Sustained Cognitive Improvement in Patients over 65 Two Years after Cochlear Implantation
Abstract
:1. Introduction
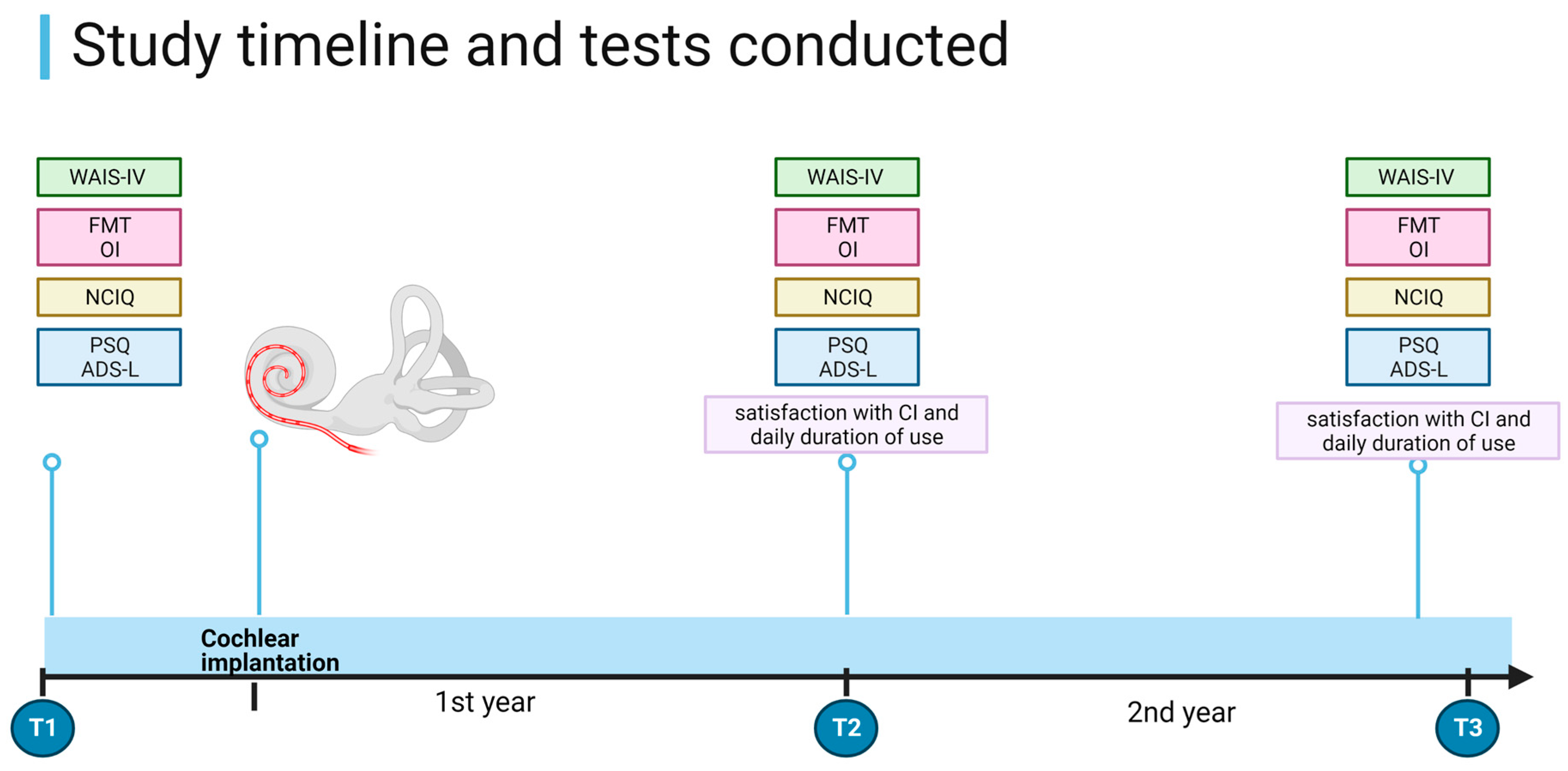
2. Materials and Methods
- Age > 65 years;
- Indication for CI according to current German guidelines (33, 34) and meeting clinical criteria for CI surgery;
- Unilateral CI;
- German mother tongue;
- Complete pre- and post-implantation data collection at all time points (Figure 1).
- Lost to follow-up appointment (due to disease and/or death);
- Severe visual impairment.
Statistical Analysis
3. Results
3.1. Patient Data
3.2. Hearing Abilities
3.3. ADS-L
3.4. PSQ
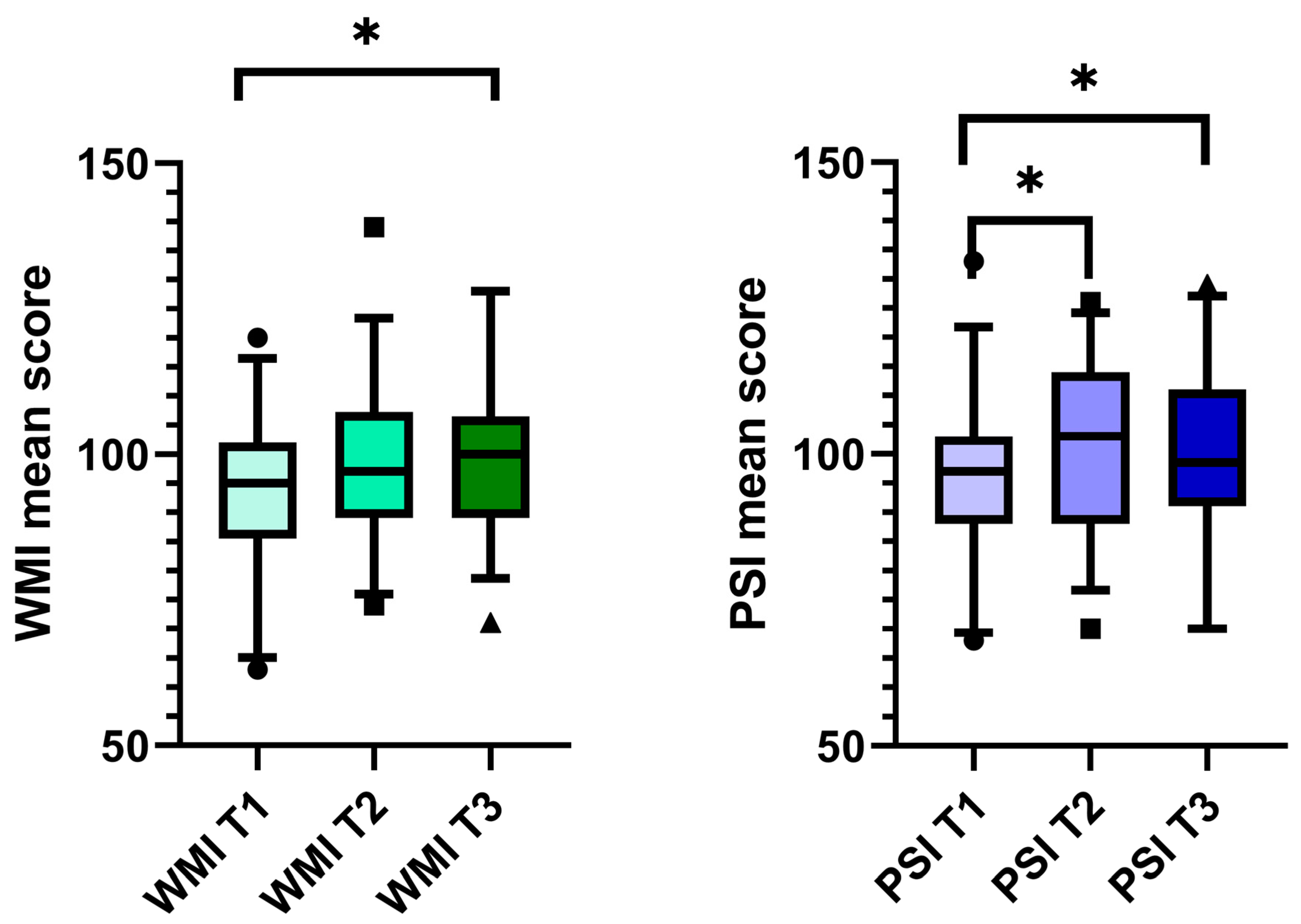
3.5. Cognition Parameters
3.6. Correlations
4. Discussion
4.1. Changes in Cognitive Parameters after CI
4.2. Correlations
4.3. Strengths, Limitations, and Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Deafness and Hearing Loss. Available online: https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss (accessed on 7 September 2023).
- Lie, A.; Skogstad, M.; Johannessen, H.A.; Tynes, T.; Mehlum, I.S.; Nordby, K.C.; Engdahl, B.; Tambs, K. Occupational noise exposure and hearing: A systematic review. Int. Arch. Occup. Environ. Health 2016, 89, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Bielefeld, E.C. Music as a unique source of noise-induced hearing loss. Hear. Res. 2023, 430, 108706. [Google Scholar] [CrossRef]
- Dillard, L.K.; Lopez-Perez, L.; Martinez, R.X.; Fullerton, A.M.; Chadha, S.; McMahon, C.M. Global burden of ototoxic hearing loss associated with platinum-based cancer treatment: A systematic review and meta-analysis. Cancer Epidemiol. 2022, 79, 102203. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, A.K.; Prince, A.A.; Chen, J.X.; Lieu, J.E.C.; Shin, J.J. Macrolide-associated sensorineural hearing loss: A systematic review. Laryngoscope 2018, 128, 228–236. [Google Scholar] [CrossRef]
- Naples, J.G.; Rice-Narusch, W.; Watson, N.W.; Ghulam-Smith, M.; Holmes, S.; Li, D.; Jalisi, S. Ototoxicity Review: A Growing Number of Non-Platinum-Based Chemo- and Immunotherapies. Otolaryngol. Head Neck Surg. 2023, 168, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Ng, M. Genetic Hearing Loss. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Gan, J.; Zhang, Y.; Wu, J.; Lei, D.; Zhang, F.; Zhao, H.; Wang, L. Current Understanding of Hearing Loss in Sporadic Vestibular Schwannomas: A Systematic Review. Front. Oncol. 2021, 11, 687201. [Google Scholar] [CrossRef] [PubMed]
- Toro, E.D.; Risbud, A.; Khosravani, N.; Vengerovich, G.; Archilla, A. Sphenoid Wing Meningioma Presenting as Sudden Sensorineural Hearing Loss: A Case Report and Literature Review. Ear Nose Throat J. 2021, 100, 352s–355s. [Google Scholar] [CrossRef]
- Simões, J.; Vlaminck, S.; Seiça, R.M.F.; Acke, F.; Miguéis, A.C.E. Cardiovascular Risk and Sudden Sensorineural Hearing Loss: A Systematic Review and Meta-Analysis. Laryngoscope 2023, 133, 15–24. [Google Scholar] [CrossRef]
- Bowl, M.R.; Dawson, S.J. Age-Related Hearing Loss. Cold Spring Harb. Perspect. Med. 2019, 9, a033217. [Google Scholar] [CrossRef]
- Sheffield, A.M.; Smith, R.J.H. The Epidemiology of Deafness. Cold Spring Harb. Perspect. Med. 2019, 9, a033258. [Google Scholar] [CrossRef]
- Deal, J.A.; Betz, J.; Yaffe, K.; Harris, T.; Purchase-Helzner, E.; Satterfield, S.; Pratt, S.; Govil, N.; Simonsick, E.M.; Lin, F.R. Hearing Impairment and Incident Dementia and Cognitive Decline in Older Adults: The Health ABC Study. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Irace, A.L.; Armstrong, N.M.; Deal, J.A.; Chern, A.; Ferrucci, L.; Lin, F.R.; Resnick, S.M.; Golub, J.S. Longitudinal Associations of Subclinical Hearing Loss with Cognitive Decline. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.Y.L.; Loo, J.H.Y. Screening an elderly hearing impaired population for mild cognitive impairment using Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA). Int. J. Geriatr. Psychiatry 2018, 33, 972–979. [Google Scholar] [CrossRef]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Dawes, P.; Emsley, R.; Cruickshanks, K.J.; Moore, D.R.; Fortnum, H.; Edmondson-Jones, M.; McCormack, A.; Munro, K.J. Hearing loss and cognition: The role of hearing AIDS, social isolation and depression. PLoS ONE 2015, 10, e0119616. [Google Scholar] [CrossRef]
- Castiglione, A.; Benatti, A.; Velardita, C.; Favaro, D.; Padoan, E.; Severi, D.; Pagliaro, M.; Bovo, R.; Vallesi, A.; Gabelli, C.; et al. Aging, Cognitive Decline and Hearing Loss: Effects of Auditory Rehabilitation and Training with Hearing Aids and Cochlear Implants on Cognitive Function and Depression among Older Adults. Audiol. Neurootol. 2016, 21 (Suppl. S1), 21–28. [Google Scholar] [CrossRef]
- Mosnier, I.; Bebear, J.P.; Marx, M.; Fraysse, B.; Truy, E.; Lina-Granade, G.; Mondain, M.; Sterkers-Artières, F.; Bordure, P.; Robier, A.; et al. Improvement of cognitive function after cochlear implantation in elderly patients. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 442–450. [Google Scholar] [CrossRef]
- Ghisletta, P.; Dahle, C.L.; Raz, N. Age-related Hearing Loss, Cognitive Performance, and Metabolic Risk in Healthy Adults: A Seven-Year Longitudinal Study. J. Gerontol. B Psychol. Sci. Soc. Sci. 2022, 78, 409–420. [Google Scholar] [CrossRef]
- Yeo, B.S.Y.; Song, H.; Toh, E.M.S.; Ng, L.S.; Ho, C.S.H.; Ho, R.; Merchant, R.A.; Tan, B.K.J.; Loh, W.S. Association of Hearing Aids and Cochlear Implants with Cognitive Decline and Dementia: A Systematic Review and Meta-analysis. JAMA Neurol. 2023, 80, 134–141. [Google Scholar] [CrossRef]
- Uchida, Y.; Sugiura, S.; Nishita, Y.; Saji, N.; Sone, M.; Ueda, H. Age-related hearing loss and cognitive decline—The potential mechanisms linking the two. Auris Nasus Larynx 2019, 46, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sweller, J.A.P.L.; Kalyuga, S. Cognitive Load Theory; Springer: New York, NY, USA; London, UK, 2011. [Google Scholar]
- Wayne, R.V.; Johnsrude, I.S. A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Ageing Res. Rev. 2015, 23, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.R.; Ferrucci, L.; An, Y.; Goh, J.O.; Doshi, J.; Metter, E.J.; Davatzikos, C.; Kraut, M.A.; Resnick, S.M. Association of hearing impairment with brain volume changes in older adults. Neuroimage 2014, 90, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Golub, J.S. Brain changes associated with age-related hearing loss. Curr. Opin. Otolaryngol. Head Neck Surg. 2017, 25, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Cacioppo, J.T.; Hawkley, L.C. Perceived social isolation and cognition. Trends Cogn. Sci. 2009, 13, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, L.E.; Palmer, C.V.; Pratt, S.; Erickson, K.I.; Moncrieff, D. The Effect of Decreased Audibility on MMSE Performance: A Measure Commonly Used for Diagnosing Dementia. J. Am. Acad. Audiol. 2016, 27, 311–323. [Google Scholar] [CrossRef]
- Amieva, H.; Ouvrard, C.; Giulioli, C.; Meillon, C.; Rullier, L.; Dartigues, J.F. Self-Reported Hearing Loss, Hearing Aids, and Cognitive Decline in Elderly Adults: A 25-Year Study. J. Am. Geriatr. Soc. 2015, 63, 2099–2104. [Google Scholar] [CrossRef]
- Pinto, T.C.C.; Machado, L.; Bulgacov, T.M.; Rodrigues-Júnior, A.L.; Costa, M.L.G.; Ximenes, R.C.C.; Sougey, E.B. Is the Montreal Cognitive Assessment (MoCA) screening superior to the Mini-Mental State Examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer’s Disease (AD) in the elderly? Int. Psychogeriatr. 2019, 31, 491–504. [Google Scholar] [CrossRef]
- Claes, A.J.; Mertens, G.; Gilles, A.; Hofkens-Van den Brandt, A.; Fransen, E.; Van Rompaey, V.; Van de Heyning, P. The Repeatable Battery for the Assessment of Neuropsychological Status for Hearing Impaired Individuals (RBANS-H) before and after Cochlear Implantation: A Protocol for a Prospective, Longitudinal Cohort Study. Front. Neurosci. 2016, 10, 512. [Google Scholar] [CrossRef]
- Völter, C.; Götze, L.; Falkenstein, M.; Dazert, S.; Thomas, J.P. Application of a computer-based neurocognitive assessment battery in the elderly with and without hearing loss. Clin. Interv. Aging 2017, 12, 1681–1690. [Google Scholar] [CrossRef]
- Wechsler, D. The Measurement and Appraisal of Adult Intelligence; Williams & Witkins: Baltimore, MD, USA, 1939. [Google Scholar]
- Knopke, S.; Schubert, A.; Häussler, S.M.; Gräbel, S.; Szczepek, A.J.; Olze, H. Improvement of Working Memory and Processing Speed in Patients over 70 with Bilateral Hearing Impairment Following Unilateral Cochlear Implantation. J. Clin. Med. 2021, 10, 3421. [Google Scholar] [CrossRef] [PubMed]
- Baddeley, A. Working memory. Science 1992, 255, 556–559. [Google Scholar] [CrossRef]
- Horning, S.; Davis, H.P. Aging and Cognition. In Encyclopedia of Human Behavior, 2nd ed.; Ramachandran, V.S., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 44–52. [Google Scholar]
- Hoth, S. The Freiburg speech intelligibility test: A pillar of speech audiometry in German-speaking countries. Hno 2016, 64, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Daseking, M.; Petermann, F. Analyse von Querschnittsdaten zur Intelligenzentwicklung im Erwachsenenalter: Eine Studie zur deutschsprachigen Version der WAIS-IV. Z. Für Neuropsychol. 2013, 24, 149–160. [Google Scholar] [CrossRef]
- Lepach, A.C.; Petermann, F. Gedächtnisdiagnostik mit der Wechsler Memory Scale–Fourth edition. Z. Für Neuropsychol. 2012, 23, 123–132. [Google Scholar] [CrossRef]
- Holube, I.K.B. Modifikation eines Fragebogesn zur Erfassung des subjektiven Hörvermögens und dessen Beziehung zur Sprachverständlichkeit in Ruhe und unter Störgeräuschen. Audiol. Akust. 1994, 33, 22–35. [Google Scholar]
- Fliege, H.; Rose, M.; Arck, P.; Walter, O.B.; Kocalevent, R.D.; Weber, C.; Klapp, B.F. The Perceived Stress Questionnaire (PSQ) reconsidered: Validation and reference values from different clinical and healthy adult samples. Psychosom. Med. 2005, 67, 78–88. [Google Scholar] [CrossRef]
- Hautzinger, M.; Bailer, M. Allgemeine Depressionsskala (ADS); manual; Beltz-Test-GmbH: Göttingen, Germany, 1993. [Google Scholar]
- Dupuis, K.; Pichora-Fuller, M.K.; Chasteen, A.L.; Marchuk, V.; Singh, G.; Smith, S.L. Effects of hearing and vision impairments on the Montreal Cognitive Assessment. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2015, 22, 413–437. [Google Scholar] [CrossRef]
- Claes, A.J.; Van de Heyning, P.; Gilles, A.; Hofkens-Van den Brandt, A.; Van Rompaey, V.; Mertens, G. Impaired Cognitive Functioning in Cochlear Implant Recipients Over the Age of 55 Years: A Cross-Sectional Study Using the Repeatable Battery for the Assessment of Neuropsychological Status for Hearing-Impaired Individuals (RBANS-H). Front. Neurosci. 2018, 12, 580. [Google Scholar] [CrossRef]
- Claes, A.J.; Van de Heyning, P.; Gilles, A.; Van Rompaey, V.; Mertens, G. Cognitive Performance of Severely Hearing-impaired Older Adults Before and After Cochlear Implantation: Preliminary Results of a Prospective, Longitudinal Cohort Study Using the RBANS-H. Otol. Neurotol. 2018, 39, e765–e773. [Google Scholar] [CrossRef]
- Calvino, M.; Sánchez-Cuadrado, I.; Gavilán, J.; Gutiérrez-Revilla, M.A.; Polo, R.; Lassaletta, L. Effect of cochlear implantation on cognitive decline and quality of life in younger and older adults with severe-to-profound hearing loss. Eur. Arch. Otorhinolaryngol. 2022, 279, 4745–4759. [Google Scholar] [CrossRef] [PubMed]
- Völter, C.; Götze, L.; Kamin, S.T.; Haubitz, I.; Dazert, S.; Thomas, J.P. Can cochlear implantation prevent cognitive decline in the long-term follow-up? Front. Neurol. 2022, 13, 1009087. [Google Scholar] [CrossRef] [PubMed]
- Boller, B.; Mellah, S.; Ducharme-Laliberté, G.; Belleville, S. Relationships between years of education, regional grey matter volumes, and working memory-related brain activity in healthy older adults. Brain Imaging Behav. 2017, 11, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Calvino, M.; Sánchez-Cuadrado, I.; Gavilán, J.; Lassaletta, L. The effect of risk factors on cognition in adult cochlear implant candidates with severe to profound hearing loss. Front. Psychol. 2022, 13, 837366. [Google Scholar] [CrossRef]
- Völter, C.; Götze, L.; Haubitz, I.; Müther, J.; Dazert, S.; Thomas, J.P. Impact of Cochlear Implantation on Neurocognitive Subdomains in Adult Cochlear Implant Recipients. Audiol. Neurootol. 2021, 26, 236–245. [Google Scholar] [CrossRef]
- Bosnes, I.; Bosnes, O.; Stordal, E.; Nordahl, H.M.; Myklebust, T.; Almkvist, O. Processing speed and working memory are predicted by components of successful aging: A HUNT study. BMC Psychol. 2022, 10, 16. [Google Scholar] [CrossRef]
| Oldenburg Inventory (OI) | The OI [41] assesses a patient’s subjective hearing ability using questions categorized into 3 subdomains, namely, “hearing in quiet”, “hearing with background noise”, and “localization”, and an overall score. |
| Perceived Stress Questionnaire (PSQ) | The PSQ is used to assess perceived stress levels [42]. It consists of 30 items measuring four subscales: worries, tension, joy, and demands. The cut-off score for low-degree stress is 0.45. |
| General Depression Scale (ADS-L) | The General Depression Scale (ADS) [43] assesses depressive symptoms. The test consists of 20 items with scores ranging from 0 to 60, with a cut-off score of 23. In this study, the long version (ADS-L) was used. |
| The total number of patients included | 33 |
| Sex | female = 19; male = 14 |
| Age (mean ± SD, range) | 75.5 ± 4.9; 65–88 |
| Years of education (mean ± SD, range) | 12.76 ± 2.36; 8–18 |
| Laterality of hearing loss Duration of deafness (mean ± SD, range) | AHL (n = 10); DSD (n = 22 *); SSD (n = 1) 12.8 ± 13.4; 0.5–58 |
| T1 | T2 | T3 | Level of Significance for T1–T2 | Level of Significance for T1–T3 | Level of Significance for T2–T3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | Two-Tailed | Two-Tailed | Two-Tailed | |
| OI-quiet | 2.6 | 2.2–3.4 | 3.4 | 2.8–4.3 | 3.6 | 2.5–4.1 | p = 0.003 * | p = 0.002 * | p = 0.572 |
| OI-noise | 2 | 1.5–2.2 | 2.5 | 2.2–3.0 | 2.4 | 1.8–3.1 | p < 0.001 * | p < 0.001 * | p = 0.224 |
| OI-localization | 2.5 | 2.0–3.0 | 3 | 2.0–3.5 | 3 | 2.0–4.0 | p = 0.051 | p = 0.025 * | p = 0.329 |
| OI-total | 2.3 | 1.9–2.6 | 3 | 2.4–3.4 | 2.96 | 2.2–3.4 | p < 0.001 * | p = 0.001 * | p = 0.526 |
| T1 | T2 | T3 | Level of Significance for T1–T2 | Level of Significance for T1–T3 | Level of Significance for T2–T3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | Two-Tailed | Two-Tailed | Two–Tailed | |
| Digit span | 22 | 19–26 | 22.5 | 20.3–23 | 23 | 20.0–26.5 | p = 0.794 | p = 0.905 | p = 0.769 |
| Arithmetic | 13 | 11–17 | 14 | 12–16.0 | 14 | 13.0–17.5 | p = 0.215 | p = 0.001 * | p = 0.009 * |
| Symbol Search | 20 | 14.5–24.0 | 22 | 16.0–26.0 | 21.0 | 18.0–26.0 | p = 0.178 | p = 0.557 | p = 0.647 |
| Coding | 49 | 38.5–56.0 | 50 | 36.0–61.0 | 50 | 37.5–58.0 | p = 0.206 | p = 0.250 | p = 0.918 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Häußler, S.M.; Stankow, E.; Knopke, S.; Szczepek, A.J.; Olze, H. Sustained Cognitive Improvement in Patients over 65 Two Years after Cochlear Implantation. Brain Sci. 2023, 13, 1673. https://doi.org/10.3390/brainsci13121673
Häußler SM, Stankow E, Knopke S, Szczepek AJ, Olze H. Sustained Cognitive Improvement in Patients over 65 Two Years after Cochlear Implantation. Brain Sciences. 2023; 13(12):1673. https://doi.org/10.3390/brainsci13121673
Chicago/Turabian StyleHäußler, Sophia Marie, Elisabeth Stankow, Steffen Knopke, Agnieszka J. Szczepek, and Heidi Olze. 2023. "Sustained Cognitive Improvement in Patients over 65 Two Years after Cochlear Implantation" Brain Sciences 13, no. 12: 1673. https://doi.org/10.3390/brainsci13121673







