Advances in Intraoperative Glioma Tissue Sampling and Infiltration Assessment
Abstract
:1. Introduction
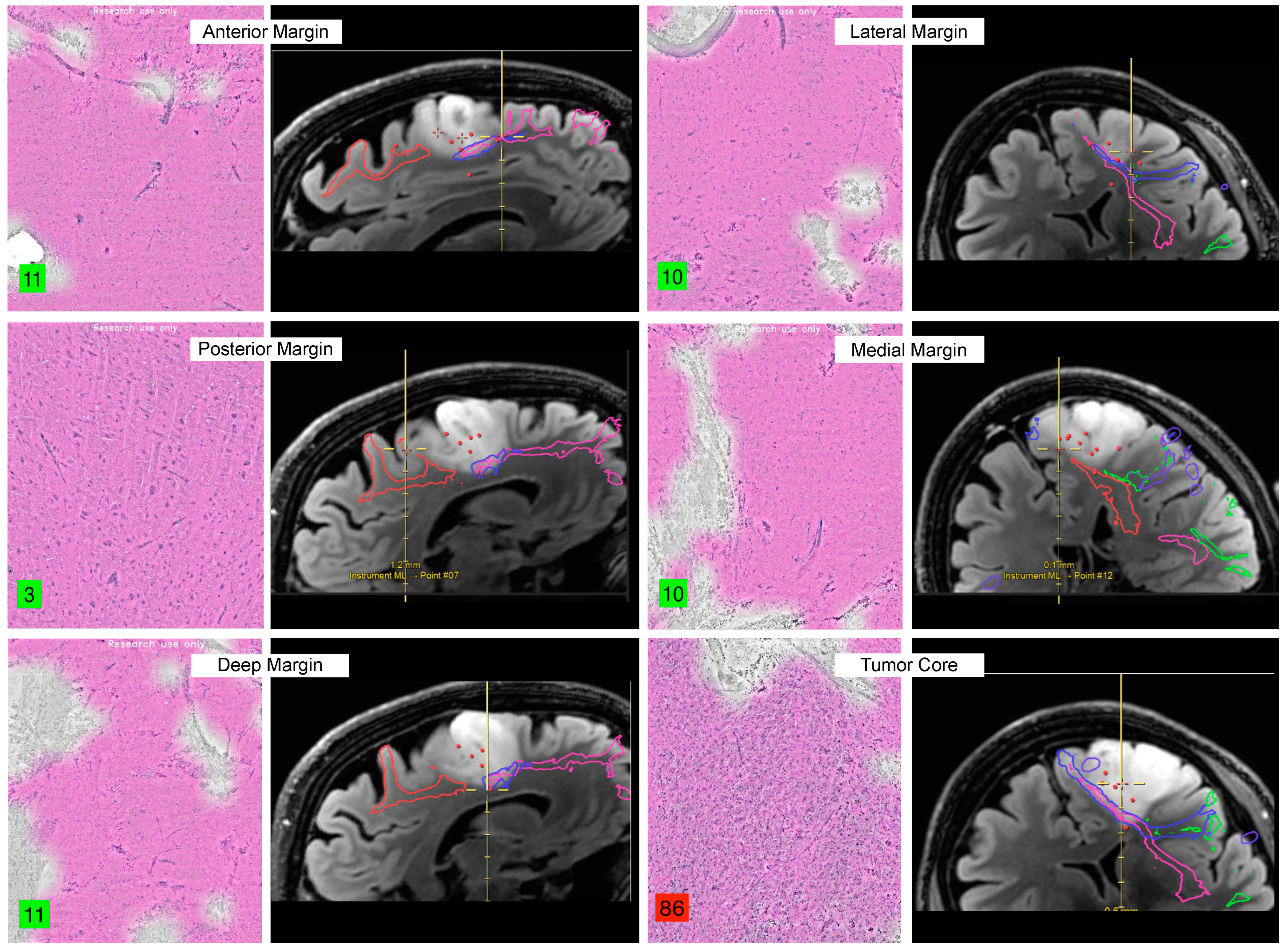
2. Neoplastic Tissue Identification
2.1. Raman Histology
2.2. Fluorescence
2.2.1. 5-Aminolevulinic Acid
2.2.2. Fluorescein
2.2.3. Indocyanine Green
2.2.4. Future Directions—Targeted Agents
2.3. Mass Spectrometry
3. Image-Guided Resection Offers Macroscopic Discrimination
3.1. Intraoperative Magnetic Resonance Imaging
3.2. Intraoperative Ultrasound
3.2.1. Contrast-Enhanced US
3.2.2. Three-Dimensional US
3.3. Fluorescence-Guided Resection
4. Nuanced Tissue Examination
4.1. Confocal Microscope
4.2. Third Harmonic Generation Microscopy
5. Analytical Methods
5.1. Big Data and Collaboration
5.2. Machine Learning
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, A.M.; Hervey-Jumper, S.; Morshed, R.A.; Young, J.; Han, S.J.; Chunduru, P.; Zhang, Y.; Phillips, J.J.; Shai, A.; Lafontaine, M.; et al. Association of Maximal Extent of Resection of Contrast-Enhanced and Non–Contrast-Enhanced Tumor with Survival within Molecular Subgroups of Patients with Newly Diagnosed Glioblastoma. JAMA Oncol. 2020, 6, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Abbatematteo, J.; Leo, E.K.D.; Kubilis, P.S.; Vaziri, S.; Bova, F.; Sayour, E.; Mitchell, D.; Quinones-Hinojosa, A. The Effects of New or Worsened Postoperative Neurological Deficits on Survival of Patients with Glioblastoma. J. Neurosurg. 2016, 127, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Aabedi, A.A.; Young, J.S.; Zhang, Y.; Ammanuel, S.; Morshed, R.A.; Dalle Ore, C.; Brown, D.; Phillips, J.J.; Oberheim Bush, N.A.; Taylor, J.W.; et al. Association of Neurological Impairment on the Relative Benefit of Maximal Extent of Resection in Chemoradiation-Treated Newly Diagnosed Isocitrate Dehydrogenase Wild-Type Glioblastoma. Neurosurgery 2022, 90, 124–130. [Google Scholar] [CrossRef]
- Young, J.S.; Morshed, R.A.; Hervey-Jumper, S.L.; Berger, M.S. The Surgical Management of Diffuse Gliomas: Current State of Neurosurgical Management and Future Directions. Neuro Oncol. 2023, noad133. [Google Scholar] [CrossRef] [PubMed]
- Al-Adli, N.N.; Young, J.S.; Sibih, Y.E.; Berger, M.S. Technical Aspects of Motor and Language Mapping in Glioma Patients. Cancers 2023, 15, 2173. [Google Scholar] [CrossRef]
- Da, N.; Rj, Z. Interinstitutional Comparison of Frozen Section Turnaround Time. A College of American Pathologists Q-Probes Study of 32868 Frozen Sections in 700 Hospitals. Arch. Pathol. Lab. Med. 1997, 121, 559–567. [Google Scholar]
- Shi, J.; Zhang, Y.; Yao, B.; Sun, P.; Hao, Y.; Piao, H.; Zhao, X. Application of Multiparametric Intraoperative Ultrasound in Glioma Surgery. BioMed Res. Int. 2021, 2021, 6651726. [Google Scholar] [CrossRef]
- Fountain, D.M.; Bryant, A.; Barone, D.G.; Waqar, M.; Hart, M.G.; Bulbeck, H.; Kernohan, A.; Watts, C.; Jenkinson, M.D. Intraoperative Imaging Technology to Maximise Extent of Resection for Glioma: A Network Meta-analysis. Cochrane Database Syst. Rev. 2021, 2021, CD013630. [Google Scholar] [CrossRef]
- Van Hese, L.; De Vleeschouwer, S.; Theys, T.; Rex, S.; Heeren, R.M.A.; Cuypers, E. The Diagnostic Accuracy of Intraoperative Differentiation and Delineation Techniques in Brain Tumours. Discov. Onc 2022, 13, 123. [Google Scholar] [CrossRef]
- Orillac, C.; Hollon, T.; Orringer, D.A. Clinical Translation of Stimulated Raman Histology. Methods Mol. Biol. 2022, 2393, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Einstein, E.H.; Ablyazova, F.; Rosenberg, A.; Harshan, M.; Wahl, S.; Har-El, G.; Constantino, P.D.; Ellis, J.A.; Boockvar, J.A.; Langer, D.J.; et al. Stimulated Raman Histology Facilitates Accurate Diagnosis in Neurosurgical Patients: A One-to-One Noninferiority Study. J. Neuro-Oncol. 2022, 159, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Straehle, J.; Erny, D.; Neidert, N. Neuropathological Interpretation of Stimulated Raman Histology Images of Brain and Spine Tumors. Part B Neurosurg. Rev. 2022, 45, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Eichberg, D.G.; Huang, K.; Shah, A.H.; Jamshidi, A.M.; Luther, E.M.; Lu, V.M.; Komotar, R.J.; Ivan, M.E.; Gultekin, S.H. Stimulated Raman Histology for Rapid Intraoperative Diagnosis of Gliomas. World Neurosurg. 2021, 150, e135–e143. [Google Scholar] [CrossRef] [PubMed]
- Movahed-Ezazi, M.; Nasir-Moin, M.; Fang, C.; Pizzillo, I.; Galbraith, K.; Drexler, S.; Krasnozhen-Ratush, O.A.; Shroff, S.; Zagzag, D.; William, C.; et al. Clinical Validation of Stimulated Raman Histology for Rapid Intraoperative Diagnosis of Central Nervous System Tumors. Mod. Pathol. 2023, 36, 100219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, Y.; Wu, B.; Zhang, S.; Zhu, K.; Liu, C.-H.; Yu, X.; Alfano, R.R. A Handheld Visible Resonance Raman Analyzer Used in Intraoperative Detection of Human Glioma. Cancers 2023, 15, 1752. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, G.; Cofano, F.; Salvati, L.F.; Monticelli, M.; Zeppa, P.; Perna, G.D.; Melcarne, A.; Altieri, R.; Rocca, G.; Sabatino, G. Fluorescence-Guided Surgery for High-Grade Gliomas: State of the Art and New Perspectives. Technol. Cancer Res. Treat. 2021, 20, 15330338211021605. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Singhal, S.; Lee, J.Y.K. Optical Principles of Fluorescence-Guided Brain Tumor Surgery: A Practical Primer for the Neurosurgeon. Neurosurgery 2019, 85, 312. [Google Scholar] [CrossRef]
- Widhalm, G.; Kiesel, B.; Woehrer, A.; Traub-Weidinger, T.; Preusser, M.; Marosi, C.; Prayer, D.; Hainfellner, J.A.; Knosp, E.; Wolfsberger, S. 5-Aminolevulinic Acid Induced Fluorescence Is a Powerful Intraoperative Marker for Precise Histopathological Grading of Gliomas with Non-Significant Contrast-Enhancement. PLoS ONE 2013, 8, e76988. [Google Scholar] [CrossRef]
- Ji, S.Y.; Kim, J.W.; Park, C.K. Experience Profiling of Fluorescence-Guided Surgery I: Gliomas. Brain Tumor Res. Treat. 2019, 7, 98–104. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J. Fluorescence-Guided Surgery with 5-Aminolevulinic Acid for Resection of Malignant Glioma: A Randomised Controlled Multicentre Phase III Trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Liu, Z.; Mela, A.; Argenziano, M.G.; Banu, M.A.; Furnari, J.; Kotidis, C.; Sperring, C.P.; Humala, N.; Mahajan, A.; Bruce, J.N.; et al. Single-Cell Analysis of 5-Aminolevulinic Acid Intraoperative Labeling Specificity for Glioblastoma. J. Neurosurg. 2023, 1, 1–11. [Google Scholar] [CrossRef]
- Ferraro, N.; Barbarite, E.; Albert, T.R.; Berchmans, E.; Shah, A.H.; Bregy, A.; Ivan, M.E.; Brown, T.; Komotar, R.J. The Role of 5-Aminolevulinic Acid in Brain Tumor Surgery: A Systematic Review. Neurosurg. Rev. 2016, 39, 545–555. [Google Scholar] [CrossRef]
- Warsi, N.; Zewude, R.; Karmur, B.; Pirouzmand, N.; Hachem, L.; Mansouri, A. The Cost-Effectiveness of 5-ALA in High-Grade Glioma Surgery: A Quality-Based Systematic Review. Can. J. Neurol. Sci. 2020, 47, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, J.; Yano, H.; Yoshimura, S.-I.; Okumura, A.; Kaku, Y.; Iwama, T.; Sakai, N. Fluorescence-Guided Resection of Glioblastoma Multiforme by Using High-Dose Fluorescein Sodium. Tech. Note J. Neurosurg. 2003, 99, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Belykh, E.; Bardonova, L.; Abramov, I.; Byvaltsev, V.A.; Kerymbayev, T.; Yu, K.; Healey, D.R.; Luna-Melendez, E.; Deneen, B.; Mehta, S. 5-Aminolevulinic Acid, Fluorescein Sodium, and Indocyanine Green for Glioma Margin Detection: Analysis of Operating Wide-Field and Confocal Microscopy in Glioma Models of Various Grades. Front. Oncol. 2023, 13, 1156812. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Yoshioka, H.; Kato, A. Fluorescence-Guided Surgery for Glioblastoma Multiforme Using High-Dose Fluorescein Sodium with Excitation and Barrier Filters. J. Clin. Neurosci. 2012, 19, 1719–1722. [Google Scholar] [CrossRef]
- Diaz, R.J.; Dios, R.R.; Hattab, E.M.; Burrell, K.; Rakopoulos, P.; Sabha, N.; Hawkins, C.; Zadeh, G.; Rutka, J.T.; Cohen-Gadol, A.A. Study of the Biodistribution of Fluorescein in Glioma-Infiltrated Mouse Brain and Histopathological Correlation of Intraoperative Findings in High-Grade Gliomas Resected under Fluorescein Fluorescence Guidance. J. Neurosurg. 2015, 122, 1360–1369. [Google Scholar] [CrossRef]
- Schebesch, K.-M.; Höhne, J.; Rosengarth, K.; Noeva, E.; Schmidt, N.O.; Proescholdt, M. Fluorescein-Guided Resection of Newly Diagnosed High-Grade Glioma: Impact on Extent of Resection and Outcome. Brain Spine 2022, 2, 101690. [Google Scholar] [CrossRef]
- Acerbi, F.; Broggi, M.; Schebesch, K.-M.; Höhne, J.; Cavallo, C.; De Laurentis, C.; Eoli, M.; Anghileri, E.; Servida, M.; Boffano, C.; et al. Fluorescein-Guided Surgery for Resection of High-Grade Gliomas: A Multicentric Prospective Phase II Study (FLUOGLIO). Clin. Cancer Res. 2018, 24, 52–61. [Google Scholar] [CrossRef]
- Smith, E.J.; Gohil, K.; Thompson, C.M.; Naik, A.; Hassaneen, W. Fluorescein-Guided Resection of High Grade Gliomas: A Meta-Analysis. World Neurosurg. 2021, 155, 181–188. [Google Scholar] [CrossRef]
- Cho, S.S.; Salinas, R.; Lee, J.Y.K. Indocyanine-Green for Fluorescence-Guided Surgery of Brain Tumors: Evidence, Techniques, and Practical Experience. Front. Surg. 2019, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.X.; Keating, J.J.; Jesus, E.M.D.; Judy, R.P.; Madajewski, B.; Venegas, O.; Okusanya, O.T.; Singhal, S. Optimization of the Enhanced Permeability and Retention Effect for Near-Infrared Imaging of Solid Tumors with Indocyanine Green. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 390–400. [Google Scholar] [PubMed]
- Sun, R.; Cuthbert, H.; Watts, C. Fluorescence-Guided Surgery in the Surgical Treatment of Gliomas: Past, Present and Future. Cancers 2021, 13, 3508. [Google Scholar] [CrossRef]
- Whitley, M.J.; Cardona, D.M.; Lazarides, A.L.; Spasojevic, I.; Ferrer, J.M.; Cahill, J.; Lee, C.-L.; Snuderl, M.; Blazer, D.G.; Hwang, E.S.; et al. A Mouse-Human Phase 1 Co-Clinical Trial of a Protease-Activated Fluorescent Probe for Imaging Cancer. Sci. Transl. Med. 2016, 8, 320ra4. [Google Scholar] [CrossRef]
- Liu, R.; Xu, Y.; Xu, K.; Dai, Z. Current Trends and Key Considerations in the Clinical Translation of Targeted Fluorescent Probes for Intraoperative Navigation. Aggregate 2021, 2, 23. [Google Scholar] [CrossRef]
- Study Details|Panitumumab-IRDye800 to Detect Pediatric Neoplasms during Neurosurgical Procedures|ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT04085887?term=IRDye800CW%20&page=4&rank=40 (accessed on 30 October 2023).
- Study Details|Safety Study of BLZ-100 in Adult Subjects with Glioma Undergoing Surgery|ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT02234297 (accessed on 30 October 2023).
- Patil, C.G.; Walker, D.G.; Miller, D.M.; Butte, P.; Morrison, B.; Kittle, D.S.; Hansen, S.J.; Nufer, K.L.; Byrnes-Blake, K.A.; Yamada, M.; et al. Phase 1 Safety, Pharmacokinetics, and Fluorescence Imaging Study of Tozuleristide (BLZ-100) in Adults with Newly Diagnosed or Recurrent Gliomas. Neurosurgery 2019, 85, E641. [Google Scholar] [CrossRef] [PubMed]
- Butte, P.V.; Mamelak, A.; Parrish-Novak, J.; Drazin, D.; Shweikeh, F.; Gangalum, P.R.; Chesnokova, A.; Ljubimova, J.Y.; Black, K. Near-Infrared Imaging of Brain Tumors Using the Tumor Paint BLZ-100 to Achieve Near-Complete Resection of Brain Tumors. Neurosurg. Focus 2014, 36, E1. [Google Scholar] [CrossRef]
- Study Details|Multispectral Bimodal Fluorescence Guided Surgery of High-Grade Glioma with Cetuximab-800CW and 5-ALA (5-Aminolevulinic Acid)|ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT05929456?term=IRDye800CW%20&page=2&rank=14 (accessed on 30 October 2023).
- Study Details|Image Guided Surgery for Margin Assessment of Head and Neck Cancer Using Cetuximab-IRDye800CW cONjugate|ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT03134846?term=IRDye800CW%20&rank=3 (accessed on 30 October 2023).
- Miller, S.E.; Tummers, W.S.; Teraphongphom, N.; van den Berg, N.S.; Hasan, A.; Ertsey, R.D.; Nagpal, S.; Recht, L.D.; Plowey, E.D.; Vogel, H.; et al. First-in-Human Intraoperative near-Infrared Fluorescence Imaging of Glioblastoma Using Cetuximab-IRDye800. J. Neuro-Oncol. 2018, 139, 135–143. [Google Scholar] [CrossRef]
- Study Details|IRDye800CW-BBN PET-NIRF Imaging Guiding Surgery in Patients with Glioblastoma|ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT02910804?term=IRDye800CW%20&rank=4 (accessed on 30 October 2023).
- Study Details|A Microdose Evaluation Study of ABY-029 in Recurrent Glioma|ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT02901925 (accessed on 30 October 2023).
- Pautke, C.; Vogt, S.; Kreutzer, K.; Haczek, C.; Wexel, G.; Kolk, A.; Imhoff, A.B.; Zitzelsberger, H.; Milz, S.; Tischer, T. Characterization of Eight Different Tetracyclines: Advances in Fluorescence Bone Labeling. J. Anat. 2010, 217, 76–82. [Google Scholar] [CrossRef]
- Study Details|Demeclocycline Fluorescence for Intraoperative Delineation Brain Tumors|ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT02740933?term=Demeclocycline%20&rank=1 (accessed on 30 October 2023).
- Senders, J.T.; Muskens, I.S.; Schnoor, R.; Karhade, A.V.; Cote, D.J.; Smith, T.R.; Broekman, M.L.D. Agents for Fluorescence-Guided Glioma Surgery: A Systematic Review of Preclinical and Clinical Results. Acta Neurochir. 2017, 159, 151–167. [Google Scholar] [CrossRef]
- Lauwerends, L.J.; van Driel, P.B.A.A.; de Jong, R.J.B.; Hardillo, J.A.U.; Koljenovic, S.; Puppels, G.; Mezzanotte, L.; Löwik, C.W.G.M.; Rosenthal, E.L.; Vahrmeijer, A.L.; et al. Real-Time Fluorescence Imaging in Intraoperative Decision Making for Cancer Surgery. Lancet Oncol. 2021, 22, e186–e195. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Chi, C.; Xiao, X.; Wang, J.; Lang, L.; Ali, I.; Niu, G.; Zhang, L.; Tian, J.; et al. First-in-Human Study of PET and Optical Dual-Modality Image-Guided Surgery in Glioblastoma Using 68Ga-IRDye800CW-BBN. Theranostics 2018, 8, 2508–2520. [Google Scholar] [CrossRef]
- Shi, X.; Xu, P.; Cao, C.; Cheng, Z.; Tian, J.; Hu, Z. PET/NIR-II Fluorescence Imaging and Image-Guided Surgery of Glioblastoma Using a Folate Receptor α-Targeted Dual-Modal Nanoprobe. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4325–4337. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.; Galstyan, A.; Sun, T.; Shatalova, E.S.; Butte, P.; Mamelak, A.N.; Carico, C.; Kittle, D.S.; Grodzinski, Z.B.; Chichi, A.; et al. Polymalic Acid Chlorotoxin Nanoconjugate for Near-Infrared Fluorescence Guided Resection of Glioblastoma Multiforme. Biomaterials 2019, 206, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.I.; Clark, P.A.; Zhang, R.R.; Kandela, I.K.; Farhoud, M.; Weichert, J.P.; Kuo, J.S. Fluorescent Cancer-Selective Alkylphosphocholine Analogs for Intraoperative Glioma Detection. Neurosurgery 2015, 76, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Tessitore, A.; Gaggiano, A.; Cicciarelli, G.; Verzella, D.; Capece, D.; Fischietti, M.; Zazzeroni, F.; Alesse, E. Serum Biomarkers Identification by Mass Spectrometry in High-Mortality Tumors. Int. J. Proteom. 2013, 2013, 125858. [Google Scholar] [CrossRef]
- Oppenheimer, S.R.; Mi, D.; Sanders, M.E.; Caprioli, R.M. A Molecular Analysis of Tumor Margins by MALDI Mass Spectrometry in Renal Carcinoma. J. Proteome Res. 2010, 9, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, C.N.; Fowler, J.W.M.; Waxer, J.F.; Gatti, R.A.; Loo, J.A. Mass Spectrometry-Based Tissue Imaging of Small Molecules. Adv. Exp. Med. Biol. 2014, 806, 283–299. [Google Scholar] [CrossRef]
- McDonnell, L.A.; Heeren, R.M.A. Imaging Mass Spectrometry. Mass Spectrom. Rev. 2007, 26, 606–643. [Google Scholar] [CrossRef]
- Reyzer, M.L.; Caprioli, R.M. Imaging Mass Spectrometry. In Proceedings of the Detection of Biological Agents for the Prevention of Bioterrorism; Banoub, J., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 267–283. [Google Scholar]
- Eberlin, L.S.; Norton, I.; Dill, A.L.; Golby, A.J.; Ligon, K.L.; Santagata, S.; Cooks, R.G.; Agar, N.Y.R. Classifying Human Brain Tumors by Lipid Imaging with Mass Spectrometry. Cancer Res. 2012, 72, 645–654. [Google Scholar] [CrossRef]
- Pirro, V.; Alfaro, C.M.; Jarmusch, A.K.; Hattab, E.M.; Cohen-Gadol, A.A.; Cooks, R.G. Intraoperative Assessment of Tumor Margins during Glioma Resection by Desorption Electrospray Ionization-Mass Spectrometry. Proc. Natl. Acad. Sci. USA 2017, 114, 6700–6705. [Google Scholar] [CrossRef]
- Schwartz, S.A.; Weil, R.J.; Johnson, M.D.; Toms, S.A.; Caprioli, R.M. Protein Profiling in Brain Tumors Using Mass Spectrometry: Feasibility of a New Technique for the Analysis of Protein Expression. Clin. Cancer Res. 2004, 10, 981–987. [Google Scholar] [CrossRef]
- Longuespée, R.; Wefers, A.K.; De Vita, E.; Miller, A.K.; Reuss, D.E.; Wick, W.; Herold-Mende, C.; Kriegsmann, M.; Schirmacher, P.; von Deimling, A.; et al. Rapid Detection of 2-Hydroxyglutarate in Frozen Sections of IDH Mutant Tumors by MALDI-TOF Mass Spectrometry. Acta Neuropathol. Commun. 2018, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Randall, E.C.; Lopez, B.G.C.; Peng, S.; Regan, M.S.; Abdelmoula, W.M.; Basu, S.S.; Santagata, S.; Yoon, H.; Haigis, M.C.; Agar, J.N.; et al. Localized Metabolomic Gradients in Patient-Derived Xenograft Models of Glioblastoma. Cancer Res. 2020, 80, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Kampa, J.M.; Kellner, U.; Marsching, C.; Ramallo Guevara, C.; Knappe, U.J.; Sahin, M.; Giampà, M.; Niehaus, K.; Bednarz, H. Glioblastoma Multiforme: Metabolic Differences to Peritumoral Tissue and IDH-Mutated Gliomas Revealed by Mass Spectrometry Imaging. Neuropathology 2020, 40, 546–558. [Google Scholar] [CrossRef]
- Cubillos, S.; Obregón, F.; Vargas, M.F.; Salazar, L.A.; Lima, L. Taurine Concentration in Human Gliomas and Meningiomas: Tumoral, Peritumoral, and Extratumoral Tissue. In Proceedings of the Taurine 6; Oja, S.S., Saransaari, P., Eds.; Springer: Boston, MA, USA, 2006; pp. 419–422. [Google Scholar]
- Balog, J.; Sasi-Szabó, L.; Kinross, J.; Lewis, M.R.; Muirhead, L.J.; Veselkov, K.; Mirnezami, R.; Dezső, B.; Damjanovich, L.; Darzi, A.; et al. Intraoperative Tissue Identification Using Rapid Evaporative Ionization Mass Spectrometry. Sci. Transl. Med. 2013, 5, 194ra93. [Google Scholar] [CrossRef] [PubMed]
- Yahanda, A.T.; Chicoine, M.R. Intraoperative MRI for Glioma Surgery: Present Overview and Future Directions. World Neurosurg. 2021, 149, 267–268. [Google Scholar] [CrossRef]
- Senft, C.; Bink, A.; Franz, A.; Vatter, H.; Gasser, T.; Seifert, V. Intraoperative MRI Guidance and Extent of Resection in Glioma Surgery: A Randomised, Controlled Trial. Lancet Oncol. 2011, 12, 997–1003. [Google Scholar] [CrossRef]
- Wu, J.-S.; Gong, X.; Song, Y.-Y.; Zhuang, D.-X.; Yao, C.-J.; Qiu, T.-M.; Lu, J.-F.; Zhang, J.; Zhu, W.; Mao, Y.; et al. 3.0-T Intraoperative Magnetic Resonance Imaging-Guided Resection in Cerebral Glioma Surgery: Interim Analysis of a Prospective, Randomized, Triple-Blind, Parallel-Controlled Trial. Neurosurgery 2014, 61, 145. [Google Scholar] [CrossRef]
- Shah, A.S.; Yahanda, A.T.; Sylvester, P.T.; Evans, J.; Dunn, G.P.; Jensen, R.L.; Honeycutt, J.; Cahill, D.P.; Sutherland, G.R.; Oswood, M.; et al. Using Histopathology to Assess the Reliability of Intraoperative Magnetic Resonance Imaging in Guiding Additional Brain Tumor Resection: A Multicenter Study. Neurosurgery 2021, 88, 49. [Google Scholar] [CrossRef] [PubMed]
- Arlt, F.; Chalopin, C.; Müns, A.; Meixensberger, J.; Lindner, D. Intraoperative 3D Contrast-Enhanced Ultrasound (CEUS): A Prospective Study of 50 Patients with Brain Tumours. Acta Neurochir. 2016, 158, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Noronha, S.; Rangaraj, N.; Moiyadi, A.; Shetty, P.; Singh, V.K. Choice of Intraoperative Ultrasound Adjuncts for Brain Tumor Surgery. BMC Med. Inform. Decis. Mak. 2022, 22, 307. [Google Scholar] [CrossRef] [PubMed]
- Moiyadi, A.V.; Shetty, P.M.; Mahajan, A.; Udare, A.; Sridhar, E. Usefulness of Three-Dimensional Navigable Intraoperative Ultrasound in Resection of Brain Tumors with a Special Emphasis on Malignant Gliomas. Acta Neurochir. 2013, 155, 2217–2225. [Google Scholar] [CrossRef]
- Noh, T.; Mustroph, M.; Golby, A.J. Intraoperative Imaging for High-Grade Glioma Surgery. Neurosurg. Clin. N. Am. 2021, 32, 47–54. [Google Scholar] [CrossRef]
- Trevisi, G.; Barbone, P.; Treglia, G.; Mattoli, M.V.; Mangiola, A. Reliability of Intraoperative Ultrasound in Detecting Tumor Residual after Brain Diffuse Glioma Surgery: A Systematic Review and Meta-Analysis. Neurosurg. Rev. 2020, 43, 1221–1233. [Google Scholar] [CrossRef]
- Jaber, M.; Wölfer, J.; Ewelt, C.; Holling, M.; Hasselblatt, M.; Niederstadt, T.; Zoubi, T.; Weckesser, M.; Stummer, W. The Value of 5-Aminolevulinic Acid in Low-Grade Gliomas and High-Grade Gliomas Lacking Glioblastoma Imaging Features: An Analysis Based on Fluorescence, Magnetic Resonance Imaging, 18F-Fluoroethyl Tyrosine Positron Emission Tomography, and Tumor Molecular Factors. Neurosurgery 2016, 78, 401–411. [Google Scholar] [CrossRef]
- Stummer, W.; Novotny, A.; Stepp, H.; Goetz, C.; Bise, K.; Reulen, H.J. Fluorescence-Guided Resection of Glioblastoma Multiforme by Using 5-Aminolevulinic Acid-Induced Porphyrins: A Prospective Study in 52 Consecutive Patients. J. Neurosurg. 2000, 93, 1003–1013. [Google Scholar] [CrossRef]
- Höhne, J.; Schebesch, K.-M.; Zoubaa, S.; Proescholdt, M.; Riemenschneider, M.J.; Schmidt, N.O. Intraoperative Imaging of Brain Tumors with Fluorescein: Confocal Laser Endomicroscopy in Neurosurgery. Clinical and User Experience. Neurosurg. Focus 2021, 50, E19. [Google Scholar] [CrossRef]
- Nabi, Z.; Reddy, D.N. Optical Biopsy in Gastroenterology: Focus on Confocal Laser Endomicroscopy. Indian. J. Gastroenterol. 2019, 38, 281–286. [Google Scholar] [CrossRef]
- Martirosyan, N.L.; Eschbacher, J.M.; Kalani, M.Y.S.; Turner, J.D.; Belykh, E.; Spetzler, R.F.; Nakaji, P.; Preul, M.C. Prospective Evaluation of the Utility of Intraoperative Confocal Laser Endomicroscopy in Patients with Brain Neoplasms Using Fluorescein Sodium: Experience with 74 Cases. Neurosurg. Focus 2016, 40, E11. [Google Scholar] [CrossRef] [PubMed]
- Kuzmin, N.V.; Wesseling, P.; Hamer, P.C.D.W.; Noske, D.P.; Galgano, G.D.; Mansvelder, H.D.; Baayen, J.C.; Groot, M.L. Third Harmonic Generation Imaging for Fast, Label-Free Pathology of Human Brain Tumors. Biomed. Opt. Express 2016, 7, 1889–1904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; de Munck, J.C.; Verburg, N.; Rozemuller, A.J.; Vreuls, W.; Cakmak, P.; van Huizen, L.M.G.; Idema, S.; Aronica, E.; de Witt Hamer, P.C.; et al. Quantitative Third Harmonic Generation Microscopy for Assessment of Glioma in Human Brain Tissue. Adv. Sci. 2019, 6, 1900163. [Google Scholar] [CrossRef] [PubMed]
- Blokker, M.; Hamer, P.C.D.W.; Wesseling, P.; Groot, M.L.; Veta, M. Fast Intraoperative Histology-Based Diagnosis of Gliomas with Third Harmonic Generation Microscopy and Deep Learning. Sci. Rep. 2022, 12, 11334. [Google Scholar] [CrossRef]
- Jiang, C.; Chowdury, A.; Hou, X.; Kondepudi, A.; Freudiger, C.W.; Conway, K.; Camelo-Piragua, S.; Orringer, D.A.; Lee, H.; Hollon, T.C. OpenSRH: Optimizing Brain Tumor Surgery Using Intraoperative Stimulated Raman Histology. Adv. Neural Inf. Process Syst. 2022, 35, 28502–28516. [Google Scholar] [PubMed]
- Xu, J.; Meng, Y.; Qiu, K.; Topatana, W.; Li, S.; Wei, C.; Chen, T.; Chen, M.; Ding, Z.; Niu, G. Applications of Artificial Intelligence Based on Medical Imaging in Glioma: Current State and Future Challenges. Front. Oncol. 2022, 12, 892056. [Google Scholar] [CrossRef]
- Cakmakci, D.; Karakaslar, E.O.; Ruhland, E.; Chenard, M.-P.; Proust, F.; Piotto, M.; Namer, I.J.; Cicek, A.E. Machine Learning Assisted Intraoperative Assessment of Brain Tumor Margins Using HRMAS NMR Spectroscopy. PLoS Comput. Biol. 2020, 16, e1008184. [Google Scholar] [CrossRef]
- Juarez-Chambi, R.M.; Kut, C.; Rico-Jimenez, J.J.; Chaichana, K.L.; Xi, J.; Campos-Delgado, D.U.; Rodriguez, F.J.; Quinones-Hinojosa, A.; Li, X.; Jo, J.A. AI-Assisted In Situ Detection of Human Glioma Infiltration Using a Novel Computational Method for Optical Coherence Tomography. Clin. Cancer Res. 2019, 25, 6329–6338. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, D.; von Spreckelsen, N.; Mawrin, C.; Ion-Margineanu, A.; Fürtjes, G.; Jünger, S.T.; Khalid, F.; Freudiger, C.W.; Timmer, M.; Ruge, M.I.; et al. Novel Rapid Intraoperative Qualitative Tumor Detection by a Residual Convolutional Neural Network Using Label-Free Stimulated Raman Scattering Microscopy. Acta Neuropathol. Commun. 2022, 10, 109. [Google Scholar] [CrossRef]
- Young, J.S.; Al-Adli, N.; Scotford, K.; Cha, S.; Berger, M.S. Pseudoprogression versus True Progression in Glioblastoma: What Neurosurgeons Need to Know. J. Neurosurg. 2023, 139, 748–759. [Google Scholar] [CrossRef]
- Hollon, T.C.; Pandian, B.; Urias, E.; Save, A.V.; Adapa, A.R.; Srinivasan, S.; Jairath, N.K.; Farooq, Z.; Marie, T.; Al-Holou, W.N.; et al. Rapid, Label-Free Detection of Diffuse Glioma Recurrence Using Intraoperative Stimulated Raman Histology and Deep Neural Networks. Neuro Oncol. 2020, 23, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, C.; Pagès-Gallego, M.; Kester, L.; Kranendonk, M.E.G.; Wesseling, P.; Verburg, N.; de Witt Hamer, P.; Kooi, E.J.; Dankmeijer, L.; van der Lugt, J.; et al. Ultra-Fast Deep-Learned CNS Tumour Classification during Surgery. Nature 2023, 622, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Djirackor, L.; Halldorsson, S.; Niehusmann, P.; Leske, H.; Capper, D.; Kuschel, L.P.; Pahnke, J.; Due-Tønnessen, B.J.; Langmoen, I.A.; Sandberg, C.J.; et al. Intraoperative DNA Methylation Classification of Brain Tumors Impacts Neurosurgical Strategy. Neurooncol. Adv. 2021, 3, vdab149. [Google Scholar] [CrossRef] [PubMed]
| Technique | Use | Method | Feasibility Requirements | ||
|---|---|---|---|---|---|
| Expertise/Training | Resources | Time | |||
| SRH | Margins | Label-free | High | High | Medium |
| FGS | Margins/EOR | Fluorescent dye | High | Medium | Low |
| MSI | Margins | Label-free | High | High | High |
| iMRI | EOR | Radiology | Medium | High | High |
| iUS | EOR | Radiology | Low | Medium | Low |
| CLE | Margins | Label-free | High | High | Medium |
| THGM | Margins | Label-free | High | High | Medium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Adli, N.N.; Young, J.S.; Scotford, K.; Sibih, Y.E.; Payne, J.; Berger, M.S. Advances in Intraoperative Glioma Tissue Sampling and Infiltration Assessment. Brain Sci. 2023, 13, 1637. https://doi.org/10.3390/brainsci13121637
Al-Adli NN, Young JS, Scotford K, Sibih YE, Payne J, Berger MS. Advances in Intraoperative Glioma Tissue Sampling and Infiltration Assessment. Brain Sciences. 2023; 13(12):1637. https://doi.org/10.3390/brainsci13121637
Chicago/Turabian StyleAl-Adli, Nadeem N., Jacob S. Young, Katie Scotford, Youssef E. Sibih, Jessica Payne, and Mitchel S. Berger. 2023. "Advances in Intraoperative Glioma Tissue Sampling and Infiltration Assessment" Brain Sciences 13, no. 12: 1637. https://doi.org/10.3390/brainsci13121637
APA StyleAl-Adli, N. N., Young, J. S., Scotford, K., Sibih, Y. E., Payne, J., & Berger, M. S. (2023). Advances in Intraoperative Glioma Tissue Sampling and Infiltration Assessment. Brain Sciences, 13(12), 1637. https://doi.org/10.3390/brainsci13121637






