Decoding Chiari Malformation and Syringomyelia: From Epidemiology and Genetics to Advanced Diagnosis and Management Strategies
Abstract
:1. Introduction
1.1. Brief Overview of Chiari Malformation, Syringomyelia and Related Disorders
1.2. Materials and Methods
1.2.1. Tonsillar Configuration
1.2.2. Magnetic Resonance Imaging (MRI)—Craniocervical Junction—Dynamic Evaluation
Dynamic Flow Studies
1.2.3. Magnetic Resonance Imaging (MRI)—Spinal Evaluation
Syringomyelia
1.3. Tethered Cord Syndrome
2. Epidemiology of Chiari Malformation, Syringomyelia and Related Disorders
2.1. Global Prevalence and Distribution: Demographics Affected
2.2. Temporal Trends
3. Pathophysiology of Chiari: Hydrodynamics of Cerebro-Spinal Fluid Flow
3.1. An Overview of the Normal Cerebro-Spinal Fluid Flow
3.2. Changes Observed in Chiari Malformation
3.3. Implications of These Changes on Overall Health
4. Pathogenesis of Syringomyelia: Lessons from Observations in Dogs
4.1. Summarized Key Findings from Canine Studies
4.2. Extrapolation to Human Pathogenesis
Caudal Cranial Fossa (CCF) Anatomy
5. The Role of Genetics in Chiari Malformation and Syringomyelia
5.1. Current Understanding of the Genetic Basis
5.2. Identified Genes and Their Impact
5.3. Potential Avenues for Future Research
6. Clinical Phenotypes in Chiari and Syringomeylia
Presentation and Clinical Features in Chiari Malformation
7. Diagnostic Investigations: Old and New Neurophysiological Methods
7.1. From Traditional Diagnostic Methods to Newer Neurophysiological Techniques—Comparison and Evaluation of Effectiveness
7.1.1. Introduction
7.1.2. Computed Tomography (CT)
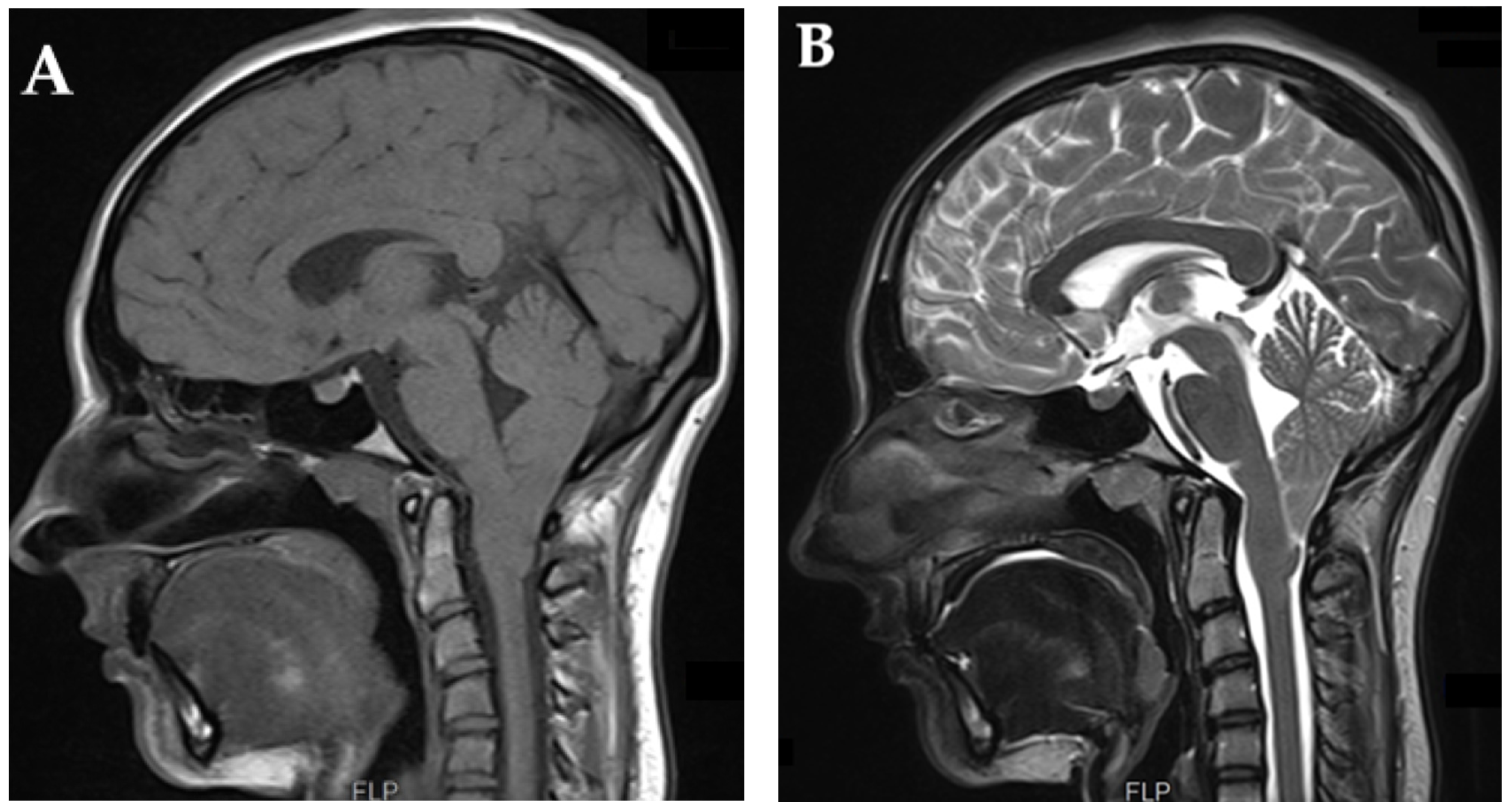
7.1.3. Magnetic Resonance Imaging (MRI)—Brain
Tonsillar Herniation
7.2. Newer Neurophysiological Techniques
7.2.1. The Size of Posterior Cranial Fossa (PCF)
7.2.2. Hydrocephalus
8. Surgery in Chiari 1 Malformation with and without Syringomyelia
8.1. Indications for Surgery
8.2. Current Surgical Techniques and Their Outcomes
8.3. Conclusions
9. Surgical Strategies in Isolated Syringomyelia
9.1. Indications for Surgery
9.2. Overview of Different Surgical Strategies/Effectiveness and Outcomes
9.2.1. Study Profile
9.2.2. Monitoring Asymptomatic Patients
9.2.3. Criteria for Surgery
9.2.4. Treatment Preferences
9.2.5. Surgical Outcomes
10. Outcome Measures in Chiari and Syringomyelia Long-Term Follow-Up
10.1. Parameters for Assessing Outcomes
10.2. Long-Term Prognosis Post-Surgical Interventions
11. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bejjani, G.K. Definition of the adult Chiari malformation: A brief historical overview. Neurosurg. Focus 2001, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Armonda, R.A.; Citrin, C.M.; Foley, K.T.; Ellenbogen, R.G. Quantitative cine-mode magnetic resonance imaging of Chiari I malformations: An analysis of cerebrospinal fluid dynamics. Neurosurgery 1994, 35, 214–223, discussion 223–224. [Google Scholar] [CrossRef] [PubMed]
- Appleby, A.; Foster, J.B.; Hankinson, J.; Hudgson, P. The diagnosis and management of the Chiari anomalies in adult life. Brain J. Neurol. 1968, 91, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Klekamp, J. The pathophysiology of syringomyelia—Historical overview and current concept. Acta Neurochir. (Wien) 2002, 144, 649–664. [Google Scholar] [CrossRef]
- Levine, D.N. The pathogenesis of syringomyelia associated with lesions at the foramen magnum: A critical review of existing theories and proposal of a new hypothesis. J. Neurol. Sci. 2004, 220, 3–21. [Google Scholar] [CrossRef]
- Greitz, D. Unraveling the riddle of syringomyelia. Neurosurg. Rev. 2006, 29, 251–264. [Google Scholar] [CrossRef]
- Catmull, S.; Ashurst, J. Tethered Cord Syndrome. Clin. Pract. Cases Emerg. Med. 2019, 3, 297–298. [Google Scholar] [CrossRef]
- Elster, A.D.; Chen, M.Y. Chiari I malformations: Clinical and radiologic reappraisal. Radiology 1992, 183, 347–353. [Google Scholar] [CrossRef]
- Meadows, J.; Kraut, M.; Guarnieri, M.; Haroun, R.I.; Carson, B.S. Asymptomatic Chiari Type I malformations identified on magnetic resonance imaging. J. Neurosurg. 2000, 92, 920–926. [Google Scholar] [CrossRef]
- Spinos, E.; Laster, D.W.; Moody, D.M.; Ball, M.R.; Witcofski, R.L.; Kelly, D.L. MR evaluation of Chiari I malformations at 0.15 T. Am. J. Neuroradiol. 1985, 6, 203–208. [Google Scholar] [CrossRef]
- Smith, B.W.; Strahle, J.; Bapuraj, J.R.; Muraszko, K.M.; Garton, H.J.; Maher, C.O. Distribution of cerebellar tonsil position: Implications for understanding Chiari malformation. J. Neurosurg. 2013, 119, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.H. Monro-Kellie 2.0: The dynamic vascular and venous pathophysiological components of intracranial pressure. J. Cereb. Blood Flow Metab. 2016, 36, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Whedon, J.M.; Glassey, D. Cerebrospinal fluid stasis and its clinical significance. Altern. Ther. Health Med. 2009, 15, 54–60. [Google Scholar] [PubMed]
- Ohara, S.; Nagai, H.; Matsumoto, T.; Banno, T. MR imaging of CSF pulsatory flow and its relation to intracranial pressure. J. Neurosurg. 1988, 69, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Haughton, V.M.; Korosec, F.R.; Medow, J.E.; Dolar, M.T.; Iskandar, B.J. Peak systolic and diastolic CSF velocity in the foramen magnum in adult patients with Chiari I malformations and in normal control participants. AJNR Am. J. Neuroradiol. 2003, 24, 169–176. [Google Scholar] [PubMed]
- Quigley, M.F.; Iskandar, B.; Quigley, M.A.; Nicosia, M.; Haughton, V. Cerebrospinal Fluid Flow in Foramen Magnum: Temporal and Spatial Patterns at MR Imaging in Volunteers and in Patients with Chiari I Malformation. Radiology 2004, 232, 229–236. [Google Scholar] [CrossRef]
- Pujol, J.; Roig, C.; Capdevila, A.; Pou, A.; Marti-Vilalta, J.L.; Kulisevsky, J.; Escartin, A.; Zannoli, G. Motion of the cerebellar tonsils in Chiari type I malformation studied by cine phase-contrast MRI. Neurology 1995, 45, 1746–1753. [Google Scholar] [CrossRef]
- Wolpert, S.M.; Bhadelia, R.A.; Bogdan, A.R.; Cohen, A.R. Chiari I malformations: Assessment with phase-contrast velocity MR. AJNR Am. J. Neuroradiol. 1994, 15, 1299–1308. [Google Scholar]
- Hofkes, S.K.; Iskandar, B.J.; Turski, P.A.; Gentry, L.R.; McCue, J.B.; Haughton, V.M. Differentiation between Symptomatic Chiari I Malformation and Asymptomatic Tonsilar Ectopia by Using Cerebrospinal Fluid Flow Imaging: Initial Estimate of Imaging Accuracy. Radiology 2007, 245, 532–540. [Google Scholar] [CrossRef]
- Krueger, K.D.; Haughton, V.M.; Hetzel, S. Peak CSF Velocities in Patients with Symptomatic and Asymptomatic Chiari I Malformation. Am. J. Neuroradiol. 2010, 31, 1837–1841. [Google Scholar] [CrossRef]
- Bhadelia, R.A.; Bogdan, A.R.; Wolpert, S.M.; Lev, S.; Appignani, B.A.; Heilman, C.B. Cerebrospinal fluid flow waveforms: Analysis in patients with Chiari I malformation by means of gated phase-contrast MR imaging velocity measurements. Radiology 1995, 196, 195–202. [Google Scholar] [CrossRef] [PubMed]
- McGirt, M.J.; Nimjee, S.M.; Fuchs, H.E.; George, T.M. Relationship of cine phase-contrast magnetic resonance imaging with outcome after decompression for Chiari I malformations. Neurosurgery 2006, 59, 140–146, discussion 140–146. [Google Scholar] [CrossRef] [PubMed]
- Ventureyra, E.C.G.; Aziz, H.A.; Vassilyadi, M. The role of cine flow MRI in children with Chiari I malformation. Childs Nerv. Syst. 2003, 19, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Holly, L.T.; Batzdorf, U. Chiari malformation and syringomyelia. J. Neurosurg. Spine 2019, 31, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Timpone, V.M.; Patel, S.H. MRI of a Syrinx: Is Contrast Material Always Necessary? Am. J. Roentgenol. 2015, 204, 1082–1085. [Google Scholar] [CrossRef]
- Strahle, J.; Muraszko, K.M.; Kapurch, J.; Bapuraj, J.R.; Garton, H.J.L.; Maher, C.O. Chiari malformation Type I and syrinx in children undergoing magnetic resonance imaging. J. Neurosurg. Pediatr. 2011, 8, 205–213. [Google Scholar] [CrossRef]
- Strahle, J.; Muraszko, K.M.; Garton, H.J.L.; Smith, B.W.; Starr, J.; Kapurch, J.R.; Maher, C.O. Syrinx location and size according to etiology: Identification of Chiari-associated syrinx. J. Neurosurg. Pediatr. 2015, 16, 21–29. [Google Scholar] [CrossRef]
- Milhorat, T.H.; Bolognese, P.A.; Nishikawa, M.; Francomano, C.A.; McDonnell, N.B.; Roonprapunt, C.; Kula, R.W. Association of Chiari malformation type I and tethered cord syndrome: Preliminary results of sectioning filum terminale. Surg. Neurol. 2009, 72, 20–35. [Google Scholar] [CrossRef]
- Hertzler, D.A.; DePowell, J.J.; Stevenson, C.B.; Mangano, F.T. Tethered cord syndrome: A review of the literature from embryology to adult presentation. Neurosurg. Focus 2010, 29, E1. [Google Scholar] [CrossRef]
- Seki, T.; Hida, K.; Yano, S.; Sasamori, T.; Hamauch, S.; Koyanagi, I.; Houkin, K. Surgical Outcome of Children and Adolescents with Tethered Cord Syndrome. Asian Spine J. 2016, 10, 940–944. [Google Scholar] [CrossRef]
- Tandon, V.; Garg, K.; Kumar, R.; Mahapatra, A.; Sharma, B. Management of adult tethered cord syndrome: Our experience and review of literature. Neurol. India 2014, 62, 137. [Google Scholar] [CrossRef]
- Kahn, E.N.; Muraszko, K.M.; Maher, C.O. Prevalence of Chiari I Malformation and Syringomyelia. Neurosurg. Clin. N. Am. 2015, 26, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Aitken, L.A.; Lindan, C.E.; Sidney, S.; Gupta, N.; Barkovich, A.J.; Sorel, M.; Wu, Y.W. Chiari Type I Malformation in a Pediatric Population. Pediatr. Neurol. 2009, 40, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Luzzi, S.; Giotta Lucifero, A.; Elsawaf, Y.; Elbabaa, S.K.; Del Maestro, M.; Savioli, G.; Galzio, R.; Gragnaniello, C. Pulsatile cerebrospinal fluid dynamics in Chiari I malformation syringomyelia: Predictive value in posterior fossa decompression and insights into the syringogenesis. J. Craniovertebral Junction Spine 2021, 12, 15–25. [Google Scholar] [CrossRef]
- Wu, J.; Ji, S.; Niu, P.; Zhang, B.; Shao, D.; Li, Y.; Xie, S.; Jiang, Z. Knowledge mapping of syringomyelia from 2003 to 2022: A bibliometric analysis. J. Clin. Neurosci. 2023, 110, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Sakka, L.; Coll, G.; Chazal, J. Anatomy and physiology of cerebrospinal fluid. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 309–316. [Google Scholar] [CrossRef]
- Spector, R.; Robert Snodgrass, S.; Johanson, C.E. A balanced view of the cerebrospinal fluid composition and functions: Focus on adult humans. Exp. Neurol. 2015, 273, 57–68. [Google Scholar] [CrossRef]
- Sekula, R.F.; Jannetta, P.J.; Casey, K.F.; Marchan, E.M.; Sekula, L.K.; McCrady, C.S. Dimensions of the posterior fossa in patients symptomatic for Chiari I malformation but without cerebellar tonsillar descent. Cerebrospinal Fluid Res. 2005, 2, 11. [Google Scholar] [CrossRef]
- Matsumae, M.; Hirayama, A.; Atsumi, H.; Yatsushiro, S.; Kuroda, K. Velocity and pressure gradients of cerebrospinal fluid assessed with magnetic resonance imaging: Clinical article. J. Neurosurg. 2014, 120, 218–227. [Google Scholar] [CrossRef]
- Yiallourou, T.I.; Kröger, J.R.; Stergiopulos, N.; Maintz, D.; Martin, B.A.; Bunck, A.C. Comparison of 4D Phase-Contrast MRI Flow Measurements to Computational Fluid Dynamics Simulations of Cerebrospinal Fluid Motion in the Cervical Spine. PLoS ONE 2012, 7, e52284. [Google Scholar] [CrossRef]
- Shaffer, N.; Martin, B.A.; Rocque, B.; Madura, C.; Wieben, O.; Iskandar, B.J.; Dombrowski, S.; Luciano, M.; Oshinski, J.N.; Loth, F. Cerebrospinal fluid flow impedance is elevated in Type I Chiari malformation. J. Biomech. Eng. 2014, 136, 021012. [Google Scholar] [CrossRef] [PubMed]
- Sarnat, H.B. Disorders of segmentation of the neural tube: Chiari malformations. Handb. Clin. Neurol. 2008, 87, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, K.L. Chiari Type II malformation: Past, present, and future. Neurosurg. Focus 2004, 16, 1–7. [Google Scholar] [CrossRef]
- Lee, R.; Tai, K.S.; Cheng, P.W.; Lui, W.M.; Chan, F.L. Chiari III malformation: Antenatal MRI diagnosis. Clin. Radiol. 2002, 57, 759–761. [Google Scholar] [CrossRef] [PubMed]
- Caldarelli, M.; Rea, G.; Cincu, R.; Di Rocco, C. Chiari type III malformation. Childs Nerv. Syst. ChNS Off. J. Int. Soc. Pediatr. Neurosurg. 2002, 18, 207–210. [Google Scholar] [CrossRef]
- Tubbs, R.S.; Demerdash, A.; Vahedi, P.; Griessenauer, C.J.; Oakes, W.J. Chiari IV malformation: Correcting an over one century long historical error. Childs Nerv. Syst. 2016, 32, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- McDougall, C.M.; Alarfaj, A.K.; Jack, A.S.; Souster, J.; Broad, R.W. Klippel-Feil syndrome in association with Chiari zero malformation in the setting of acute traumatic spinal cord injury. Interdiscip. Neurosurg. 2017, 9, 1–3. [Google Scholar] [CrossRef]
- Bogdanov, E.I.; Faizutdinova, A.T.; Heiss, J.D. Posterior cranial fossa and cervical spine morphometric abnormalities in symptomatic Chiari type 0 and Chiari type 1 malformation patients with and without syringomyelia. Acta Neurochir. (Wien) 2021, 163, 3051–3064. [Google Scholar] [CrossRef]
- Sandu, R.B.; Pantiru, M.I.; Cosman, M.; Poeata, I. Syringobulbia and syringomyelia in a case with Chiari 0 malformation successfully treated by posterior fossa reconstruction: Case presentation and literature review. Romanian Neurosurg. 2019, 33, 239–242. [Google Scholar] [CrossRef]
- Czosnyka, M.; Czosnyka, Z.; Momjian, S.; Pickard, J.D. Cerebrospinal fluid dynamics. Physiol. Meas. 2004, 25, R51–R76. [Google Scholar] [CrossRef]
- Williams, H. A unifying hypothesis for hydrocephalus, Chiari malformation, syringomyelia, anencephaly and spina bifida. Cerebrospinal Fluid Res. 2008, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.E.; Knowler, S.P.; Rusbridge, C.; Noorman, E.; Jeffery, N.D. Prevalence of asymptomatic syringomyelia in Cavalier King Charles spaniels. Vet. Rec. 2011, 168, 667. [Google Scholar] [CrossRef] [PubMed]
- Wijnrocx, K.; Van Bruggen, L.W.L.; Eggelmeijer, W.; Noorman, E.; Jacques, A.; Buys, N.; Janssens, S.; Mandigers, P.J.J. Twelve years of chiari-like malformation and syringomyelia scanning in Cavalier King Charles Spaniels in the Netherlands: Towards a more precise phenotype. PLoS ONE 2017, 12, e0184893. [Google Scholar] [CrossRef] [PubMed]
- Hechler, A.C.; Moore, S.A. Understanding and Treating Chiari-like Malformation and Syringomyelia in Dogs. Top. Companion Anim. Med. 2018, 33, 1–11. [Google Scholar] [CrossRef]
- Ono, A.; Numasawa, T.; Wada, K.; Yokoyama, T.; Takeuchi, K.; Suetsuna, F.; Ueyama, K.; Toh, S. Surgical outcomes of foramen magnum decompression for syringomyelia associated with Chiari I malformation: Relation between the location of the syrinx and body pain. J. Orthop. Sci. 2010, 15, 299–304. [Google Scholar] [CrossRef]
- Sgouros, S.; Williams, B. Management and outcome of posttraumatic syringomyelia. J. Neurosurg. 1996, 85, 197–205. [Google Scholar] [CrossRef]
- Ushewokunze, S.O.S.; Gan, Y.C.; Phillips, K.; Thacker, K.; Flint, G. Surgical treatment of post-traumatic syringomyelia. Spinal Cord 2010, 48, 710–713. [Google Scholar] [CrossRef]
- Krieger, M.D.; McComb, J.G.; Levy, M.L. Toward a simpler surgical management of Chiari I malformation in a pediatric population. Pediatr. Neurosurg. 1999, 30, 113–121. [Google Scholar] [CrossRef]
- Akbari, S.H.A.; Rizvi, A.A.; CreveCoeur, T.S.; Han, R.H.; Greenberg, J.K.; Torner, J.; Brockmeyer, D.L.; Wellons, J.C.; Leonard, J.R.; Mangano, F.T.; et al. Socioeconomic and demographic factors in the diagnosis and treatment of Chiari malformation type I and syringomyelia. J. Neurosurg. Pediatr. 2022, 29, 288–297. [Google Scholar] [CrossRef]
- Sakushima, K.; Tsuboi, S.; Yabe, I.; Hida, K.; Terae, S.; Uehara, R.; Nakano, I.; Sasaki, H. Nationwide survey on the epidemiology of syringomyelia in Japan. J. Neurol. Sci. 2012, 313, 147–152. [Google Scholar] [CrossRef]
- Ciaramitaro, P.; Migliaretti, G.; Ferraris, M.; Garnero, A.; Morana, G.; Carucci, P.; Stura, I.; Massaro, F.; Garbossa, D. Syringomyelia Associated with Chiari 1 Malformation in Adults: Positive Outcome Predictors after Posterior Fossa Decompression with Duraplasty. J. Clin. Med. 2023, 12, 3019. [Google Scholar] [CrossRef]
- Siasios, J.; Kapsalaki, E.Z.; Fountas, K.N. Surgical management of patients with Chiari I malformation. Int. J. Pediatr. 2012, 2012, 640127. [Google Scholar] [CrossRef]
- Haroun, R.I.; Guarnieri, M.; Meadow, J.J.; Kraut, M.; Carson, B.S. Current Opinions for the Treatment of Syringomyelia and Chiari Malformations: Survey of the Pediatric Section of the American Association of Neurological Surgeons. Pediatr. Neurosurg. 2000, 33, 311–317. [Google Scholar] [CrossRef]
- Attenello, F.J.; McGirt, M.J.; Gathinji, M.; Datoo, G.; Atiba, A.; Weingart, J.; Carson, B.; Jallo, G.I. Outcome of Chiari-associated syringomyelia after hindbrain decompression in children: Analysis of 49 consecutive cases. Neurosurgery 2008, 62, 1307–1313, discussion 1313. [Google Scholar] [CrossRef]
- Sgouros, S.; Kountouri, M.; Natarajan, K. Posterior fossa volume in children with Chiari malformation Type I. J. Neurosurg. Pediatr. 2006, 105, 101–106. [Google Scholar] [CrossRef]
- Driver, C.J.; Rusbridge, C.; Cross, H.R.; McGonnell, I.; Volk, H.A. Relationship of brain parenchyma within the caudal cranial fossa and ventricle size to syringomyelia in cavalier King Charles spaniels. J. Small Anim. Pract. 2010, 51, 382–386. [Google Scholar] [CrossRef]
- Shaw, T.A.; McGonnell, I.M.; Driver, C.J.; Rusbridge, C.; Volk, H.A. Increase in Cerebellar Volume in Cavalier King Charles Spaniels with Chiari-like Malformation and Its Role in the Development of Syringomyelia. PLoS ONE 2012, 7, e33660. [Google Scholar] [CrossRef]
- Olsen, E.; Suiter, E.J.; Pfau, T.; McGonnell, I.M.; Matiasek, K.; Giejda, A.; Volk, H.A. Cavalier King Charles Spaniels with Chiari-like malformation and Syringomyelia have increased variability of spatio-temporal gait characteristics. BMC Vet. Res. 2017, 13, 159. [Google Scholar] [CrossRef]
- Cinalli, G.; Spennato, P.; Sainte-Rose, C.; Arnaud, E.; Aliberti, F.; Brunelle, F.; Cianciulli, E.; Renier, D. Chiari malformation in craniosynostosis. Childs Nerv. Syst. 2005, 21, 889–901. [Google Scholar] [CrossRef]
- Carrera, I.; Dennis, R.; Mellor, D.J.; Penderis, J.; Sullivan, M. Use of magnetic resonance imaging for morphometric analysis of the caudal cranial fossa in Cavalier King Charles Spaniels. Am. J. Vet. Res. 2009, 70, 340–345. [Google Scholar] [CrossRef] [PubMed]
- McBratney-Owen, B.; Iseki, S.; Bamforth, S.D.; Olsen, B.R.; Morriss-Kay, G.M. Development and tissue origins of the mammalian cranial base. Dev. Biol. 2008, 322, 121–132. [Google Scholar] [CrossRef]
- Driver, C.J.; Volk, H.A.; Rusbridge, C.; Van Ham, L.M. An update on the pathogenesis of syringomyelia secondary to Chiari-like malformations in dogs. Vet. J. 2013, 198, 551–559. [Google Scholar] [CrossRef]
- Nagy, L.; Mobley, J.; Ray, C. Familial Aggregation of Chiari Malformation: Presentation, Pedigree, and Review of the Literature. Turk. Neurosurg. 2016, 26, 315–320. [Google Scholar] [CrossRef]
- AvŞar, T.; ÇaliŞ, Ş.; Yilmaz, B.; Demİrcİ OtluoĞlu, G.; Holyavkİn, C.; KiliÇ, T. Genome-wide identification of Chiari malformation type I associated candidate genes and chromosomal variations. Turk. J. Biol. Turk Biyol. Derg. 2020, 44, 449–456. [Google Scholar] [CrossRef]
- Merello, E.; Tattini, L.; Magi, A.; Accogli, A.; Piatelli, G.; Pavanello, M.; Tortora, D.; Cama, A.; Kibar, Z.; Capra, V.; et al. Exome sequencing of two Italian pedigrees with non-isolated Chiari malformation type I reveals candidate genes for cranio-facial development. Eur. J. Hum. Genet. EJHG 2017, 25, 952–959. [Google Scholar] [CrossRef]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018, 46, D1062–D1067. [Google Scholar] [CrossRef]
- Ancot, F.; Lemay, P.; Knowler, S.P.; Kennedy, K.; Griffiths, S.; Cherubini, G.B.; Sykes, J.; Mandigers, P.J.J.; Rouleau, G.A.; Rusbridge, C.; et al. A genome-wide association study identifies candidate loci associated to syringomyelia secondary to Chiari-like malformation in Cavalier King Charles Spaniels. BMC Genet. 2018, 19, 16. [Google Scholar] [CrossRef]
- Urbizu, A.; Toma, C.; Poca, M.A.; Sahuquillo, J.; Cuenca-León, E.; Cormand, B.; Macaya, A. Chiari Malformation Type I: A Case-Control Association Study of 58 Developmental Genes. PLoS ONE 2013, 8, e57241. [Google Scholar] [CrossRef]
- Urbizu, A.; Garrett, M.E.; Soldano, K.; Drechsel, O.; Loth, D.; Marcé-Grau, A.; Mestres I Soler, O.; Poca, M.A.; Ossowski, S.; Macaya, A.; et al. Rare functional genetic variants in COL7A1, COL6A5, COL1A2 and COL5A2 frequently occur in Chiari Malformation Type 1. PLoS ONE 2021, 16, e0251289. [Google Scholar] [CrossRef]
- Xiaoshuai, L.; Qiushi, W.; Rui, W. Advantages of CRISPR-Cas9 combined organoid model in the study of congenital nervous system malformations. Front. Bioeng. Biotechnol. 2022, 10, 932936. [Google Scholar] [CrossRef]
- McVige, J.W.; Leonardo, J. Imaging of Chiari type I malformation and syringohydromyelia. Neurol. Clin. 2014, 32, 95–126. [Google Scholar] [CrossRef]
- Stephen, D.; Silberstein, R.B.; Lipton, D.W.; Dodick, S.D.; Lipton, R.B.; Dodick, D.; Wolff, H.G. (Eds.) Wolff’s Headache and Other Head Pain, 8th ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2008; ISBN 978-0-19-532656-7. [Google Scholar]
- Pascual, J.; Oterino, A.; Berciano, J. Headache in type I Chiari malformation. Neurology 1992, 42, 1519. [Google Scholar] [CrossRef]
- Toldo, I.; Tangari, M.; Mardari, R.; Perissinotto, E.; Sartori, S.; Gatta, M.; Calderone, M.; Battistella, P.A. Headache in Children With C hiari I Malformation. Headache J. Head Face Pain 2014, 54, 899–908. [Google Scholar] [CrossRef]
- Wu, Y.W.; Chin, C.T.; Chan, K.M.; Barkovich, A.J.; Ferriero, D.M. Pediatric Chiari I malformations: Do clinical and radiologic features correlate? Neurology 1999, 53, 1271–1276. [Google Scholar] [CrossRef]
- Kim, H.G.; Oh, H.S.; Kim, T.W.; Park, K.H. Clinical Features of Post-Traumatic Syringomyelia. Korean J. Neurotrauma 2014, 10, 66–69. [Google Scholar] [CrossRef]
- Tam, S.K.P.; Chia, J.; Brodbelt, A.; Foroughi, M. Assessment of patients with a Chiari malformation type I. Brain Spine 2022, 2, 100850. [Google Scholar] [CrossRef]
- Hayward, R. VOMIT (victims of modern imaging technology)—An acronym for our times. Bmj 2003, 326, 1273. [Google Scholar] [CrossRef]
- Langridge, B.; Phillips, E.; Choi, D. Chiari Malformation Type 1: A Systematic Review of Natural History and Conservative Management. World Neurosurg. 2017, 104, 213–219. [Google Scholar] [CrossRef]
- Chazen, J.L.; Talbott, J.F.; Lantos, J.E.; Dillon, W.P. MR myelography for identification of spinal CSF leak in spontaneous intracranial hypotension. AJNR Am. J. Neuroradiol. 2014, 35, 2007–2012. [Google Scholar] [CrossRef]
- Aboulezz, A.O.; Sartor, K.; Geyer, C.A.; Gado, M.H. Position of cerebellar tonsils in the normal population and in patients with Chiari malformation: A quantitative approach with MR imaging. J. Comput. Assist. Tomogr. 1985, 9, 1033–1036. [Google Scholar] [CrossRef]
- Mikulis, D.J.; Diaz, O.; Egglin, T.K.; Sanchez, R. Variance of the position of the cerebellar tonsils with age: Preliminary report. Radiology 1992, 183, 725–728. [Google Scholar] [CrossRef]
- Jussila, M.-P.; Nissilä, J.; Vakkuri, M.; Olsén, P.; Niinimäki, J.; Leinonen, V.; Serlo, W.; Salokorpi, N.; Suo-Palosaari, M. Preoperative measurements on MRI in Chiari 1 patients fail to predict outcome after decompressive surgery. Acta Neurochir. (Wien) 2021, 163, 2005–2014. [Google Scholar] [CrossRef]
- Milhorat, T.H.; Chou, M.W.; Trinidad, E.M.; Kula, R.W.; Mandell, M.; Wolpert, C.; Speer, M.C. Chiari I malformation redefined: Clinical and radiographic findings for 364 symptomatic patients. Neurosurgery 1999, 44, 1005–1017. [Google Scholar] [CrossRef]
- Nishikawa, M.; Sakamoto, H.; Hakuba, A.; Nakanishi, N.; Inoue, Y. Pathogenesis of Chiari malformation: A morphometric study of the posterior cranial fossa. J. Neurosurg. 1997, 86, 40–47. [Google Scholar] [CrossRef]
- Karagöz, F.; Izgi, N.; Sencer, S.K. Morphometric measurements of the cranium in patients with Chiari type I malformation and comparison with the normal population. Acta Neurochir. (Wien) 2002, 144, 165–171. [Google Scholar] [CrossRef]
- Milhorat, T.H.; Nishikawa, M.; Kula, R.W.; Dlugacz, Y.D. Mechanisms of cerebellar tonsil herniation in patients with Chiari malformations as guide to clinical management. Acta Neurochir. (Wien) 2010, 152, 1117–1127. [Google Scholar] [CrossRef]
- Urbizu, A.; Poca, M.-A.; Vidal, X.; Rovira, A.; Sahuquillo, J.; Macaya, A. MRI-based Morphometric Analysis of Posterior Cranial Fossa in the Diagnosis of Chiari Malformation Type I. J. Neuroimaging 2014, 24, 250–256. [Google Scholar] [CrossRef]
- Dufton, J.A.; Habeeb, S.Y.; Heran, M.K.; Mikulis, D.J.; Islam, O. Posterior fossa measurements in patients with and without Chiari I malformation. Can. J. Neurol. Sci. 2011, 38, 452–455. [Google Scholar] [CrossRef]
- Stovner, L.J.; Bergan, U.; Nilsen, G.; Sjaastad, O. Posterior cranial fossa dimensions in the Chiari I malformation: Relation to pathogenesis and clinical presentation. Neuroradiology 1993, 35, 113–118. [Google Scholar] [CrossRef]
- Badie, B.; Mendoza, D.; Batzdorf, U. Posterior fossa volume and response to suboccipital decompression in patients with Chiari I malformation. Neurosurgery 1995, 37, 214–218. [Google Scholar] [CrossRef]
- Noudel, R.; Gomis, P.; Sotoares, G.; Bazin, A.; Pierot, L.; Pruvo, J.-P.; Bordet, R.; Roche, P.-H. Posterior fossa volume increase after surgery for Chiari malformation Type I: A quantitative assessment using magnetic resonance imaging and correlations with the treatment response: Clinical article. J. Neurosurg. 2011, 115, 647–658. [Google Scholar] [CrossRef]
- Tubbs, R.S.; McGirt, M.J.; Oakes, W.J. Surgical experience in 130 pediatric patients with Chiari I malformations. J. Neurosurg. 2003, 99, 291–296. [Google Scholar] [CrossRef]
- Orakdogen, M.; Emon, S.T.; Erdogan, B.; Somay, H. Fourth Ventriculostomy in Occlusion of the Foramen of Magendie Associated with Chiari Malformation and Syringomyelia. NMC Case Rep. J. 2015, 2, 72–75. [Google Scholar] [CrossRef]
- Decq, P.; Guérinel, C.L.; Sol, J.-C.; Brugières, P.; Djindjian, M.; Nguyen, J.-P. Chiari I malformation: A rare cause of noncommunicating hydrocephalus treated by third ventriculostomy. J. Neurosurg. 2001, 95, 783–790. [Google Scholar] [CrossRef]
- Hayhurst, C.; Osman-Farah, J.; Das, K.; Mallucci, C. Initial management of hydrocephalus associated with Chiari malformation Type I-syringomyelia complex via endoscopic third ventriculostomy: An outcome analysis. J. Neurosurg. 2008, 108, 1211–1214. [Google Scholar] [CrossRef]
- Hankinson, T.; Tubbs, R.S.; Wellons, J.C. Duraplasty or not? An evidence-based review of the pediatric Chiari I malformation. Childs Nerv. Syst. ChNS Off. J. Int. Soc. Pediatr. Neurosurg. 2011, 27, 35–40. [Google Scholar] [CrossRef]
- Nash, J.; Cheng, J.S.; Meyer, G.A.; Remler, B.F. Chiari type I malformation: Overview of diagnosis and treatment. WMJ Off. Publ. State Med. Soc. Wis. 2002, 101, 35–40. [Google Scholar]
- Sindou, M.; Gimbert, E. Decompression for Chiari type I-malformation (with or without syringomyelia) by extreme lateral foramen magnum opening and expansile duraplasty with arachnoid preservation: Comparison with other technical modalities (Literature review). Adv. Tech. Stand. Neurosurg. 2009, 34, 85–110. [Google Scholar] [CrossRef]
- Alden, T.D.; Ojemann, J.G.; Park, T.S. Surgical treatment of Chiari I malformation: Indications and approaches. Neurosurg. Focus 2001, 11, E2. [Google Scholar] [CrossRef]
- Sindou, M.; Chávez-Machuca, J.; Hashish, H. Cranio-cervical decompression for Chiari type I-malformation, adding extreme lateral foramen magnum opening and expansile duroplasty with arachnoid preservation. Technique and long-term functional results in 44 consecutive adult cases—Comparison with literature data. Acta Neurochir. (Wien) 2002, 144, 1005–1019. [Google Scholar] [CrossRef]
- Tubbs, R.S.; Lyerly, M.J.; Loukas, M.; Shoja, M.M.; Oakes, W.J. The pediatric Chiari I malformation: A review. Childs Nerv. Syst. ChNS Off. J. Int. Soc. Pediatr. Neurosurg. 2007, 23, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Valentini, L.; Visintini, S.; Saletti, V.; Chiapparini, L.; Estienne, M.; Solero, C.L. Treatment for Chiari 1 malformation (CIM): Analysis of a pediatric surgical series. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2011, 32 (Suppl. S3), S321–S324. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.J.; Neill, J.; Crone, K.R.; Hendrick, E.B.; Humphreys, R.P. Hydrosyringomyelia and its management in childhood. Neurosurgery 1987, 21, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Munshi, I.; Frim, D.; Stine-Reyes, R.; Weir, B.K.; Hekmatpanah, J.; Brown, F. Effects of posterior fossa decompression with and without duraplasty on Chiari malformation-associated hydromyelia. Neurosurgery 2000, 46, 1384–1389, discussion 1389–1390. [Google Scholar] [CrossRef] [PubMed]
- Ono, A.; Suetsuna, F.; Ueyama, K.; Yokoyama, T.; Aburakawa, S.; Takeuchi, K.; Numasawa, T.; Wada, K.; Toh, S. Cervical spinal motion before and after surgery in patients with Chiari malformation type I associated with syringomyelia. J. Neurosurg. Spine 2007, 7, 473–477. [Google Scholar] [CrossRef]
- Milhorat, T.H.; Kotzen, R.M.; Mu, H.T.; Capocelli, A.L.; Milhorat, R.H. Dysesthetic pain in patients with syringomyelia. Neurosurgery 1996, 38, 940–946, discussion 946–947. [Google Scholar] [CrossRef]
- Nakamura, M.; Chiba, K.; Nishizawa, T.; Maruiwa, H.; Matsumoto, M.; Toyama, Y. Retrospective study of surgery-related outcomes in patients with syringomyelia associated with Chiari I malformation: Clinical significance of changes in the size and localization of syrinx on pain relief. J. Neurosurg. 2004, 100, 241–244. [Google Scholar] [CrossRef]
- Goel, A. Basilar invagination, syringomyelia and Chiari formation and their relationship with atlantoaxial instability. Neurol. India 2018, 66, 940. [Google Scholar] [CrossRef]
- Goel, A.; Kaswa, A.; Shah, A. Atlantoaxial Fixation for Treatment of Chiari Formation and Syringomyelia with No Craniovertebral Bone Anomaly: Report of an Experience with 57 Cases. Acta Neurochir. Suppl. 2019, 125, 101–110. [Google Scholar] [CrossRef]
- Goel, A.; Jadhav, D.; Shah, A.; Rai, S.; Dandpat, S.; Vutha, R.; Dhar, A.; Prasad, A. Chiari 1 Formation Redefined-Clinical and Radiographic Observations in 388 Surgically Treated Patients. World Neurosurg. 2020, 141, e921–e934. [Google Scholar] [CrossRef]


| Preoperative Symptoms | Postoperative Outcomes | Atrophy and Spasticity |
|---|---|---|
| Dissociated sensory loss, headache, lower cranial nerve dysfunction, and weakness. | The headache immediately disappeared after surgery, indicating a successful resolution of this symptom. | Atrophy and spasticity were largely unaffected by surgery, suggesting that the treatment may not have a significant impact on these symptoms. |
| The involvement of C2–C5 metameres | All treated patients experienced a full recovery within 6 months after surgery. |
| Fundamental Characteristic | Mechanism | Supportive Findings | Citations |
|---|---|---|---|
| The early fusion of the spheno-occipital synchondrosis leads to brachycephalicism and miniaturization. | Easly fusion leads to reduced skull length, triggering compensatory elongation of other calvarial bones. |
| [55,56] |
| Overcrowding of the whole brain leads to the displacement of the cerebellum and brainstem towards the caudal region. |
| [57,58] | |
| The cause of overcrowding is linked to a smaller caudal cranial fossa. |
| [27,57,59] | |
| CM leads to secondary effects that raise uncertainty about their impact on the development of syringomyelia. | CM leads to the herniation of the cerebellum and brainstem. |
| [60,61] |
| Occipital hypoplasia undergoes gradual development. |
| [60] | |
| A shortened skull base can decrease the size of the jugular foramen and increase ICP. |
| [26,62] | |
| Clinical signs related to CM/SM are impacted by issues in the craniocervical junction. | Simultaneous CJA influence both symptoms and SM progression |
| [63,64] |
| Brain parenchyma size results in overcrowding. | Overcrowding occurs as a consequence of an enlarged cerebellum |
| [58] |
| Chr. Location | Gene | Biological Process/Gene Ontologya | Variant Classc | Enhanced Expressiond |
|---|---|---|---|---|
| 9q34.11 | USP20 | Endocytosis, Ubl conjugation pathway | Intronic | Low tissue specificity |
| 5q31.1 | TRPC7 | Calcium transport | Intronic | Adrenal gland, brain, intestine, kidney, pituitary gland, testis |
| 9q33.2 | TRAF1 | Apoptosis | Intronic | Low tissue specificity |
| 5q31.3 | SLC4A9 | Anion transmembrane transporter activity | Missense | Kidney, heart |
| 9q33.2 | PHF19 | Chromatin regulator | Intronic | Low tissue specificity |
| 9q33.3 | OLFML2A | Protein homodimerization activity | Missense | Low tissue specificity |
| 5q31.3 | NR3C1 | Apoptosis, cell cycle, transcription regulation | Intronic | Low Tissue Specificity |
| 13q33.3 | MYO16 | Motor activity, actin binding | Intronic | Brain |
| 9q33.3 | MVB12B | Protein transport | Intronic | Brain |
| 9q34.11 | LOC101929331 | N/A | Intronic | N/A |
| 5q31.3 | LOC101926941 | N/A | Intronic | N/A |
| 5q31.1 | LOC100996485 | N/A | Intronic | N/A |
| 17q21.33 | LOC100288866 | N/A | Intronic | Low tissue specificity |
| 5q23.1 | LINC00992 | N/A | Intronic | Pancreas, colon |
| 3p24.1 | LINC00693 | N/A | Intronic | Brain |
| 7q22.3 | LHFPL3–AS2 | N/A | Intronic | Kidney |
| 7q22.2 | LHFPL3 | N/A | Intronic | Brain |
| 5q32 | HTR4 | G protein-coupled receptor activity | Intronic | Brain, heart muscle, intestine, pituitary gland |
| 5q31.1 | FSTL4 | Calcium ion binding, metal ion binding | Intronic | Brain |
| 5q31.3 | FGF1 | Angiogenesis, differentiation | Intronic | Brain, heart muscle, kidney |
| 13q33.3 | FAM155A | Calcium ion import across plasma membrane | Intronic | Brain, pituitary gland |
| 13q.34 | COL4A2 | Basal membrane formation | Intronic | Placenta |
| 13q.34 | COL4A1 | Basal membrane formation | 5’UTR | Placenta |
| 9q32 | COL27A1 | Extracellular matrix structural constituent | Intronic | Brain, uterine, cervix |
| 9q33.2 | CNTRL | Cell cycle, cell division | Intronic | Low tissue specificity |
| 9q33.1 | BRINP1 | Inhibits cell proliferation with negative regulation of the G1/S transition | Intronic | Brain |
| 9q33.1 | ASTN2 | Protein transport | Intronic | Low tissue specificity |
| 5q31.3 | ARHGAP26 | GTPase activity | Intronic | Low tissue specificity |
| 5q32 | ADRB2 | G protein-coupled receptor activity | Intronic | Blood |
| Category | Details |
|---|---|
| Patient Cohort | 388 patients with Chiari formation |
| Surgical Approach | Atlantoaxial fixation |
| Clinical Outcomes | 99.4% of patients showed immediate postoperative and sustained improvement |
| Radiological Outcomes | Reduction in syrinx size in 65 out of 221 patients in the immediate post-operative phase; significant syrinx size reduction in 95 out of 110 cases on delayed post-operative scans |
| Pathogenesis Perspective | Proposed atlantoaxial instability as a nodal point of pathogenesis for Chiari 1 formation |
| Treatment Goals | Achieve firm atlantoaxial fixation resulting in segmental arthrodesis; no foramen magnum decompression or syrinx manipulation |
| Surgical Technique | Lateral mass plate and screw fixation; avoidance of metal spacers post-2013 in favor of bone grafts for realignment and arthrodesis |
| Postoperative Management | Hard cervical collar for 3 months to facilitate bone fusion |
| Complications | Vertebral artery injury in a few cases; technical difficulties due to complex craniovertebral junction anatomy |
| Improvement Indicators | Immediate postoperative improvements in clinical symptoms such as voice volume, breathing, pain relief, and motor function; progressive improvement over time |
| Long-term Observations | Reversal of spinal deformities and recovery from major presenting symptoms in the im-mediate postoperative period |
| Clinical Assessment | Utilized Goel clinical grading scale, JOA score, VAS, and patient self-assessment; reviewed by independent neurosurgeons |
| Radiological Assessment | Postoperative CT and MRI to evaluate syrinx size reduction and tonsillar herniation re-gression |
| Considerations for Pediatric Patients | Symptoms and alterations in pediatric cases likely depend on the onset and degree of at-lantoaxial instability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toader, C.; Ples, H.; Covache-Busuioc, R.-A.; Costin, H.P.; Bratu, B.-G.; Dumitrascu, D.-I.; Glavan, L.A.; Ciurea, A.V. Decoding Chiari Malformation and Syringomyelia: From Epidemiology and Genetics to Advanced Diagnosis and Management Strategies. Brain Sci. 2023, 13, 1658. https://doi.org/10.3390/brainsci13121658
Toader C, Ples H, Covache-Busuioc R-A, Costin HP, Bratu B-G, Dumitrascu D-I, Glavan LA, Ciurea AV. Decoding Chiari Malformation and Syringomyelia: From Epidemiology and Genetics to Advanced Diagnosis and Management Strategies. Brain Sciences. 2023; 13(12):1658. https://doi.org/10.3390/brainsci13121658
Chicago/Turabian StyleToader, Corneliu, Horia Ples, Razvan-Adrian Covache-Busuioc, Horia Petre Costin, Bogdan-Gabriel Bratu, David-Ioan Dumitrascu, Luca Andrei Glavan, and Alexandru Vlad Ciurea. 2023. "Decoding Chiari Malformation and Syringomyelia: From Epidemiology and Genetics to Advanced Diagnosis and Management Strategies" Brain Sciences 13, no. 12: 1658. https://doi.org/10.3390/brainsci13121658
APA StyleToader, C., Ples, H., Covache-Busuioc, R.-A., Costin, H. P., Bratu, B.-G., Dumitrascu, D.-I., Glavan, L. A., & Ciurea, A. V. (2023). Decoding Chiari Malformation and Syringomyelia: From Epidemiology and Genetics to Advanced Diagnosis and Management Strategies. Brain Sciences, 13(12), 1658. https://doi.org/10.3390/brainsci13121658







