Prefrontal and Motor Planning Cortical Activity during Stepping Tasks Is Related to Task Complexity but Not Concern about Falling in Older People: A fNIRS Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Registration
2.2. Participants
2.3. Descriptive Measures
2.4. Concern about Falling
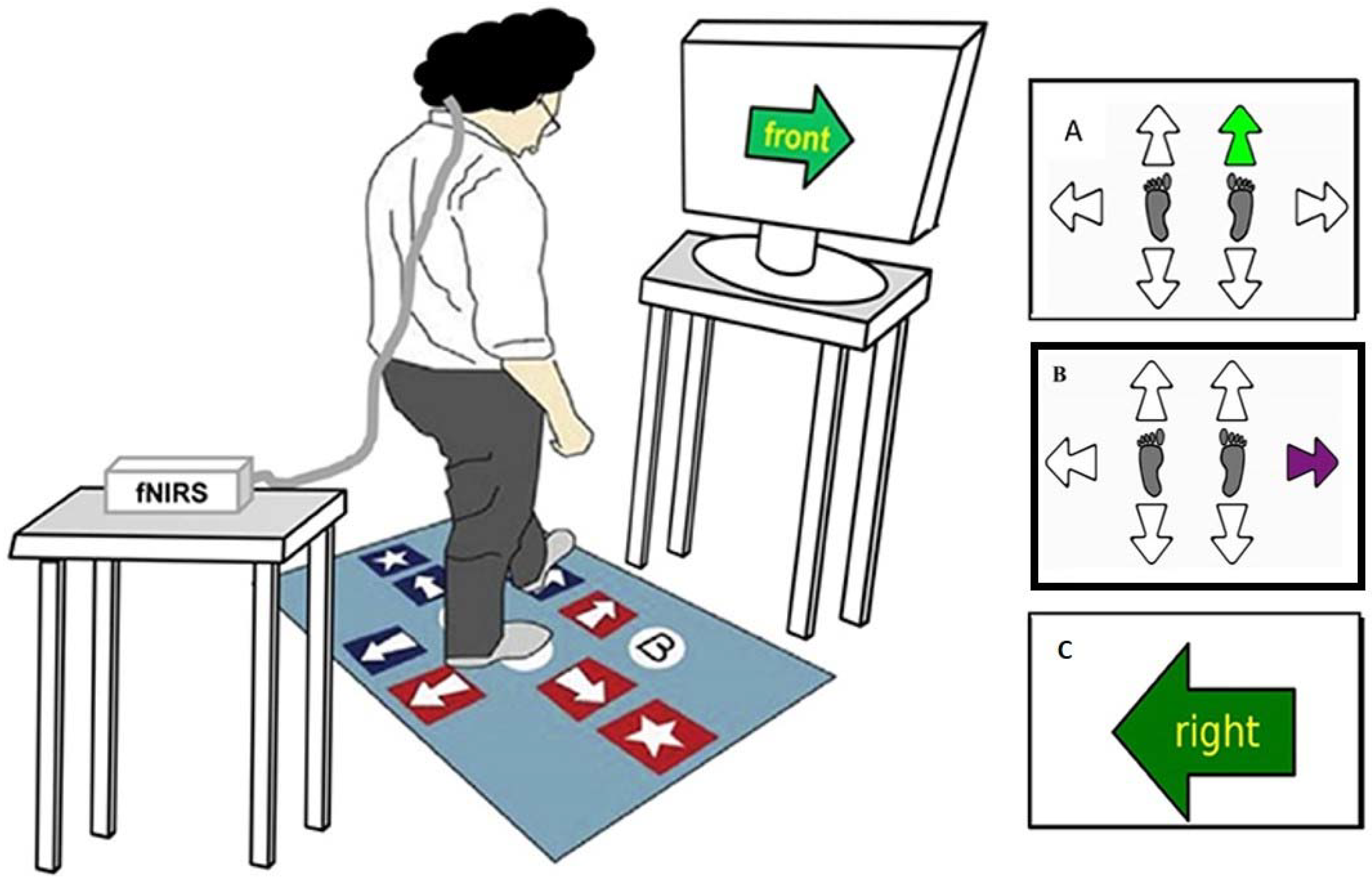
2.5. Experimental Protocol
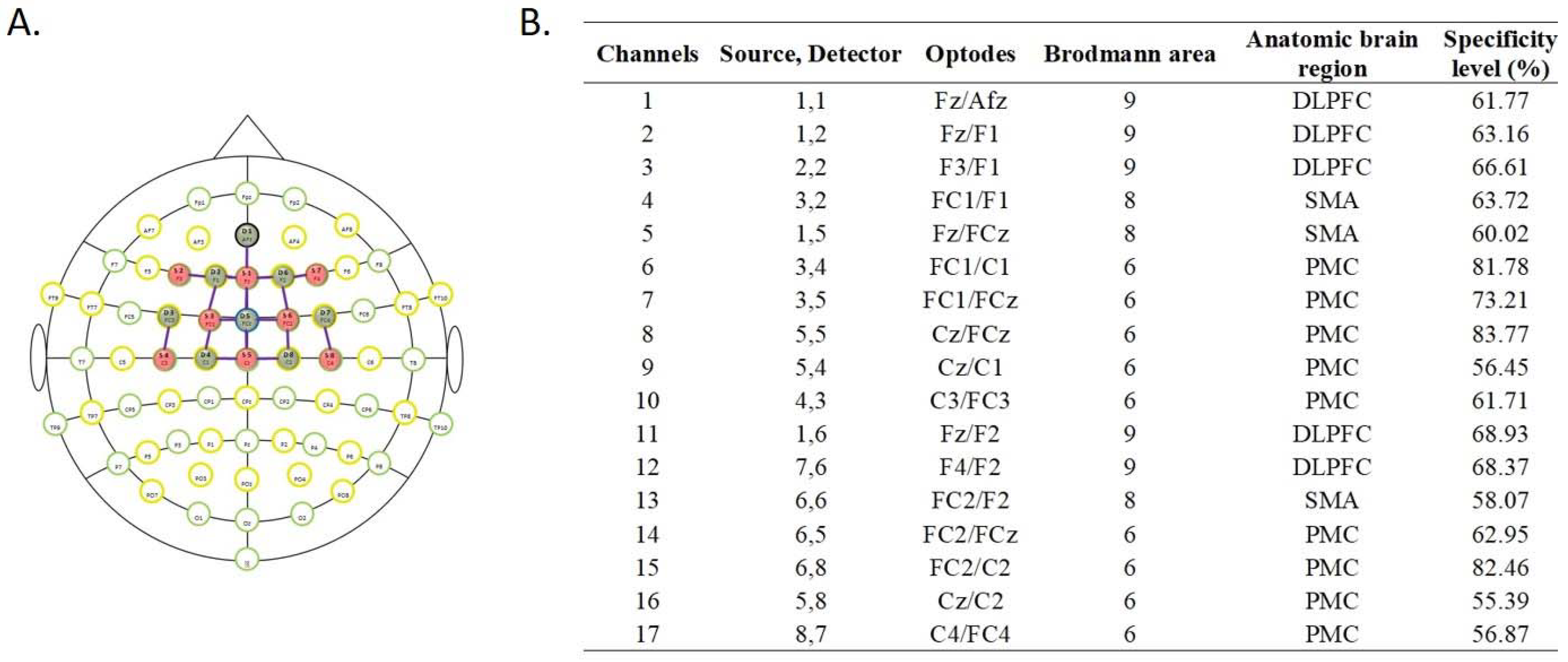
2.6. fNIRS Data Acquisition and Analysis
2.7. Statistical Analysis
3. Results
3.1. Haemodynamics
3.2. Stepping Response Times
3.3. Stepping Errors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montero-Odasso, M.; van der Velde, N.; Martin, F.C.; Petrovic, M.; Tan, M.P.; Ryg, J.; Aguilar-Navarro, S.; Alexander, N.B.; Becker, C.; Blain, H.; et al. World guidelines for falls prevention and management for older adults: A global initiative. Age Ageing 2022, 51, afac205. [Google Scholar] [CrossRef]
- Amboni, M.; Barone, P.; Hausdorff, J.M. Cognitive contributions to gait and falls: Evidence and implications. Mov. Disord. 2013, 28, 1520–1533. [Google Scholar] [CrossRef]
- Hamacher, D.; Herold, F.; Wiegel, P.; Hamacher, D.; Schega, L. Brain activity during walking: A systematic review. Neurosci. Biobehav. Rev. 2015, 57, 310–327. [Google Scholar] [CrossRef]
- Seidler, R.D.; Bernard, J.A.; Burutolu, T.B.; Fling, B.W.; Gordon, M.T.; Gwin, J.T.; Kwak, Y.; Lipps, D.B. Motor control and aging: Links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev. 2010, 34, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Miyai, I.; Suzuki, M.; Kubota, K. Gait capacity affects cortical activation patterns related to speed control in the elderly. Exp. Brain Res. 2009, 193, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Holtzer, R.; Epstein, N.; Mahoney, J.R.; Izzetoglu, M.; Blumen, H.M. Neuroimaging of mobility in aging: A targeted review. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- Nóbrega-Sousa, P.; Gobbi, L.T.B.; Orcioli-Silva, D.; Conceição, N.R.D.; Beretta, V.S.; Vitório, R. Prefrontal Cortex Activity During Walking: Effects of Aging and Associations With Gait and Executive Function. Neurorehabil. Neural Repair 2020, 34, 915–924. [Google Scholar] [CrossRef] [PubMed]
- MacKay, S.; Ebert, P.; Harbidge, C.; Hogan, D.B. Fear of Falling in Older Adults: A Scoping Review of Recent Literature. Can. Geriatr. J. 2021, 24, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Schoene, D.; Heller, C.; Aung, Y.N.; Sieber, C.C.; Kemmler, W.; Freiberger, E. A systematic review on the influence of fear of falling on quality of life in older people: Is there a role for falls? Clin. Interv. Aging 2019, 14, 701–719. [Google Scholar] [CrossRef] [PubMed]
- Ellmers, T.J.; Wilson, M.R.; Kal, E.C.; Young, W.R. The perceived control model of falling: Developing a unified framework to understand and assess maladaptive fear of falling. Age Ageing 2023, 52, afad093. [Google Scholar] [CrossRef]
- Eysenck, M.W.; Derakshan, N.; Santos, R.; Calvo, M.G. Anxiety and cognitive performance: Attentional control theory. Emotion 2007, 7, 336–353. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.A.; White, P.; Doan, J.B.; de Bruin, N. Selective attentional processing to fall-relevant stimuli among older adults who fear falling. Exp. Aging Res. 2011, 37, 330–345. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.O.; Knight, B.G. Attentional bias for threat in older adults: Moderation of the positivity bias by trait anxiety and stimulus modality. Psychol. Aging 2009, 24, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Young, W.R.; Mark Williams, A. How fear of falling can increase fall-risk in older adults: Applying psychological theory to practical observations. Gait Posture 2015, 41, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Holtzer, R.; Kraut, R.; Izzetoglu, M.; Ye, K. The effect of fear of falling on prefrontal cortex activation and efficiency during walking in older adults. Geroscience 2019, 41, 89–100. [Google Scholar] [CrossRef]
- St George, R.J.; Jayakody, O.; Healey, R.; Breslin, M.; Hinder, M.R.; Callisaya, M.L. Cognitive inhibition tasks interfere with dual-task walking and increase prefrontal cortical activity more than working memory tasks in young and older adults. Gait Posture 2022, 95, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.R.; Fitzpatrick, R.C. Choice stepping reaction time: A composite measure of falls risk in older people. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M627–M632. [Google Scholar] [CrossRef]
- Scarpina, F.; Tagini, S. The Stroop Color and Word Test. Front. Psychol. 2017, 8, 557. [Google Scholar] [CrossRef]
- Schoene, D.; Delbaere, K.; Lord, S.R. Impaired Response Selection During Stepping Predicts Falls in Older People-A Cohort Study. J. Am. Med. Dir. Assoc. 2017, 18, 719–725. [Google Scholar] [CrossRef]
- Liu, X.; Banich, M.T.; Jacobson, B.L.; Tanabe, J.L. Common and distinct neural substrates of attentional control in an integrated Simon and spatial Stroop task as assessed by event-related fMRI. Neuroimage 2004, 22, 1097–1106. [Google Scholar] [CrossRef]
- Jacobs, J.V.; Lou, J.S.; Kraakevik, J.A.; Horak, F.B. The supplementary motor area contributes to the timing of the anticipatory postural adjustment during step initiation in participants with and without Parkinson’s disease. Neuroscience 2009, 164, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Nachev, P.; Kennard, C.; Husain, M. Functional role of the supplementary and pre-supplementary motor areas. Nat. Rev. Neurosci. 2008, 9, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.P.; Merino, D.M.; Beyer, K.B.; McIlroy, W.E. Cortical control of anticipatory postural adjustments prior to stepping. Neuroscience 2016, 313, 99–109. [Google Scholar] [CrossRef]
- Halsband, U.; Ito, N.; Tanji, J.; Freund, H.J. The role of premotor cortex and the supplementary motor area in the temporal control of movement in man. Brain 1993, 116 Pt 1, 243–266. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, A.C.; Fink, A. Intelligence and neural efficiency. Neurosci. Biobehav. Rev. 2009, 33, 1004–1023. [Google Scholar] [CrossRef]
- Sturnieks, D.L.; Menant, J.; Valenzuela, M.; Delbaere, K.; Sherrington, C.; Herbert, R.D.; Lampit, A.; Broadhouse, K.; Turner, J.; Schoene, D.; et al. Effect of cognitive-only and cognitive-motor training on preventing falls in community-dwelling older people: Protocol for the smart±step randomised controlled trial. BMJ Open 2019, 9, e029409. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J. Am. Geriatr. Soc. 1975, 23, 433–441. [Google Scholar] [CrossRef]
- Mioshi, E.; Dawson, K.; Mitchell, J.; Arnold, R.; Hodges, J.R. The Addenbrooke’s Cognitive Examination Revised (ACE-R): A brief cognitive test battery for dementia screening. Int. J. Geriatr. Psychiatry 2006, 21, 1078–1085. [Google Scholar] [CrossRef]
- Strauss, E.; Sherman, E.M.; Spreen, O. A Compendium of Neuropsychological Tests: Administration, Tests, Norms, and Commentary, 3rd ed.; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Lord, S.R.; Menz, H.B.; Tiedemann, A. A physiological profile approach to falls risk assessment and prevention. Phys. Ther. 2003, 83, 237–252. [Google Scholar] [CrossRef]
- Lim, M.L.; van Schooten, K.S.; Radford, K.A.; Menant, J.; Lord, S.R.; Sachdev, P.S.; Brodaty, H.; Delbaere, K. The Iconographical Falls Efficacy Scale (IconFES) in community-dwelling older people—A longitudinal validation study. Age Ageing 2021, 50, 822–829. [Google Scholar] [CrossRef]
- Schoene, D.; Smith, S.T.; Davies, T.A.; Delbaere, K.; Lord, S.R. A Stroop Stepping Test (SST) using low-cost computer game technology discriminates between older fallers and non-fallers. Age Ageing 2014, 43, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Zimeo Morais, G.A.; Balardin, J.B.; Sato, J.R. fNIRS Optodes’ Location Decider (fOLD): A toolbox for probe arrangement guided by brain regions-of-interest. Sci. Rep. 2018, 8, 3341. [Google Scholar] [CrossRef] [PubMed]
- Pelicioni, P.H.S.; Lord, S.R.; Okubo, Y.; Menant, J.C. Cortical activation during gait adaptability in people with Parkinson’s disease. Gait Posture 2022, 91, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Bray, S.; Reiss, A.L. Functional near infrared spectroscopy (NIRS) signal improvement based on negative correlation between oxygenated and deoxygenated hemoglobin dynamics. Neuroimage 2010, 49, 3039–3046. [Google Scholar] [CrossRef] [PubMed]
- Gage, W.H.; Sleik, R.J.; Polych, M.A.; McKenzie, N.C.; Brown, L.A. The allocation of attention during locomotion is altered by anxiety. Exp. Brain Res. 2003, 150, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Steffener, J.; Stern, Y. Exploring the neural basis of cognitive reserve in aging. Biochim. Biophys. Acta 2012, 1822, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Mirelman, A.; Maidan, I.; Bernad-Elazari, H.; Shustack, S.; Giladi, N.; Hausdorff, J.M. Effects of aging on prefrontal brain activation during challenging walking conditions. Brain Cogn. 2017, 115, 41–46. [Google Scholar] [CrossRef]
- Menant, J.C.; Maidan, I.; Alcock, L.; Al-Yahya, E.; Cerasa, A.; Clark, D.J.; de Bruin, E.D.; Fraser, S.; Gramigna, V.; Hamacher, D.; et al. A consensus guide to using functional near-infrared spectroscopy in posture and gait research. Gait Posture 2020, 82, 254–265. [Google Scholar] [CrossRef]
- Reuter-Lorenz, P.A.; Cappell, K.A. Neurocognitive Aging and the Compensation Hypothesis. Curr. Dir. Psychol. Sci. 2008, 17, 177–182. [Google Scholar] [CrossRef]
- Sebastian, A.; Baldermann, C.; Feige, B.; Katzev, M.; Scheller, E.; Hellwig, B.; Lieb, K.; Weiller, C.; Tüscher, O.; Klöppel, S. Differential effects of age on subcomponents of response inhibition. Neurobiol. Aging 2013, 34, 2183–2193. [Google Scholar] [CrossRef]
- Cohen, R.G.; Nutt, J.G.; Horak, F.B. Errors in postural preparation lead to increased choice reaction times for step initiation in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Verhaeghen, P. Aging and Executive Control: Reports of a Demise Greatly Exaggerated. Curr. Dir. Psychol. Sci. 2011, 20, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Eggenberger, P.; Wolf, M.; Schumann, M.; de Bruin, E.D. Exergame and Balance Training Modulate Prefrontal Brain Activity during Walking and Enhance Executive Function in Older Adults. Front. Aging Neurosci. 2016, 8, 66. [Google Scholar] [CrossRef] [PubMed]

| Variable | Participants (n = 99) | Low Concern about Falling (n = 71) | High Concern about Falling (n = 28) | Mean Difference (95% Confidence Interval) |
|---|---|---|---|---|
| Age (years) | 71.6 ± 4.8 | 71.1 ± 4.4 | 73.0 ± 5.5 | −1.9 (−4.0, 0.2) |
| Gender (female) | 71 (72%) | 50 (70%) | 21 (75%) | n/a |
| Height (cm) | 165.6 ± 9.6 | 165.9 ± 9.7 | 164.7 ± 9.7 | 1.3 (−3.0, 5.6) |
| Weight (cm) | 78.0 ± 15.3 | 77.2 ± 14.4 | 80.0 ± 17.4 | −2.8 (−9.6, 3.9) |
| Education (years) | 15.7 ± 4.4 | 15.9 ± 4.2 | 15.1 ± 5.0 | 0.7 (−1.2, 2.7) |
| ACER 1 | 95.3 ± 3.8 | 95.3 ± 4.0 | 95.1 ± 3.3 | 0.2 (−1.5, 1.9) |
| TMT-A (seconds) 2 | 34.3 ± 11.2 | 32.7 ± 11.0 | 37.9 ± 11.1 | −5.2 (−11.6, 1.3) |
| TMT-B (seconds) 3 | 77.2 ± 27.5 | 75.2 ± 26.7 | 81.7 ± 29.6 | −6.5 (−22.7, 9.7) |
| PPA score 4 | −0.05 ± 1.12 | −0.18 ± 1.08 | 0.26 ± 1.31 | −0.43 (−0.98, 0.13) |
| Region of Interest | Test | Low Concern about Falling (n = 71) | High Concern about Falling (n = 28) | Main Effect of Group | Main Effect of Test | Group x Test Interaction |
|---|---|---|---|---|---|---|
| Dorsolateral prefrontal cortex | CSRT a | 0.021 ± 0.006 | 0.009 ± 0.009 | F1, 97 = 1.124, p = 0.292 η2 = 0.011 | F2, 194 = 5.405, p = 0.005 η2 = 0.053 | F2, 194 = 0.175, p = 0.840 η2 = 0.002 |
| iCSRT | 0.026 ± 0.005 | 0.017 ± 0.009 | ||||
| SST a | 0.037 ± 0.006 | 0.032 ± 0.010 | ||||
| Premotor cortex | CSRT b | 0.036 ± 0.007 | 0.029 ± 0.011 | F1, 97 = 0.545, p = 0.462 η2 = 0.006 | F2, 194 = 7.348, p = 0.001 η2 = 0.070 | F2, 194 = 0.039, p = 0.961 η2 < 0.001 |
| iCSRT b | 0.043 ± 0.007 | 0.033 ± 0.011 | ||||
| SST b | 0.061 ± 0.007 | 0.054 ± 0.012 | ||||
| Supplementary motor area | CSRT c | 0.023 ± 0.005 | 0.025 ± 0.008 | F1, 97 = 0.043, p = 0.837 η2 < 0.001 | F2, 194 = 4.253, p = 0.016 η2 = 0.042 | F2, 194 = 0.198, p = 0.820 η2 = 0.002 |
| iCSRT | 0.030 ± 0.006 | 0.026 ± 0.009 | ||||
| SST c | 0.042 ± 0.006 | 0.038 ± 0.006 |
| Test | Low Concern About Falling (n = 71) | High Concern About Falling (n = 28) | Main Effect of Group | Main Effect of Test | Group x Test Interaction |
|---|---|---|---|---|---|
| CSRT | 777 ± 10 | 816 ± 17 | F1, 94 = 1.986, p = 0.162 η2 = 0.021 | F2, 188 = 180.253, p <0.001 η2 = 0.657 | F2, 188 = 0.257, p = 0.774 η2 = 0.003 |
| iCSRT | 858 ± 12 | 880 ± 19 | |||
| SST | 1079 ± 24 | 1124 ± 39 |
| Low Concern about Falling | High Concern about Falling | Total | |
|---|---|---|---|
| CSRT | |||
| No error | 25 | 9 | 34 |
| 1 or more errors | 46 | 19 | 65 |
| Total | 71 | 28 | 99 |
| iCSRT | |||
| No error | 42 | 8 | 50 |
| 1 or more errors a | 29 | 20 | 49 |
| Total | 71 | 28 | 99 |
| SST | |||
| No error | 47 | 23 | 70 |
| 1 or more errors | 24 | 5 | 29 |
| Total | 71 | 28 | 99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tung, C.; Lord, S.R.; Pelicioni, P.H.S.; Sturnieks, D.L.; Menant, J.C.C. Prefrontal and Motor Planning Cortical Activity during Stepping Tasks Is Related to Task Complexity but Not Concern about Falling in Older People: A fNIRS Study. Brain Sci. 2023, 13, 1675. https://doi.org/10.3390/brainsci13121675
Tung C, Lord SR, Pelicioni PHS, Sturnieks DL, Menant JCC. Prefrontal and Motor Planning Cortical Activity during Stepping Tasks Is Related to Task Complexity but Not Concern about Falling in Older People: A fNIRS Study. Brain Sciences. 2023; 13(12):1675. https://doi.org/10.3390/brainsci13121675
Chicago/Turabian StyleTung, Carmen, Stephen Ronald Lord, Paulo Henrique Silva Pelicioni, Daina Louise Sturnieks, and Jasmine Charlotte Christiane Menant. 2023. "Prefrontal and Motor Planning Cortical Activity during Stepping Tasks Is Related to Task Complexity but Not Concern about Falling in Older People: A fNIRS Study" Brain Sciences 13, no. 12: 1675. https://doi.org/10.3390/brainsci13121675
APA StyleTung, C., Lord, S. R., Pelicioni, P. H. S., Sturnieks, D. L., & Menant, J. C. C. (2023). Prefrontal and Motor Planning Cortical Activity during Stepping Tasks Is Related to Task Complexity but Not Concern about Falling in Older People: A fNIRS Study. Brain Sciences, 13(12), 1675. https://doi.org/10.3390/brainsci13121675







