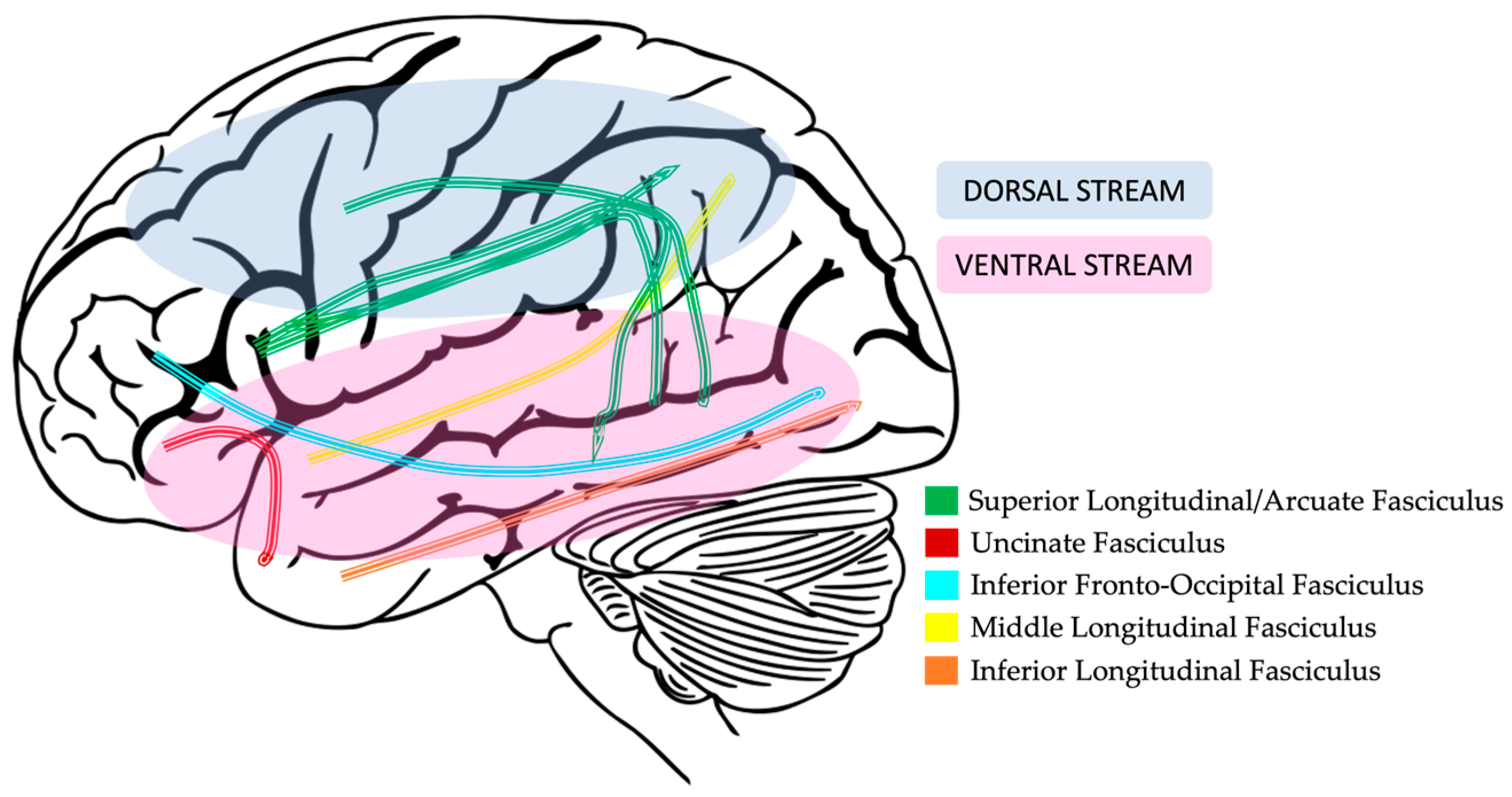
2.3.3. Dorsal and Ventral Language Pathways
2.3.4. Dorsal Pathway Findings in DLD
2.3.5. Ventral Pathway Findings in DLD
3. Functional Neuroimaging Findings in DLD
3.1. Functional Magnetic Resonance Imaging (fMRI) Studies
3.1.1. Regional Activation Patterns for Expressive Language Tasks
3.1.2. Regional Activation Patterns for Receptive Language Tasks
3.2. Cerebral Blood Flow Patterns
Cerebral Blood Flow Patterns in DLD
4. Interim Summary: Neuroimaging Patterns in DLD
5. Neuroimaging Evidence Supporting Theoretical Accounts of Language Impairment Patterns in DLD
5.1. Theoretical Approach: Linguistic Knowledge
| Error Type | Theoretical Account |
|---|---|
| Agreement/Tense/Number Marking | |
| Structural Complexity | |
| Application of Rules |
|
Neuroimaging Evidence Supporting Linguistic Knowledge Theories
5.2. Theoretical Approach: Language Processing Accounts
| Error Type | Theoretical Account |
|---|---|
| Temporal Processing | |
| Perceptual Processing | |
| Phonological Short-Term Memory |
|
Neuroimaging Evidence Supporting Language Processing Theories
5.3. Theoretical Approach: Non-Linguistic Cognitive Processing
| Error Type | Theoretical Account |
|---|---|
| Reduced Resource |
|
| Reduced Processing Speed |
|
| Impaired Learning System |
|
Neuroimaging Evidence Supporting Non-Linguistic Cognitive Processing Theories
5.4. Interim Summary: Linking Theory to Brain
6. Discussion: The Current State (and Limitations) in Linking Theory to Brain
7. Future Directions: New Approaches in Linking Theory to Brain
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Referenced DLD Neuroimaging Studies
| Reference | N (M/F), Mean Age (SD or Range) | Method | Key Findings in DLD (Compared to TD) |
|---|---|---|---|
| Structural Imaging Studies | |||
| Plante et al., 1991 [160] | SLI: 8 (8 M), 5.2 months TD: 8 (8 M), 4.2–9.6 months | Manual Morphometry | Children with SLI had significantly larger right hemisphere perisylvian volumes and left hemisphere volumes were as expected, showing abnormal asymmetry. No significant differences were found for extra-perisylvian regions between SLI and TD due to individual differences. |
| Jernigan et al., 1991 [57] | LI: 20 (13 M/7 F), 8.9 (±0.7) TD: 12 (8 M/4 F), 9.0 (±0.7) | Semi-automatic and Manual Morphometry | Children with LI had significantly smaller volumes in the L posterior perisylvian region compared to the R region and significantly greater volume in the R prefrontal regions compared to the L region. The authors found weak statistical evidence for reversed asymmetries in the perisylvian region (right > left). |
| Preis et al., 1998 [58] | DLD: 21 (14 M/7 F), 8.33 (±21 months) TD: 21 (14 M/7 F), 8.25 (±21 months) | Manual Morphometry | There were no differences in the planum temporale or the planum parietal asymmetry between DLD and TD children. DLD children had smaller forebrain sizes, which accounted for smaller planum temporale bilaterally in DLD. |
| Gauger et al., 1997 [32] | SLI: 11 (8 M/3 F); 9.0 (±2.3) TD: 19 (14 M, 5 F), 8.9 (±1.6) | Manual Morphometry | The pars triangularis was significantly smaller in the L hemisphere of children with SLI. Children with SLI were more likely to have rightward asymmetry of language structures. |
| Herbert et al., 2003 [36] | DLD: 24 (16 M/8 F), 8.31 (5.7–11.3) TD: 9.1 (6.5–11) | Semi-automatic and Manual Morphometry | Total brain volume was greater in children with DLD as compared to controls. A significant main effect of sex was found, with males showing significantly larger total brain volumes as compared with females. Regional differences between DLD and control children revealed cerebral white matter volume was 13% larger in the DLD sample. |
| De Fossé et al., 2004 [59] | ALI: 16 (16 M), 9.8 (±2.1) ALN: 6 (6 M), 8.3 (±0.9) SLI: 9 (9 M), 9.9 (±2.3) TD: 11 (11 M), 10.4 (±2.7) | Semi-automatic and Manual Morphometry | Non-language-impaired individuals had larger cerebral volumes than language-impaired individuals (both SLI and ALI). The IFG was larger on the R side in LI and larger on the L side in LN participants. The PT was equal in size for language normal participants and larger in the L for language-impaired individuals. |
| Herbert et al., 2005 [66] | DLD: 15 (15 M) ASD: 16 (16 M) TD: 15 (15 M) Total: 46 between 5.7 and 11.3 years. | Semi-automatic and Manual Morphometry | Cerebral symmetry patterns in children with DLD and ASD were similar but differed from TD patterns. In both autistic and DLD samples, there was a substantial increase compared with controls in the aggregate amount of cortical parcellation unit (PU) asymmetry. The ASD and DLD brains showed an increase in the number (and volume) of cortical PUs with rightward asymmetry, but at the same time, they showed either no loss (ASD) or only a small loss (DLD) in the number (and volume) of cortical PUs with leftward asymmetry. |
| Soriano-Mas et al., 2009 [37] | SLI: 36 (24 M/12 F), 10.58 (±2.80) TD: 36 (24 M/12 F), 10.88 (±2.83) | VBM | Global Volume Results: Children with SLI showed larger gray and white matter volumes than controls, but no differences were observed in CSF spaces. Global gray matter and white matter was increased in children with SLI when compared to TD children, but this finding was not consistent in the older children subgroup. Regional Volume Results: Children with SLI had increased gray matter in the R perisylvian region and the occipital petalia, in the L middle occipital gyrus. Findings indicated that in younger children, several regions of gray matter increase in SLI (entorhinal area bilaterally, and the temporopolar cortex, the caudate nucleus, the motor-precentral cortex, and the precuneus of the left hemisphere). There was a specific volume increase in the left middle occipital gyrus for children with SLI. The R perisylvian region, in comparison to controls, was increased during the whole age range (i.e., no interaction between gray matter volume and age), and so was the left temporopolar cortex, with a specific volume increase in younger children with SLI (i.e., interaction between gray matter volume and age). Significant volume increases were observed in younger children with SLI in the juxtacortical WM of the right medial frontal cortex and bilaterally in the middle temporal gyrus. There were no notable findings in the older group in regard to WM. |
| Badcock et al., 2012 [38] | SLI: 10 (9 M/1 F), 13.5 (±2.6) SLI-Sibling (SIB): 6 (4 M/2 F), 18.0 (±3.8) TD: 16 (7 M/9 F), 12.5 (±4.7) | VBM | Total GM volume did not differ. DLD had more GM than TD in L IFG, R insula, and L intraparietal. DLD has less GM than TD in pSTS bilaterally, R STG, R caudate, and R side of midbrain at substantia nigra, medial frontal polar cortex, R medial superior parietal, and L Occipital. DLD had more GM than SIB in L anterior intraparietal. There was less GM in the R parietal opercular and L occipital. SIB compared to TD, with more GM in ventral extent of central sulcus and retro splenial area bilaterally. SIBs had less GM in bilateral caudate, R putamen, R medial geniculate, and L fusiform than TDs. |
| Girbau-Massana et al., 2014 [33] | SLI: 10 (6 M/4 F), 8.5–10.9 (6 of the SLI participants had SLI + Reading Disorder or RD) TD: 14 (10 M/4 F), 8.2–11.8 | VBM | Children with SLI had significantly lower overall GM volume, especially at the right postcentral parietal gyrus, and greater CSF volume. Children with SLI and RD exhibited a significant smaller volume of GM in right postcentral parietal gyrus than children with TD. The CSF volumes were significantly greater in children with SLI and RD and SLI than in children with TD. Ratios of gray to white matter were not significantly different in children with SLI and RD when compared to children with TD. |
| Lee et al., 2020 [64] | 2 cohorts of subjects: Cohort 1: DLD: 14 (5 M/9 F), 22.42 (±2.02) TD: 12 (4 M/8 F), 22.10 (±0.51) Cohort 2: DLD: 19 (8 M/11 F), 16.96 (±1.49) TD: 61 (25 M/36 F), 16.62 (±1.40) | Semi-Automatic Morphometry and Diffusion Tensor Imaging (DTI) | Dorsal Pathway: The DLD group had significantly larger relative volumes of the L transverse temporal gyrus in the dorsal pathway than the comparison group. None of the other ROIs in the dorsal pathway showed significant age or group effects. Ventral Pathway: The ROIs comprising the ventral pathway showed significantly larger volumes for DLD in the R pars orbitalis, of the R temporal pole, and the L temporal pole for DLD subjects. Significant increases in relative volumes were seen across age for the L MTG, and for the L pars triangularis in DLD. DTI: Significantly reduced FA in the DLD group compared with the comparison group was found in the L and R SLF. There was an age-by-group interaction effect on the R SLF only for the comparison group. Significantly reduced FA values were found in the DLD group for the L IFOF and left UF. DLD group had no significantly increased FA across age in the L ILF and R UF (although the controls showed increased FA here). The DLD group also had smaller intracranial volumes. |
| Krishnan et al., 2022 [74] | DLD: 33 (22 M/11 F), 12.48 (±1.80) HSL: 20 (17 M/3 F), 12.40 (±1.67) TD: 56 (28 M/28 F), 12.41 (±1.62) | MPM Quantitative Image (Myelin and Iron Concentration), and T1-Weighted structural | Lower myelin content in bilateral caudate nuclei and L IFG. No group differences were found for iron concentration. |
| Bahar et al., 2023 [41] | DLD: 54 (39 M/15 F), 12.4 (±0.25) TD: 74 (41 M/33 F), 12.6 (±0.2) | T1-Weighted Structural (Surface Area, Cortical Thickness, and Gray Matter Volume) | Surface Area: DLD showed smaller surface area bilaterally in the IFG extending to the anterior insula, in the posterior temporal and ventral occipito-temporal cortex, and in portions of the anterior cingulate and superior frontal cortex. Cortical Thickness: No differences in cortical thickness, or asymmetry of these cortical metrics. Gray Matter Volume: DLD showed smaller volumes in the R pars triangularis, L MTG, and PTG. |
| Lee et al., 2013 [35] | DLI: 12 (4 M/8 F), 21.99 (±1.56) TD: 12 (4 M/8 F), 22.06 (±0.51) | Semi-Automatic Morphometry and Diffusion Tensor Imaging | The DLI group showed smaller bilateral caudate nucleus, globus pallidus, and bilateral thalamus, as well as smaller cerebral lobes including the occipital lobe and frontal lobe. The DLI group was significantly larger than the control group in the relative volumes of the hippocampus, putamen, the R globus pallidus, and the nucleus accumbens. The DLI group showed lower FA values in the cerebral lobes including the frontal and occipital lobes (no significant findings for other ROIs). |
| Roberts et al., 2014 [90] | SLI: 14 (8 M/6 F), 9.73 (±2.69) ALI: 16 (14 M/2 F), 9.80 (±2.57) ANL: 18 (16 M/2 F), 11.47 (±3.25) TD: 25 (16 M, 9 F), 11.42 (±2.92) | Diffusion Tensor Imaging | Both ASD and LI were associated with elevated mean diffusivity of the LH arcuate fasciculus. There was a main effect of ASD on the axial, but not radial, component of diffusivity, and a main effect of LI on the radial, but not axial, component of diffusivity, suggesting that although ASD plus LI and SLI may share a microstructural anomaly of elevated radial diffusivity, they differ in axial diffusivity. No differences were found in L arcuate fasciculus FA for SLI or ASD. A main effect of ASD was found on axial diffusivity but not radial diffusivity; conversely, there was a main effect of LI on radial diffusivity but not axial diffusivity. There was also a significant ASD vs LI interaction on axial diffusivity. No group differences were observed in the RH arcuate fasciculus for any of the DTI measures. |
| Vydrova et al., 2015 [91] | SLI: 37 (25 M/12 F), 8.4 (±1.6) TD: 34 (18 M/16 F), 8.9 (±1.6) | Diffusion Tensor Imaging | Children with SLI had reduced FA of the investigated tracts, and corresponding increases in mean diffusivity values (AF bilaterally, L IFOF and L ILF), increases in radial diffusivity component (AF bilaterally, L IFOF, L ILF and UF bilaterally), and decreases in axial diffusivity component (R IFOF and L UF). Children with SLI showed bilaterally larger volumes of the ILF compared to controls. |
| Verly et al., 2019 [93] | DLD: 17 (11 M/6 F), 10.07 (±2.01) TD: 22 (15 M/7 F), 11.00 (±1.11) | Diffusion Tensor Imaging | DTI Metrics: Participants with DLD showed decreased FA values in the L ILF and middle longitudinal fascicle (MdLF). A higher tract volume of the SLF, MdLF, and the longitudinal posterior part of the superior longitudinal fascicle (SLF) was detected in children with DLD compared to TD children. Also, lower apparent diffusion coefficient (ADC) values of the ILF and a higher FA of the SLF were detected in children with DLD. There was a pattern towards increased RH FA values in patients with DLD relative to controls across the SLF, SLF_anterior, MdLF, ILF, and ECFS. A trend towards lower ADC values was observed in children with DLD relative to controls across the SLF, SLF_longitudinal, SLF_posterior, and all ventral language-related WM tracts. Finally, a trend towards a higher tract volume was observed for all dorsal and ventral WM tracts in patients with DLD. Lateralization: TD children showed LH lateralization of FA for all language-related WM tracts, except for the posterior part of the SLF and the ECFS. A significant RH lateralization was found for the anterior part of the SLF. None of the studied dorsal and ventral language-related WM tracts showed a significant L or R lateralization pattern, except for the MdLF in children with DLD. |
| Functional Imaging Studies | |||
| Liegeois et al., 2003 [96] | KE Family Affected: 5 (3 M/2 F), 34.8 (±17.0) Unaffected: 5 (3 M/2 F), 25.3 (±3.4) | fMRI Covert and Overt Verb Generation and Word Repetition | Overall: Affected members showed significant under-activation bilaterally in Broca’s area and the putamen. Covert Task: The distribution of activation was the same for the unaffected and control group in L IFG, but for affected members, there was more diffuse involvement of RH compared to LH and posterior compared with anterior bilaterally. ROIs: Under-activation for affected most notably in L BA44, but also R putamen/globus pallidus, L SMG, R IFG, and L precentral gyrus. Bilateral overactivation for affected in pSTG, L precentral, R temporal pole, and a number of parietal ROIs. Overt Task: Activation was mainly bilateral. There was activation in L IFG and bilateral putamen. No significant overactivation was detected. Repetition Task: Significant overactivation in L BA44 and L anterior insula. |
| Hugdahl et al., 2004 [101] | SLI: 5 (2 M/3 F), 11–70 years TD: 6 (2 M/4 F), 15–61 years | fMRI Passive Listening | Reduced activation in MTG and STS. Unlike TD, SLI also activated areas in the R inferior frontal lobe. |
| Weismer et al., 2005 [161] | DLD: 8 (5 M/3 F), 13.10 (±6 months) TD: 8 (6 M/2 F), 14.0 (±7 months) | fMRI Encoding and Recognition Tasks | No significant differences in activation patterns or laterality. There were two distinct activations in the IFG (BA44/45) and the insular portion of BA44. Encoding Task: Significant difference in precentral sulcus and parietal for encoding. DLDs showed hypoactivation in the insular portion of IFG during recognition. Less coactivation between IFG and STG during encoding. DLD had significant correlations between activation in parietal and frontal memory ROIs and parietal and STG during encoding. Recognition Task: DLD had a weak association between the STG and frontal ROIs and STG and parietal. |
| Dibbets et al., 2006 [103] | SLI: 6 (all male); 6.10 (±8.4 months) TD: 7 (all male), 6.10 (±6.3 months); | fMRI Switch Task | Non-Switch Task: Increased activation in L and R frontal area and superior parietal and occipital lobe. Switch Task: Increased activation in L/R frontal, temporal, and cingulate regions. |
| De Guibert et al., 2011 [97] | SLI: 21 (9 M/12 F), 11.4 (±3.3) TD: 18 (9 M/9 F), 12.7 (±3.0) | fMRI Lexico-Semantic Tasks (category fluency and responsive naming) and two Visual Phonological Tasks | Category Fluency Task: Lack of left lateralization in BA44 and reduced activation. Naming Task: More activation in RH, with no activation in some of the regions that TDs activated. Insula was bilateral for SLI, whereas it was only left-lateralized for TD. L hypoactivation in posterior STG/SMG. Minimal Pair Triplets Task: SLI showed RH activation, but TD did not. There was R hyperactivation in the insula. Segmentation Triplets Task: SLI showed no activation in the ROIs that were expected (IFG, STG, SMG, or insula). Lack of L lateralization in BA44. Overall, there was a lack of lateralization in the LH, with no differences in lateralization of the rest of the brain. The L posterior STG/SMG was hypoactivated, while the R anterior insula was hyperactivated. |
| Badcock et al., 2012 [38] | SLI: 10 (9 M/1 F), 13.5 (±2.6 years) SLI-Sibling (SIB): 6 (4 M/2 F), 18.0 (±3.8) TD: 16 (7 M/9 F), 12.5 (±4.7) | fMRI Overt Word Generation and Passive Listening Tasks | TD and SIB showed a similar pattern of activation. DLD showed no activation in L IFG like other groups and much lower levels of activation in bilateral STG. TD and SIB showed L lateralization for speech but DLDs had less (due to a couple of individuals) in frontal and temporal areas. Structure–Function Relationship: Increased GM and decreased activation in inferior frontal region for DLDs and decreased GM and activation in posterior temporal region. |
| Plante et al., 2017 [102] | LI: 16 (7 M/9 F), 20.0 (±2.2 years) NL: 16 (7 M/9 F), 20 (±3.5 years) | fMRI Implicit Language Learning Task | There were no differences in the location of activation, but the level of activation was increased in L BA44, L SMG, L STG. |
| Pigdon et al., 2020 [98] | DLD: 18 (10 M/8 F), 124 months (±3) DSD: 15 (5 M/10 F), 123 months (±4) TD: 42 (22 M/20 F), 123 (±6) TD-matched subset: 17 (8 M/9 F), 123 (±5) | fMRI Nonword Repetition Task | DLD and DSD groups primarily activated subcortical regions (thalamus and globus pallidus), while TDs primarily activated cortical regions that are similar to adult activation patterns for nonword repetition, namely bilateral STS/STG, anterior insula, L IFG, SMA, precentral gyrus and motor cortex, L TPJ, anterior cingulate, and thalamus. However, there were no regions that survived thresholding when comparing the two groups; thus, there were no statistically significant differences. |
| Krishnan et al., 2021 [159] | DLD: 50 (35 M/15 F), 12.0 (10–15) TD: 67 (38 M/29 F), 12.1 (10–15) | fMRI Verb generation task | No activation differences in the L IFG or putamen and no lateralization differences. Sub-threshold activation differences in bilateral caudate and L IFG in a subset of children with poor task performance. |
| Cerebral Blood Flow (CBF) | |||
| Denays et al., 1989 [110] | Dysphasia: 14 (10 M/4 F), aged 5–16 years | SPECT | Expression Deficit: Hypoperfusion in frontal convolution of LH (Broca’s area). Comprehension and Expression Deficits: Hypoperfusion in the L tempo-parietal and hypoperfusion in upper and middle R frontal lobe. Three children with comprehension/expression deficits showed no abnormality. And 11 out of 14 were hypoperfused in language regions; there was hypoperfusion in areas for expressive deficits only, but for those with both, there was nothing in L IFG. |
| Lou et al., 1990 [111] | 24 patients (median 10 yr, range 6–15) all from special school and with antenatal and perinatal events. ADHD: 9 ADHD + Phonological-syntactic dysphasia: 8 Severe phonological syntactic dysphasia: 3 Severe verbal decoding issues: 3 Severe lexical-semantic: 1 | SPECT | For the language-impaired group, differences were less obvious than in other groups, but overall CBF was lower in the L perisylvian region than the R. For the phonological-syntactic only group, CBF to L prefrontal was lower than R. |
| Tzourio et al., 1994 [113] | Expressive–Receptive: 7 (4 M/3 F), 10 (±1) Expressive: 7 (6 M/1 F), 8 (±1) ADHD: 6 (6 M), 8 (±1) | SPECT | There was an absence of LH activation during phonemic discrimination task in children with expressive–receptive deficits compared to those with only expressive difficulties and those with ADHD. Abnormal lateralization patterns were found for language in the more severely impaired group. |
| Chiron et al., 1999 [109] | Dysphasia: 8 (8 M), 10.2 Duchenne Muscular Dystrophy: 8 (8 M), 10.7 | SPECT | Dichotic Task: Lack of activation in Broca’s but CBF increase in R homologue compared to Duchenne Muscular Dystrophy group. Dichaptic Task: Bilateral increase in CBF as opposed to only in RH for Duchenne group. |
| Ors et al., 2005 [112] | SLI: 19 (11 M/8 F), 9.7 (7–15) ADHD: 12 (11 M/1 F), 9.4 (7–14) | SPECT | Children with SLI had lower CBF values in R parietal and subcortical regions and no L asymmetry as is typical in development. |
| Hwang et al., 2006 [114] | DLD: 21 (14 M/7 F), 4.2 (±0.8) Tension Headaches: 17 (13 M/4 F), 11.0 (±1.9) | SPECT | Reduced CBF patterns were found in the R putamen, R inferior parietal cortex and the L globus pallidus (though this region did not survive correction for multiple comparisons). There were no regions of increased CBF in DLD compared to controls. |
| Im et al., 2007 [115] | DLD: 17 (15 M/2 F), 4.7 (±2.1) Split into 2 groups: >5 years and <5 years. ADHD: 10 (9 M/1 F), 7.0 (±1.8) | PET | >5 Years: Abnormal findings in the thalamus, cerebellum, basal ganglia, caudate nucleus, and left temporal lobe. <5 Years: Abnormal findings in the thalamus, basal ganglia, cerebellum, caudate nucleus, and frontal lobe. Overall, the children with DLD showed decreased glucose metabolism in the frontal, temporal, and right parietal areas. |
| Whitehouse & Bishop, 2008 [116] | SLI: 11 (7 M/4 F), 18.15 (±3.75) SLI-History: 9 (7 M/2 F), 18.33 (±14.33) ASD: 11, (10 M/1 F), 19.98 (±5.03) TD: 11 (7 M/11 F), 20.88 (±3.29) | Functional Transcranial Doppler (fTCD) Ultrasound Covert and Overt Word Generation | The SLI-History, ASD, and TD groups all demonstrated greater activation in the LH compared to the RH during the task, indicating typical LH dominance for language. The SLI group demonstrated an altered pattern of either RH dominance or bilateral activation. |
Appendix B. Diffusion Tensor Imaging (DTI) Indices
| Measure | What Is It Measuring? | Clinical Findings |
|---|---|---|
| Fractional Anisotropy (FA) | Amount of diffusion along primary axis (axon) compared to secondary and tertiary axes (perpendicular to the axon). FA increases as anisotropy increases (between 0 and 1; [102]). FA may reflect axonal microstructure (diameter and density), myelination concentration, and degree of fiber crossings [109]. | Clinical Populations: Decreased FA values in patients with neurodegenerative diseases, such as Alzheimer’s Disease and in normal aging [107,109]. DLD: The evidence is mixed, but findings suggest decreased FA in the superior longitudinal fasciculus and left hemisphere ventral (see Figure 4) tracts. There is limited evidence for decreased FA in the right hemisphere [62,83,110,111,112]. |
| Mean Diffusivity (MD) | Average diffusion across all three axes (λ1 + λ2 + λ3/3) [108]. MD may reflect structural integrity of axons [107]. | Clinical Populations: Increased MD in patients with disturbed structural integrity such as those with epilepsy and chronic stroke [107,113]. DLD: Increased MD in the arcuate fasciculus [112,114]. |
| Axial Diffusivity (AD) | Amount of diffusion along the primary axis (λ1) [108]. AD may reflect axonal integrity/damage [115]. | Clinical Populations: Decreased AD in patients with axonal degeneration and inflammation, such as individuals with multiple sclerosis (MS) [104,116]. DLD: No evidence of changes in AD. More research is needed. |
| Radial Diffusivity (RD) | Average diffusion perpendicular to the primary axis (λ2 + λ3/2) [108]. RD may reflect myelin integrity/damage [115]. | Clinical Populations: Increased RD in patients with myelin loss, such as individuals with MS [113]. DLD: Increased RD in the arcuate fasciculus [112,114]. |
Appendix C. Regional Activation Patterns by Task
| Left Hemisphere Regions | Right Hemisphere Regions | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Task | Reference | Inferior Frontal Gyrus (IFG) | Non-IFG Frontal | Supramarginal/Angular Gyrus | Precentral Gyrus/ Sulcus | Superior Temporal Gyrus/ Sulcus | Middle/ Inferior Temporal Gyrus | Sub-Cortical | IFG | Sub-Cortical |
| Covert Naming | Liegeois et al., 2003 [96] | Hypo-activation | N.S. | Hypo-activation | Hyper-activation | Hyper-activation | Hyper-activation | N.S. | Hypo-activation | Hypo-activation (putamen/ GP) |
| De Guibert et al., 2011 [97] | Hypo-activation | N.S. | Hypo-activation | N.S. | Hypo-activation (PT) | N.S. | ----- | Activation found (IFG and Insula) | ----- | |
| Badcock et al., 2012 [38] | No activation found | ----- | ----- | ----- | Hypo-activation | ----- | N.S. | Activation found | Hypo-activation (putamen) | |
| Overt Naming | Liegeois et al., 2003 [96] | Hypo-activation | N.S. | ----- | N.S. | N.S. | N.S. | Hypo-activation (putamen) | N.S. | Hypo-activation (putamen) |
| Krishnan et al., 2021 [159] | N.S. | N.S. | Hyper-activation (at lowered voxel threshold only in RH) | ----- | N.S. | ----- | Hyper-activation (in caudate at lowered voxel threshold in low-performing subset) | N.S. | Hyper-activation (in caudate at lowered voxel threshold in low-performing subset) | |
| Nonword Repetition | Pigdon et al., 2020 [98] | No activation found | No activation found | No activation found | No activation found | No activation found | ----- | Hyper-activation (thalamus/ GP–uncorrected) | No activation found | Hyper-activation (thalamus/ GP–uncorrected) |
| Left Hemisphere | Right Hemisphere | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Task | Reference | Inferior Frontal Gyrus (IFG) | Non-IFG Frontal | Supra-marginal/Angular Gyrus | Precentral Gyrus/ Sulcus | Superior Temporal Gyrus/ Sulcus | Middle/ Inferior Temporal Gyrus | Sub-cortical | IFG | Sub-cortical | Other |
| Passive Listening | Hugdahl et al., 2004 [101] | ----- | ----- | ----- | ----- | Hypo-activation (PT) | Hypo-activation | ----- | N.S. | ----- | Hypo-activation (PT, MTG) |
| Implicit Learning | Plante et al., 2017 [102] | Hyper-activation | N.S. | Hyper-activation | N.S. | Hyper-activation | ----- | ----- | ----- | ----- | N.S. (STG/S, MTG) |
| Verbal Working Memory | Weismer et al., 2005 [161] | Hypo-activation (insular portion) | N.S. | ----- | Hypo-activation (parietal) | N.S. | ----- | ----- | N.S. | ----- | ----- |
| Executive Control | Dibbets et al., 2006 [103] | ----- | Hyper-activation (medial, orbito, superior) | ----- | N.S. | Hyper-activation | Hyper-activation | Hyper-activation (cingulate) | ----- | Hyper-activation (cingulate) | Hyper-activation (intraparietal, AG) |
References
- Tomblin, J.B.; Records, N.L.; Buckwalter, P.; Zhang, X.; Smith, E.; O’Brien, M. Prevalence of Specific Language Impairment in Kindergarten Children. J. Speech Lang. Hear. Res. 1997, 40, 1245–1260. [Google Scholar] [CrossRef] [PubMed]
- Maenner, M.J.; Shaw, K.A.; Baio, J. Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2016. MMWR Surveill. Summ. 2020, 69, 1. [Google Scholar] [CrossRef] [PubMed]
- Norbury, C.F.; Gooch, D.; Wray, C.; Baird, G.; Charman, T.; Simonoff, E.; Vamvakas, G.; Pickles, A. The impact of nonverbal ability on prevalence and clinical presentation of language disorder: Evidence from a population study. J. Child Psychol. Psychiatry 2016, 57, 1247–1257. [Google Scholar] [CrossRef]
- Zablotsky, B.; Black, L.I.; Maenner, M.J.; Schieve, L.A.; Danielson, M.L.; Bitsko, R.H.; Blumberg, S.J.; Kogan, M.D.; Boyle, C.A. Prevalence and trends of developmental disabilities among children in the United States: 2009–2017. Pediatrics 2019, 144, e20190811. [Google Scholar] [CrossRef] [PubMed]
- Clegg, J.; Hollis, C.; Mawhood, L.; Rutter, M. Developmental language disorders—A follow-up in later adult life. Cognitive, language and psychosocial outcomes. J. Child Psychol. Psychiatry 2005, 46, 128–149. [Google Scholar] [CrossRef]
- Maggio, V.; Grañana, N.E.; Richaudeau, A.; Torres, S.; Giannotti, A.; Suburo, A.M. Behavior problems in children with specific language impairment. J. Child Neurol. 2014, 29, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Sansavini, A.; Favilla, M.E.; Guasti, M.T.; Marini, A.; Millepiedi, S.; Di Martino, M.V.; Vecchi, S.; Battajon, N.; Bertolo, L.; Capirci, O.; et al. Developmental Language Disorder: Early Predictors, Age for the Diagnosis, and Diagnostic Tools. A Scoping Review. Brain Sci. 2021, 11, 654. [Google Scholar] [CrossRef]
- Bishop, D.V. Cerebral asymmetry and language development: Cause, correlate, or consequence? Science 2013, 340, 1230531. [Google Scholar] [CrossRef]
- Schwartz, R.G. Handbook of Child Language Disorders, 2nd ed.; Psychology Press: London, UK, 2017. [Google Scholar] [CrossRef]
- Bishop, D.V.; Norbury, C.F. Exploring the borderlands of autistic disorder and specific language impairment: A study using standardised diagnostic instruments. J. Child Psychol. Psychiatry 2002, 43, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, H.S.; Camarata, S. Reconceptualizing developmental language disorder as a spectrum disorder: Issues and evidence. Int. J. Lang. Commun. Disord. 2019, 54, 79–94. [Google Scholar] [CrossRef]
- Tager-Flusberg, H. Do autism and specific language impairment represent overlapping language disorders? In Developmental Language Disorders; Psychology Press: London, UK, 2004; pp. 42–63. [Google Scholar]
- Pena, E.D.; Spaulding, T.J.; Plante, E. The composition of normative groups and diagnostic decision making: Shooting ourselves in the foot. Am. J. Speech Lang. Pathol. 2006, 15, 247–254. [Google Scholar] [CrossRef]
- Spaulding, T.J.; Plante, E.; Farinella, K.A. Eligibility criteria for language impairment. Lang. Speech Hear. Serv. Sch. 2006, 37, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Bishop, D.V.; Snowling, M.J.; Thompson, P.A.; Greenhalgh, T.; Consortium, C.; Adams, C.; Archibald, L.; Baird, G.; Bauer, A.; Bellair, J. Phase 2 of CATALISE: A multinational and multidisciplinary Delphi consensus study of problems with language development: Terminology. J. Child Psychol. Psychiatry 2017, 58, 1068–1080. [Google Scholar] [CrossRef] [PubMed]
- Mayes, A.K.; Reilly, S.; Morgan, A.T. Neural correlates of childhood language disorder: A systematic review. Dev. Med. Child Neurol. 2015, 57, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.L.; Brown, T.T. Specific language impairment. In Neurobiology of Language; Elsevier: Amsterdam, The Netherlands, 2016; pp. 899–912. [Google Scholar]
- Webster, R.I.; Shevell, M.I. Topical review: Neurobiology of specific language impairment. J. Child Neurol. 2004, 19, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Lebel, C.; Deoni, S. The development of brain white matter microstructure. Neuroimage 2018, 182, 207–218. [Google Scholar] [CrossRef]
- Stiles, J.; Jernigan, T.L. The Basics of Brain Development. Neuropsychol. Rev. 2010, 20, 327–348. [Google Scholar] [CrossRef]
- Yap, Q.J.; Teh, I.; Fusar-Poli, P.; Sum, M.Y.; Kuswanto, C.; Sim, K. Tracking cerebral white matter changes across the lifespan: Insights from diffusion tensor imaging studies. J. Neural Transm. 2013, 120, 1369–1395. [Google Scholar] [CrossRef]
- Jernigan, T.L.; Gamst, A.C. Changes in volume with age—Consistency and interpretation of observed effects. Neurobiol. Aging 2005, 26, 1271–1274. [Google Scholar] [CrossRef]
- Pirozzi, F.; Nelson, B.; Mirzaa, G. From microcephaly to megalencephaly: Determinants of brain size. Dialogues Clin. Neurosci. 2018, 20, 267–282. [Google Scholar] [CrossRef]
- Bayard, F.; Nymberg Thunell, C.; Abé, C.; Almeida, R.; Banaschewski, T.; Barker, G.; Bokde, A.L.W.; Bromberg, U.; Büchel, C.; Quinlan, E.B.; et al. Distinct brain structure and behavior related to ADHD and conduct disorder traits. Mol. Psychiatry 2020, 25, 3020–3033. [Google Scholar] [CrossRef] [PubMed]
- Brieber, S.; Neufang, S.; Bruning, N.; Kamp-Becker, I.; Remschmidt, H.; Herpertz-Dahlmann, B.; Fink, G.R.; Konrad, K. Structural brain abnormalities in adolescents with autism spectrum disorder and patients with attention deficit/hyperactivity disorder. J. Child Psychol. Psychiatry 2007, 48, 1251–1258. [Google Scholar] [CrossRef]
- Hasan, K.M.; Molfese, D.L.; Walimuni, I.S.; Stuebing, K.K.; Papanicolaou, A.C.; Narayana, P.A.; Fletcher, J.M. Diffusion tensor quantification and cognitive correlates of the macrostructure and microstructure of the corpus callosum in typically developing and dyslexic children. NMR Biomed. 2012, 25, 1263–1270. [Google Scholar] [CrossRef]
- Krain, A.L.; Castellanos, F.X. Brain development and ADHD. Clin. Psychol. Rev. 2006, 26, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Nickl-Jockschat, T.; Habel, U.; Maria Michel, T.; Manning, J.; Laird, A.R.; Fox, P.T.; Schneider, F.; Eickhoff, S.B. Brain structure anomalies in autism spectrum disorder-a meta-analysis of VBM studies using anatomic likelihood estimation. Hum. Brain Mapp. 2012, 33, 1470–1489. [Google Scholar] [CrossRef]
- Xia, Z.; Hoeft, F.; Zhang, L.; Shu, H. Neuroanatomical anomalies of dyslexia: Disambiguating the effects of disorder, performance, and maturation. Neuropsychologia 2016, 81, 68–78. [Google Scholar] [CrossRef]
- Lange, N.; Travers, B.G.; Bigler, E.D.; Prigge, M.B.D.; Froehlich, A.L.; Nielsen, J.A.; Cariello, A.N.; Zielinski, B.A.; Anderson, J.S.; Fletcher, P.T.; et al. Longitudinal Volumetric Brain Changes in Autism Spectrum Disorder Ages 6–35 Years. Autism Res. 2015, 8, 82–93. [Google Scholar] [CrossRef]
- Bethlehem, R.A.I.; Seidlitz, J.; White, S.R.; Vogel, J.W.; Anderson, K.M.; Adamson, C.; Adler, S.; Alexopoulos, G.S.; Anagnostou, E.; Areces-Gonzalez, A.; et al. Brain charts for the human lifespan. Nature 2022, 604, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Gauger, L.M.; Lombardino, L.J.; Leonard, C.M. Brain morphology in children with specific language impairment. J. Speech Lang. Hear. Res. 1997, 40, 1272–1284. [Google Scholar] [CrossRef]
- Girbau-Massana, D.; Garcia-Marti, G.; Marti-Bonmati, L.; Schwartz, R.G. Gray–white matter and cerebrospinal fluid volume differences in children with specific language impairment and/or reading disability. Neuropsychologia 2014, 56, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Herbert, M.R.; Ziegler, D.A.; Makris, N.; Filipek, P.A.; Kemper, T.L.; Normandin, J.J.; Sanders, H.A.; Kennedy, D.N.; Caviness, V.S., Jr. Localization of white matter volume increase in autism and developmental language disorder. Ann. Neurol. 2004, 55, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Nopoulos, P.C.; Tomblin, J.B. Abnormal subcortical components of the corticostriatal system in young adults with DLI: A combined structural MRI and DTI study. Neuropsychologia 2013, 51, 2154–2161. [Google Scholar] [CrossRef]
- Herbert, M.R.; Ziegler, D.A.; Makris, N.; Bakardjiev, A.; Hodgson, J.; Adrien, K.T.; Kennedy, D.N.; Filipek, P.A.; Caviness, V.S., Jr. Larger brain and white matter volumes in children with developmental language disorder. Dev. Sci. 2003, 6, F11–F22. [Google Scholar] [CrossRef]
- Soriano-Mas, C.; Pujol, J.; Ortiz, H.; Deus, J.; López-Sala, A.; Sans, A. Age-related brain structural alterations in children with specific language impairment. Hum. Brain Mapp. 2009, 30, 1626–1636. [Google Scholar] [CrossRef]
- Badcock, N.A.; Bishop, D.V.; Hardiman, M.J.; Barry, J.G.; Watkins, K.E. Co-localisation of abnormal brain structure and function in specific language impairment. Brain Lang. 2012, 120, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Carper, R.A.; Moses, P.; Tigue, Z.D.; Courchesne, E. Cerebral Lobes in Autism: Early Hyperplasia and Abnormal Age Effects. NeuroImage 2002, 16, 1038–1051. [Google Scholar] [CrossRef]
- Wierenga, L.M.; Langen, M.; Oranje, B.; Durston, S. Unique developmental trajectories of cortical thickness and surface area. Neuroimage 2014, 87, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Bahar, N.; Cler, G.J.; Krishnan, S.; Asaridou, S.S.; Smith, H.J.; Willis, H.E.; Healy, M.P.; Watkins, K.E. Differences in cortical surface area in developmental language disorder. bioRxiv 2023. [Google Scholar] [CrossRef]
- White, T.; Su, S.; Schmidt, M.; Kao, C.-Y.; Sapiro, G. The development of gyrification in childhood and adolescence. Brain Cogn. 2010, 72, 36–45. [Google Scholar] [CrossRef]
- Fedorenko, E.; Thompson-Schill, S.L. Reworking the language network. Trends Cogn. Sci. 2014, 18, 120–126. [Google Scholar] [CrossRef]
- Friederici, A.D.; Chomsky, N.; Berwick, R.C.; Moro, A.; Bolhuis, J.J. Language, mind and brain. Nat. Hum. Behav. 2017, 1, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Hertrich, I.; Dietrich, S.; Ackermann, H. The margins of the language network in the brain. Front. Commun. 2020, 5, 519955. [Google Scholar] [CrossRef]
- Price, C.J. The anatomy of language: A review of 100 fMRI studies published in 2009. Ann. N. Y. Acad. Sci. 2010, 1191, 62–88. [Google Scholar] [CrossRef] [PubMed]
- Ocklenburg, S.; Friedrich, P.; Fraenz, C.; Schlüter, C.; Beste, C.; Güntürkün, O.; Genç, E. Neurite architecture of the planum temporale predicts neurophysiological processing of auditory speech. Sci. Adv. 2018, 4, eaar6830. [Google Scholar] [CrossRef] [PubMed]
- Galuske, R.A.; Schlote, W.; Bratzke, H.; Singer, W. Interhemispheric asymmetries of the modular structure in human temporal cortex. Science 2000, 289, 1946–1949. [Google Scholar] [CrossRef]
- Dorsaint-Pierre, R.; Penhune, V.B.; Watkins, K.E.; Neelin, P.; Lerch, J.P.; Bouffard, M.; Zatorre, R.J. Asymmetries of the planum temporale and Heschl’s gyrus: Relationship to language lateralization. Brain 2006, 129, 1164–1176. [Google Scholar] [CrossRef]
- Foundas, A.L.; Leonard, C.M.; Gilmore, R.; Fennell, E.; Heilman, K.M. Planum temporale asymmetry and language dominance. Neuropsychologia 1994, 32, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Geschwind, N.; Levitsky, W. Human brain: Left-right asymmetries in temporal speech region. Science 1968, 161, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Rojas, D.C.; Camou, S.L.; Reite, M.L.; Rogers, S.J. Planum temporale volume in children and adolescents with autism. J. Autism Dev. Disord. 2005, 35, 479–486. [Google Scholar] [CrossRef]
- Eckert, M.A.; Leonard, C.M. Structural imaging in dyslexia: The planum temporale. Ment. Retard. Dev. Disabil. Res. Rev. 2000, 6, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Plante, E.; Swisher, L.; Vance, R.; Rapcsak, S. MRI findings in boys with specific language impairment. Brain Lang. 1991, 41, 52–66. [Google Scholar] [CrossRef]
- Cohen, M.; Campbell, R.; Yaghmai, F. Neuropathological abnormalities in developmental dysphasia. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1989, 25, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Galaburda, A.M.; Sherman, G.F.; Rosen, G.D.; Aboitiz, F.; Geschwind, N. Developmental dyslexia: Four consecutive patients with cortical anomalies. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1985, 18, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Jernigan, T.L.; Hesselink, J.R.; Sowell, E.; Tallal, P.A. Cerebral structure on magnetic resonance imaging in language-and learning-impaired children. Arch. Neurol. 1991, 48, 539–545. [Google Scholar] [CrossRef]
- Preis, S.; Jäncke, L.; Schittler, P.; Huang, Y.; Steinmetz, H. Normal intrasylvian anatomical asymmetry in children with developmental language disorder. Neuropsychologia 1998, 36, 849–855. [Google Scholar] [CrossRef]
- De Fossé, L.; Hodge, S.M.; Makris, N.; Kennedy, D.N.; Caviness, V.S.; McGrath, L.; Steele, S.; Ziegler, D.A.; Herbert, M.R.; Frazier, J.A.; et al. Language-association cortex asymmetry in autism and specific language impairment. Ann. Neurol. 2004, 56, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Rogalsky, C.; Matchin, W.; Hickok, G. Broca’s area, sentence comprehension, and working memory: An fMRI study. Front. Hum. Neurosci. 2008, 2, 237. [Google Scholar] [CrossRef]
- Grodzinsky, Y. The neurology of syntax: Language use without Broca’s area. Behav. Brain Sci. 2000, 23, 1–21. [Google Scholar] [CrossRef]
- Martin, R.C. Language processing: Functional organization and neuroanatomical basis. Annu. Rev. Psychol. 2003, 54, 55–89. [Google Scholar] [CrossRef] [PubMed]
- Hope, T.M.; Prejawa, S.; Parker Jones, Ō.; Oberhuber, M.; Seghier, M.L.; Green, D.W.; Price, C.J. Dissecting the functional anatomy of auditory word repetition. Front. Hum. Neurosci. 2014, 8, 246. [Google Scholar] [CrossRef]
- Lee, J.C.; Dick, A.S.; Tomblin, J.B. Altered brain structures in the dorsal and ventral language pathways in individuals with and without developmental language disorder (DLD). Brain Imaging Behav. 2020, 14, 2569–2586. [Google Scholar] [CrossRef]
- Watkins, K.E.; Vargha-Khadem, F.; Ashburner, J.; Passingham, R.E.; Connelly, A.; Friston, K.J.; Frackowiak, R.S.; Mishkin, M.; Gadian, D.G. MRI analysis of an inherited speech and language disorder: Structural brain abnormalities. Brain 2002, 125, 465–478. [Google Scholar] [CrossRef]
- Herbert, M.R.; Ziegler, D.A.; Deutsch, C.; O’Brien, L.M.; Kennedy, D.N.; Filipek, P.; Bakardjiev, A.; Hodgson, J.; Takeoka, M.; Makris, N. Brain asymmetries in autism and developmental language disorder: A nested whole-brain analysis. Brain 2005, 128, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Booth, J.R.; Wood, L.; Lu, D.; Houk, J.C.; Bitan, T. The role of the basal ganglia and cerebellum in language processing. Brain Res. 2007, 1133, 136–144. [Google Scholar] [CrossRef]
- Crosson, B.; Benefield, H.; Cato, M.A.; Sadek, J.R.; Moore, A.B.; Wierenga, C.E.; Gopinath, K.; Soltysik, D.; Bauer, R.M.; Auerbach, E.J.; et al. Left and right basal ganglia and frontal activity during language generation: Contributions to lexical, semantic, and phonological processes. J. Int. Neuropsychol. Soc. 2003, 9, 1061–1077. [Google Scholar] [CrossRef]
- Tan, A.P.; Ngoh, Z.M.; Yeo, S.S.P.; Koh, D.X.P.; Gluckman, P.; Chong, Y.S.; Daniel, L.M.; Rifkin-Graboi, A.; Fortier, M.V.; Qiu, A.; et al. Left lateralization of neonatal caudate microstructure affects emerging language development at 24 months. Eur. J. Neurosci. 2021, 54, 4621–4637. [Google Scholar] [CrossRef]
- Thibault, S.; Py, R.; Gervasi, A.M.; Salemme, R.; Koun, E.; Lövden, M.; Boulenger, V.; Roy, A.C.; Brozzoli, C. Tool use and language share syntactic processes and neural patterns in the basal ganglia. Science 2021, 374, eabe0874. [Google Scholar] [CrossRef] [PubMed]
- Wahl, M.; Marzinzik, F.; Friederici, A.D.; Hahne, A.; Kupsch, A.; Schneider, G.-H.; Saddy, D.; Curio, G.; Klostermann, F. The Human Thalamus Processes Syntactic and Semantic Language Violations. Neuron 2008, 59, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Watkins, K.E.; Bishop, D.V.M. Neurobiological Basis of Language Learning Difficulties. Trends Cogn. Sci. 2016, 20, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Ullman, M.T.; Pierpont, E.I. Specific language impairment is not specific to language: The procedural deficit hypothesis. Cortex 2005, 41, 399–433. [Google Scholar] [CrossRef]
- Krishnan, S.; Cler, G.J.; Smith, H.J.; Willis, H.E.; Asaridou, S.S.; Healy, M.P.; Papp, D.; Watkins, K.E. Quantitative MRI reveals differences in striatal myelin in children with DLD. eLife 2022, 11, e74242. [Google Scholar] [CrossRef]
- Corrigan, N.M.; Yarnykh, V.L.; Hippe, D.S.; Owen, J.P.; Huber, E.; Zhao, T.C.; Kuhl, P.K. Myelin development in cerebral gray and white matter during adolescence and late childhood. Neuroimage 2021, 227, 117678. [Google Scholar] [CrossRef]
- Timmler, S.; Simons, M. Grey matter myelination. Glia 2019, 67, 2063–2070. [Google Scholar] [CrossRef]
- Zhao, T.; Xu, Y.; He, Y. Graph theoretical modeling of baby brain networks. NeuroImage 2019, 185, 711–727. [Google Scholar] [CrossRef] [PubMed]
- Dubois, J.; Dehaene-Lambertz, G.; Kulikova, S.; Poupon, C.; Hüppi, P.S.; Hertz-Pannier, L. The early development of brain white matter: A review of imaging studies in fetuses, newborns and infants. Neuroscience 2014, 276, 48–71. [Google Scholar] [CrossRef]
- Lebel, C.; Gee, M.; Camicioli, R.; Wieler, M.; Martin, W.; Beaulieu, C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage 2012, 60, 340. [Google Scholar] [CrossRef]
- Lebel, C.; Treit, S.; Beaulieu, C. A review of diffusion MRI of typical white matter development from early childhood to young adulthood. NMR Biomed. 2019, 32, e3778. [Google Scholar] [CrossRef]
- Kaestner, E.; Balachandra, A.R.; Bahrami, N.; Reyes, A.; Lalani, S.J.; Macari, A.C.; Voets, N.L.; Drane, D.L.; Paul, B.M.; Bonilha, L. The white matter connectome as an individualized biomarker of language impairment in temporal lobe epilepsy. NeuroImage Clin. 2020, 25, 102125. [Google Scholar] [CrossRef]
- Langer, N.; Peysakhovich, B.; Zuk, J.; Drottar, M.; Sliva, D.D.; Smith, S.; Becker, B.L.; Grant, P.E.; Gaab, N. White matter alterations in infants at risk for developmental dyslexia. Cereb. Cortex 2017, 27, 1027–1036. [Google Scholar] [CrossRef]
- Olivé, G.; Slušná, D.; Vaquero, L.; Muchart-López, J.; Rodríguez-Fornells, A.; Hinzen, W. Structural connectivity in ventral language pathways characterizes non-verbal autism. Brain Struct. Funct. 2022, 227, 1817–1829. [Google Scholar] [CrossRef] [PubMed]
- Vanderauwera, J.; Wouters, J.; Vandermosten, M.; Ghesquière, P. Early dynamics of white matter deficits in children developing dyslexia. Dev. Cogn. Neurosci. 2017, 27, 69–77. [Google Scholar] [CrossRef]
- Fletcher, P.T.; Whitaker, R.T.; Tao, R.; Dubray, M.B.; Froehlich, A.; Ravichandran, C.; Alexander, A.L.; Bigler, E.D.; Lange, N.; Lainhart, J.E. Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. NeuroImage 2010, 51, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Y.; Tachibana, M.; Rahman, S.; Kagitani-Shimono, K. Atypical structural connectivity of language networks in autism spectrum disorder: A meta-analysis of diffusion tensor imaging studies. Autism Res. 2022, 15, 1585–1602. [Google Scholar] [CrossRef]
- Dick, A.S.; Bernal, B.; Tremblay, P. The language connectome: New pathways, new concepts. Neuroscientist 2014, 20, 453–467. [Google Scholar] [CrossRef]
- Hickok, G.; Poeppel, D. Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition 2004, 92, 67–99. [Google Scholar] [CrossRef]
- Saur, D.; Kreher, B.W.; Schnell, S.; Kümmerer, D.; Kellmeyer, P.; Vry, M.-S.; Umarova, R.; Musso, M.; Glauche, V.; Abel, S.; et al. Ventral and dorsal pathways for language. Proc. Natl. Acad. Sci. USA 2008, 105, 18035–18040. [Google Scholar] [CrossRef]
- Roberts, T.; Heiken, K.; Zarnow, D.; Dell, J.; Nagae, L.; Blaskey, L.; Solot, C.; Levy, S.; Berman, J.; Edgar, J. Left hemisphere diffusivity of the arcuate fasciculus: Influences of autism spectrum disorder and language impairment. Am. J. Neuroradiol. 2014, 35, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Vydrova, R.; Komarek, V.; Sanda, J.; Sterbova, K.; Jahodova, A.; Maulisova, A.; Zackova, J.; Reissigova, J.; Krsek, P.; Kyncl, M. Structural alterations of the language connectome in children with specific language impairment. Brain Lang. 2015, 151, 35–41. [Google Scholar] [CrossRef]
- Simmonds, D.J.; Hallquist, M.N.; Asato, M.; Luna, B. Developmental stages and sex differences of white matter and behavioral development through adolescence: A longitudinal diffusion tensor imaging (DTI) study. NeuroImage 2014, 92, 356–368. [Google Scholar] [CrossRef]
- Verly, M.; Gerrits, R.; Sleurs, C.; Lagae, L.; Sunaert, S.; Zink, I.; Rommel, N. The mis-wired language network in children with developmental language disorder: Insights from DTI tractography. Brain Imaging Behav. 2019, 13, 973–984. [Google Scholar] [CrossRef]
- Liu, J.; Tsang, T.; Jackson, L.; Ponting, C.; Jeste, S.S.; Bookheimer, S.Y.; Dapretto, M. Altered lateralization of dorsal language tracts in 6-week-old infants at risk for autism. Dev. Sci. 2019, 22, e12768. [Google Scholar] [CrossRef]
- Baum, G.L.; Cui, Z.; Roalf, D.R.; Ciric, R.; Betzel, R.F.; Larsen, B.; Cieslak, M.; Cook, P.A.; Xia, C.H.; Moore, T.M.; et al. Development of structure–function coupling in human brain networks during youth. Proc. Natl. Acad. Sci. USA 2020, 117, 771–778. [Google Scholar] [CrossRef]
- Liégeois, F.; Baldeweg, T.; Connelly, A.; Gadian, D.G.; Mishkin, M.; Vargha-Khadem, F. Language fMRI abnormalities associated with FOXP2 gene mutation. Nat. Neurosci. 2003, 6, 1230–1237. [Google Scholar] [CrossRef]
- De Guibert, C.; Maumet, C.; Jannin, P.; Ferré, J.-C.; Tréguier, C.; Barillot, C.; Le Rumeur, E.; Allaire, C.; Biraben, A. Abnormal functional lateralization and activity of language brain areas in typical specific language impairment (developmental dysphasia). Brain 2011, 134, 3044–3058. [Google Scholar] [CrossRef]
- Pigdon, L.; Willmott, C.; Reilly, S.; Conti-Ramsden, G.; Liegeois, F.; Connelly, A.; Morgan, A.T. The neural basis of nonword repetition in children with developmental speech or language disorder: An fMRI study. Neuropsychologia 2020, 138, 107312. [Google Scholar] [CrossRef]
- Abbott, N.T.; Baker, C.J.; Chen, C.; Liu, T.T.; Love, T.E. Defining Hypoperfusion in Chronic Aphasia: An Individualized Thresholding Approach. Brain Sci. 2021, 11, 491. [Google Scholar] [CrossRef] [PubMed]
- Kiran, S.; Thompson, C.K. Neuroplasticity of Language Networks in Aphasia: Advances, Updates, and Future Challenges. Front. Neurol. 2019, 10, 295. [Google Scholar] [CrossRef]
- Hugdahl, K.; Gundersen, H.; Brekke, C.; Thomsen, T.; Rimol, L.M.; Ersland, L.; Niemi, J. fMRI brain activation in a Finnish family with specific language impairment compared with a normal control group. J. Speech Lang. Hear. Res. 2004, 47, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Plante, E.; Patterson, D.; Sandoval, M.; Vance, C.J.; Asbjørnsen, A.E. An fMRI study of implicit language learning in developmental language impairment. NeuroImage Clin. 2017, 14, 277–285. [Google Scholar] [CrossRef]
- Dibbets, P.; Bakker, K.; Jolles, J. Functional MRI of task switching in children with specific language impairment (SLI). Neurocase 2006, 12, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Schmithorst, V.J.; Vannest, J.; Lee, G.; Hernandez-Garcia, L.; Plante, E.; Rajagopal, A.; Holland, S.K.; Consortium, C.A. Evidence that neurovascular coupling underlying the BOLD effect increases with age during childhood. Hum. Brain Mapp. 2015, 36, 1–15. [Google Scholar] [CrossRef]
- Shaw, K.; Bell, L.; Boyd, K.; Grijseels, D.M.; Clarke, D.; Bonnar, O.; Crombag, H.S.; Hall, C.N. Neurovascular coupling and oxygenation are decreased in hippocampus compared to neocortex because of microvascular differences. Nat. Commun. 2021, 12, 3190. [Google Scholar] [CrossRef] [PubMed]
- Bonakdarpour, B.; Parrish, T.B.; Thompson, C.K. Hemodynamic response function in patients with stroke-induced aphasia: Implications for fMRI data analysis. NeuroImage 2007, 36, 322–331. [Google Scholar] [CrossRef]
- Brumm, K.P.; Perthen, J.E.; Liu, T.T.; Haist, F.; Ayalon, L.; Love, T. An arterial spin labeling investigation of cerebral blood flow deficits in chronic stroke survivors. Neuroimage 2010, 51, 995–1005. [Google Scholar] [CrossRef]
- Love, T.; Swinney, D.; Wong, E.; Buxton, R. Perfusion imaging and stroke: A more sensitive measure of the brain bases of cognitive deficits. Aphasiology 2002, 16, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Chiron, C.; Pinton, F.; Masure, M.; Duvelleroy-Hommet, C.; Leon, F.; Billard, C. Hemispheric specialization using SPECT and stimulation tasks in children with dysphasia and dystrophia. Dev. Med. Child Neurol. 1999, 41, 512–520. [Google Scholar] [CrossRef]
- Denays, R.; Tondeur, M.; Foulon, M.; Verstraeten, F.; Ham, H.; Piepsz, A.; Noël, P. Regional brain blood flow in congenital dysphasia: Studies with technetium-99m HM-PAO SPECT. J. Nucl. Med. 1989, 30, 1825–1829. [Google Scholar]
- Lou, H.; Henriksen, L.; Bruhn, P. Focal cerebral dysfunction in developmental learning disabilities. Lancet 1990, 335, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Ors, M.; Ryding, E.; Lindgren, M.; Gustafsson, P.; Blennow, G.; Rosén, I. SPECT findings in children with specific language impairment. Cortex 2005, 41, 316–326. [Google Scholar] [CrossRef]
- Tzourio, N.; Heim, A.; Zilbovicius, M.; Gerard, C.; Mazoyer, B.M. Abnormal regional CBF response in left hemisphere of dysphasic children during a language task. Pediatr. Neurol. 1994, 10, 20–26. [Google Scholar] [CrossRef]
- Hwang, J.W.; Lee, J.-B.; Kim, B.-N.; Lee, H.-Y.; Lee, D.-S.; Shin, M.-S.; Cho, S.-C. Regional cerebral perfusion abnormalities in developmental language disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2006, 256, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Im, S.-H.; Park, E.S.; Kim, D.Y.; Song, D.H.; Lee, J.D. The neuroradiological findings of children with developmental language disorder. Yonsei Med. J. 2007, 48, 405–411. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Whitehouse, A.J.O.; Bishop, D.V.M. Cerebral dominance for language function in adults with specific language impairment or autism. Brain 2008, 131, 3193–3200. [Google Scholar] [CrossRef]
- Paniukov, D.; Lebel, R.; Giesbrecht, G.; Lebel, C. Cerebral Blood Flow Increases Across Early Childhood. Neuroimage 2020, 204, 116224. [Google Scholar] [CrossRef]
- Avants, B.B.; Duda, J.T.; Kilroy, E.; Krasileva, K.; Jann, K.; Kandel, B.T.; Tustison, N.J.; Yan, L.; Jog, M.; Smith, R. The pediatric template of brain perfusion. Sci. Data 2015, 2, 150003. [Google Scholar] [CrossRef]
- Moses, P.; Hernandez, L.M.; Orient, E. Age-related differences in cerebral blood flow underlie the BOLD fMRI signal in childhood. Front. Psychol. 2014, 5, 300. [Google Scholar] [CrossRef]
- Wu, C.; Honarmand, A.R.; Schnell, S.; Kuhn, R.; Schoeneman, S.E.; Ansari, S.A.; Carr, J.; Markl, M.; Shaibani, A. Age-related changes of normal cerebral and cardiac blood flow in children and adults aged 7 months to 61 years. J. Am. Heart Assoc. 2016, 5, e002657. [Google Scholar] [CrossRef] [PubMed]
- Purkayastha, S.; Sorond, F. Transcranial Doppler ultrasound: Technique and application. Semin. Neurol. 2012, 32, 411–420. [Google Scholar] [CrossRef]
- Leonard, L.B. Children with specific language impairment and their contribution to the study of language development. J. Child Lang. 2014, 41, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.L.; Wexler, K.; Cleave, P.L. Specific language impairment as a period of extended optional infinitive. J. Speech Lang. Hear. Res. 1995, 38, 850–863. [Google Scholar] [CrossRef]
- Wexler, K.; Lightfoot, D.; Homstein, N. Verb Movement; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Rice, M.L.; Wexler, K.; Hershberger, S. Tense over time: The longitudinal course of tense acquisition in children with specific language impairment. J. Speech Lang. Hear. Res. 1998, 41, 1412–1431. [Google Scholar] [CrossRef]
- Wexler, K.; Schütze, C.T.; Rice, M. Subject case in children with SLI and unaffected controls: Evidence for the Agr/Tns omission model. Lang. Acquis. 1998, 7, 317–344. [Google Scholar] [CrossRef]
- Wexler, K. Very early parameter setting and the unique checking constraint: A new explanation of the optional infinitive stage. Lingua 1998, 106, 23–79. [Google Scholar] [CrossRef]
- Van der Lely, H.K. SLI in children: Movement, economy, and deficits in the computational-syntactic system. Lang. Acquis. 1998, 7, 161–192. [Google Scholar] [CrossRef]
- Marshall, C.R.; Van Der Lely, H.K. The impact of phonological complexity on past tense inflection in children with Grammatical-SLI. Adv. Speech Lang. Pathol. 2007, 9, 191–203. [Google Scholar] [CrossRef]
- Ingram, D.; Carr, L. When morphology ability exceeds syntactic ability: A case study. In Proceedings of the Convention of the American Speech-Language-Hearing Association, New Orleans, LA, USA, 17–21 November 1994. [Google Scholar]
- Marshall, C.R.; van der Lely, H.K. A challenge to current models of past tense inflection: The impact of phonotactics. Cognition 2006, 100, 302–320. [Google Scholar] [CrossRef]
- Leonard, L.B.; Kueser, J.B. Five overarching factors central to grammatical learning and treatment in children with developmental language disorder. Int. J. Lang. Commun. Disord. 2019, 54, 347–361. [Google Scholar] [CrossRef]
- Tagarelli, K.M.; Shattuck, K.F.; Turkeltaub, P.E.; Ullman, M.T. Language learning in the adult brain: A neuroanatomical meta-analysis of lexical and grammatical learning. NeuroImage 2019, 193, 178–200. [Google Scholar] [CrossRef]
- Kuhnke, P.; Meyer, L.; Friederici, A.D.; Hartwigsen, G. Left posterior inferior frontal gyrus is causally involved in reordering during sentence processing. Neuroimage 2017, 148, 254–263. [Google Scholar] [CrossRef]
- Copland, D.A.; Brownsett, S.; Iyer, K.; Angwin, A.J. Corticostriatal regulation of language functions. Neuropsychol. Rev. 2021, 31, 472–494. [Google Scholar] [CrossRef]
- Tallal, P.; Piercy, M. Defects of Non-Verbal Auditory Perception in Children with Developmental Aphasia. Nature 1973, 241, 468–469. [Google Scholar] [CrossRef]
- Tallal, P.; Piercy, M. Developmental aphasia: Rate of auditory processing and selective impairment of consonant perception. Neuropsychologia 1974, 12, 83–93. [Google Scholar] [CrossRef]
- Schwartz, R.G.; Scheffler, F.L.V.; Lopez, K. Speech perception and lexical effects in specific language impairment. Clin. Linguist. Phon. 2013, 27, 339–354. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leonard, L.B.; McGregor, K.K.; Allen, G.D. Grammatical morphology and speech perception in children with specific language impairment. J. Speech Lang. Hear. Res. 1992, 35, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Baddeley, A. The episodic buffer: A new component of working memory? Trends Cogn. Sci. 2000, 4, 417–423. [Google Scholar] [CrossRef]
- Baddeley, A. Working memory and language: An overview. J. Commun. Disord. 2003, 36, 189–208. [Google Scholar] [CrossRef]
- Baddeley, A.D.; Hitch, G. Working memory. In Psychology of Learning and Motivation; Elsevier: Amsterdam, The Netherlands, 1974; Volume 8, pp. 47–89. [Google Scholar]
- Gathercole, S.E.; Baddeley, A.D. Evaluation of the role of phonological STM in the development of vocabulary in children: A longitudinal study. J. Mem. Lang. 1989, 28, 200–213. [Google Scholar] [CrossRef]
- Gathercole, S.E.; Baddeley, A.D. Phonological memory deficits in language disordered children: Is there a causal connection? J. Mem. Lang. 1990, 29, 336–360. [Google Scholar] [CrossRef]
- Estes, K.G.; Evans, J.L.; Else-Quest, N.M. Differences in the nonword repetition performance of children with and without specific language impairment: A meta-analysis. J. Speech Lang. Hear. Res. 2007, 50, 177–195. [Google Scholar] [CrossRef]
- Ferrill, M.; Love, T.; Walenski, M.; Shapiro, L.P. The time-course of lexical activation during sentence comprehension in people with aphasia. Am. J. Speech-Lang. Pathol. 2012, 21, S179–S189. [Google Scholar] [CrossRef] [PubMed]
- Robertson, E.K.; Gallant, J.E. Eye tracking reveals subtle spoken sentence comprehension problems in children with dyslexia. Lingua 2019, 228, 102708. [Google Scholar] [CrossRef]
- Brauer, J.; Anwander, A.; Perani, D.; Friederici, A.D. Dorsal and ventral pathways in language development. Brain Lang. 2013, 127, 289–295. [Google Scholar] [CrossRef]
- Perani, D.; Saccuman, M.C.; Scifo, P.; Anwander, A.; Spada, D.; Baldoli, C.; Poloniato, A.; Lohmann, G.; Friederici, A.D. Neural language networks at birth. Proc. Natl. Acad. Sci. USA 2011, 108, 16056–16061. [Google Scholar] [CrossRef]
- Labache, L.; Joliot, M.; Saracco, J.; Jobard, G.; Hesling, I.; Zago, L.; Mellet, E.; Petit, L.; Crivello, F.; Mazoyer, B.; et al. A SENtence Supramodal Areas AtlaS (SENSAAS) based on multiple task-induced activation mapping and graph analysis of intrinsic connectivity in 144 healthy right-handers. Brain Struct. Funct. 2019, 224, 859–882. [Google Scholar] [CrossRef] [PubMed]
- Love, T.; Swinney, D.; Walenski, M.; Zurif, E. How left inferior frontal cortex participates in syntactic processing: Evidence from aphasia. Brain Lang. 2008, 107, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Hula, W.D.; McNeil, M.R. Models of attention and dual-task performance as explanatory constructs in aphasia. Semin. Speech Lang. 2008, 29, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.W. Relation of working memory to off-line and real-time sentence processing in children with specific language impairment. Appl. Psycholinguist. 2000, 21, 117–148. [Google Scholar] [CrossRef]
- Just, M.A.; Carpenter, P.A. A capacity theory of comprehension: Individual differences in working memory. Psychol. Rev. 1992, 99, 122. [Google Scholar] [CrossRef]
- Kail, R. A method for studying the generalized slowing hypothesis in children with specific language impairment. J. Speech Lang. Hear. Res. 1994, 37, 418–421. [Google Scholar] [CrossRef]
- Windsor, J.; Kohnert, K. The Search for Common Ground. J. SpeechLang. Hear. Res. 2004, 47, 877–890. [Google Scholar] [CrossRef]
- Jednoróg, K.; Marchewka, A.; Altarelli, I.; Monzalvo Lopez, A.K.; Van Ermingen-Marbach, M.; Grande, M.; Grabowska, A.; Heim, S.; Ramus, F. How reliable are gray matter disruptions in specific reading disability across multiple countries and languages? insights from a large-scale voxel-based morphometry study. Hum. Brain Mapp. 2015, 36, 1741–1754. [Google Scholar] [CrossRef]
- Friedrich, P.; Fraenz, C.; Schlüter, C.; Ocklenburg, S.; Mädler, B.; Güntürkün, O.; Genç, E. The relationship between axon density, myelination, and fractional anisotropy in the human corpus callosum. Cereb. Cortex 2020, 30, 2042–2056. [Google Scholar] [CrossRef]
- Krishnan, S.; Asaridou, S.S.; Cler, G.J.; Smith, H.J.; Willis, H.E.; Healy, M.P.; Thompson, P.A.; Bishop, D.V.M.; Watkins, K.E. Functional organisation for verb generation in children with developmental language disorder. NeuroImage 2021, 226, 117599. [Google Scholar] [CrossRef]
- Plante, E. MRI findings in the parents and siblings of specifically language-impaired boys. Brain Lang. 1991, 41, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Weismer, S.E.; Plante, E.; Jones, M.; Tomblin, J.B. A functional magnetic resonance imaging investigation of verbal working memory in adolescents with specific language impairment. J. Speech Lang. Hear. Res. 2005, 48, 405–425. [Google Scholar] [CrossRef]


| Phonology | Syntax/Morphology | Word Finding/ Semantics | Pragmatics/Language Use | Discourse | Verbal Learning/Memory |
|---|---|---|---|---|---|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbott, N.; Love, T. Bridging the Divide: Brain and Behavior in Developmental Language Disorder. Brain Sci. 2023, 13, 1606. https://doi.org/10.3390/brainsci13111606
Abbott N, Love T. Bridging the Divide: Brain and Behavior in Developmental Language Disorder. Brain Sciences. 2023; 13(11):1606. https://doi.org/10.3390/brainsci13111606
Chicago/Turabian StyleAbbott, Noelle, and Tracy Love. 2023. "Bridging the Divide: Brain and Behavior in Developmental Language Disorder" Brain Sciences 13, no. 11: 1606. https://doi.org/10.3390/brainsci13111606
APA StyleAbbott, N., & Love, T. (2023). Bridging the Divide: Brain and Behavior in Developmental Language Disorder. Brain Sciences, 13(11), 1606. https://doi.org/10.3390/brainsci13111606







