The Effects of Acetazolamide on Cerebral Hemodynamics in Adult Patients with an Acute Brain Injury: A Systematic Review
Abstract
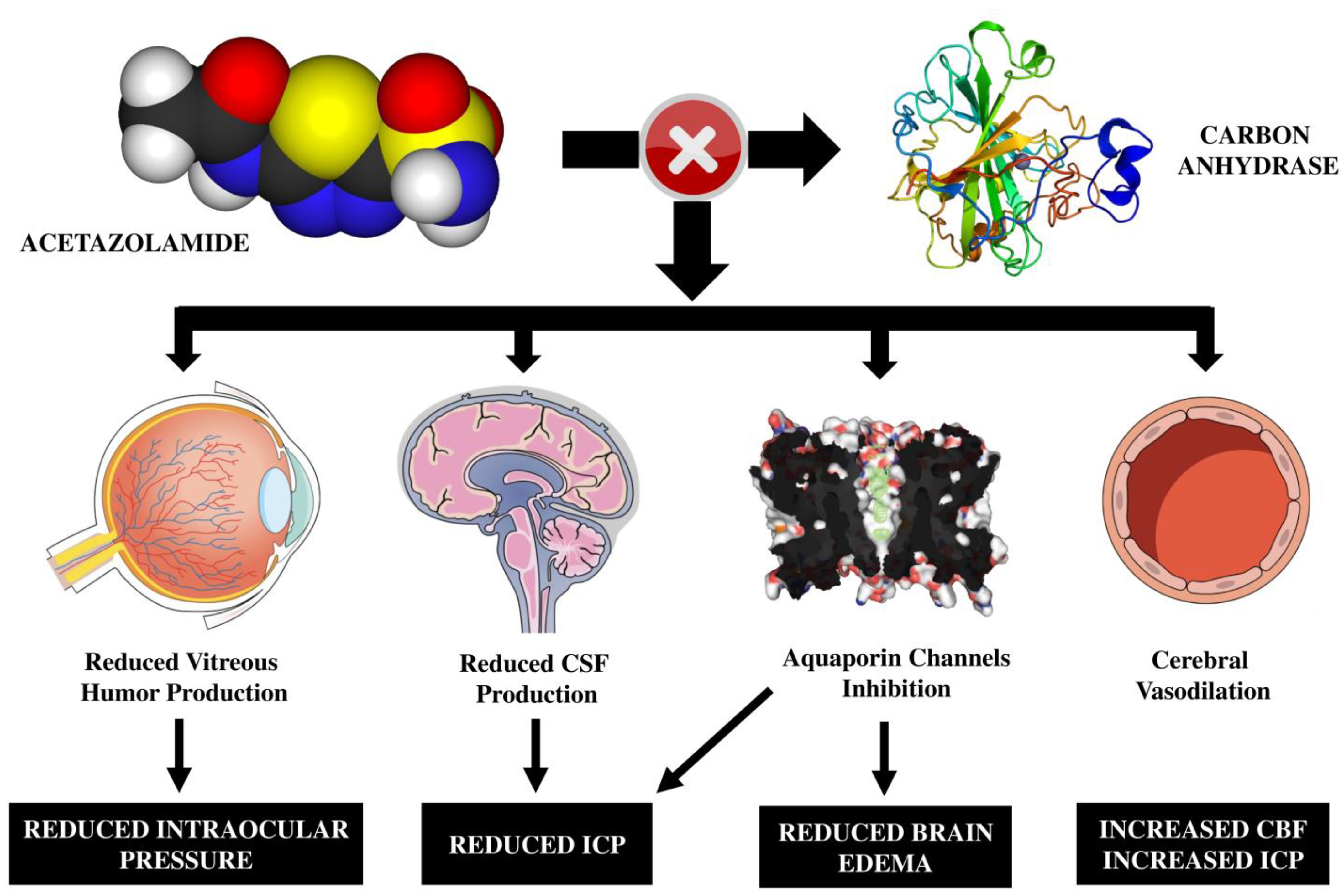
:1. Introduction
2. Materials and Methods
2.1. Population, Interventions, Comparatives, and Outcomes (PICOs) and Eligibility Criteria
2.2. Search Strategies
2.3. Selection of Studies
2.4. Assessment of Risk of Bias of Included Studies
2.5. Data Extraction
3. Results
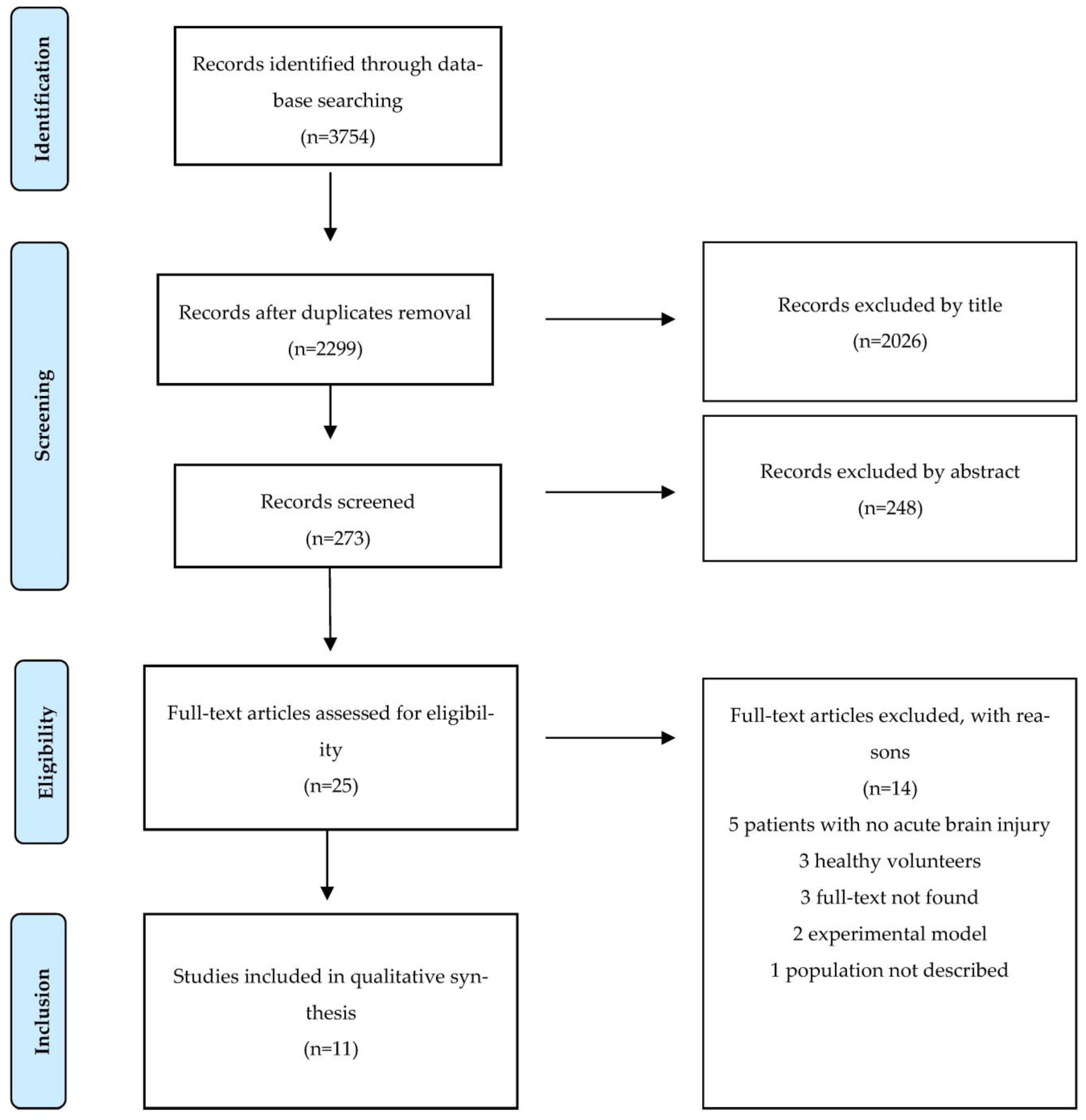
3.1. Study Selection and Characteristics
3.2. Summary of Main Results
| Author (Year) | Study Design and Population | Outcomes | Measuring Method | Main Results |
|---|---|---|---|---|
| Shinoda et al. (1991) [23] | Prospective interventional, single-center, Japan 42 patients with SAH | Cerebral perfusion after acetazolamide associated with different findings in the acute and subacute stages of SAH. | 123I–IMP–SPECT | Diffuse brain swelling: no perfusion in the acute-subacute stages. VMR was reduced in all patients in the acute stage after surgical intervention but improved over time. VMR decreased more in the subacute stage (9–21 days), and it was more frequently reduced in patient with worse clinical status. |
| Kimura et al. (1993) [24] | Prospective interventional, single-center, Japan 79 patients with SAH | Correlation between VMR and development of DCI due to vasospasms. | 123I–IMP–SPECT | Reduced VMR by day 8 after SAH in several territories is associated with DCI due to vasospasms. |
| Yoshida et al. (1996) [25] | Prospective interventional, single-center, Japan 50 patients with SAH | Relationship between VMR in the acute phase and clinical outcome. | Xe-CT | Higher VMR was associated with better clinical outcomes, while there were no differences according to SAH grades or the development of vasospasms. |
| Kitahara et al. (1996) [18] | Prospective interventional, single-center, Japan 22 patients with ICH | VMR in patients with HPH compared to hypertensive patients with no ICH (non-HPH). | Xe-CT | VMR was preoperatively reduced in the ipsilateral hemisphere compared to non-HPH, while in the thalamus, there were no significant differences. Contralateral VMR in the hemisphere was lower in HPH during the preoperative phase compared to non-HPH, though it significantly rose chronically. |
| Tanaka et al. (1996) [19] | Prospective interventional, single-center, Japan 15 patients with ICH | VMR in the chronic stage of putaminal vs. thalamic hemorrhages. | Xe-CT | VMR was statistically significant in the chronic stages in both types of hemorrhage. |
| Szabo et al. (1997) [26] | Prospective interventional, single-center, Hungary 27 patients with aSAH | VMR in a 1–8-year follow-up after a vasospasm in aSAH. | TCD | VMR was restored to normal values. |
| Tanaka et al. (1998) [27] | Prospective interventional, single-center, Japan 18 patients symptomatic for vasospasms after aSAH and 27 patients asymptomatic for vasospasm after aSAH (tot = 45 patients) | VMR in patients with and without ischemic symptoms due to vasospasms in the acute, subacute, and chronic stages of aSAH. | Xe-CT | In symptomatic patients, VMR was normal in the acute stage, while it was significantly higher in the chronic phase compared to healthy controls (subacute stage was not tested). In asymptomatic patients, VMR was significantly reduced from the acute to the subacute stage, though it rose chronically. |
| Chang et al. (2003) [21] | Prospective interventional, single-center, Japan 48 patients with ventriculomegaly after aSAH | VMR in a follow-up (1–12 months) compared to healthy subjects and after surgical shunting in patients who developed symptomatic NPH. | Radionuclide angiography with 99mTc-HMPAO | VMR was reduced in asymptomatic and symptomatic patients, except in non-responders to surgery. After shunting, VMR was increased in clinically recovered patients but stable in those who remained symptomatic. |
| Nogami et al. (2004) [20] | Prospective interventional, single-center, Japan 17 patients with HIE in the subacute stage | Correlation of VMR with clinical outcome; correlation of MRI findings and clinical outcome; correlation of VMR with MRI patterns. | Xe-CT | VMR resulted in more patients with a good clinical outcome. Patients with an unfavorable MRI pattern (hyperintense lesions in T1 and T2) had lower VMR. |
| Jarus-Dziedzic et al. (2011) [28] | Prospective interventional, single-center, Poland 24 patients with aSAH | BFV and CVR in a long-term follow-up; CO2 reactivity in patients treated with clipping, coiling or conservatively. | TCD | VMR, after acetazolamide administration, was restored in the chronic stage. Reactivity to CO2 was preserved in the three groups with not statistically significant differences. |
| Bøthun et al. (2019) [22] | Prospective interventional, single-center, Norway 42 patients with aSAH and 37 patients with UIA (tot = 79 patients) | CVR as a potential predictor of DCI; relationship between CVR and rupture status of the aneurysm (UIA vs. aSAH). | TCD | A reduction in contralateral VMR is predictive of the development of clinical DCI but not radiological infarction. VMR is reduced in patients with aSAH compared to UIA. |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Berkel, M.A.; Elefritz, J.L. Evaluating off-label uses of acetazolamide. Am. J. Health Pharm. 2018, 75, 524–531. [Google Scholar] [CrossRef]
- Shukralla, A.A.; Dolan, E.; Delanty, N. Acetazolamide: Old drug, new evidence? Epilepsia Open 2022, 7, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Barbuskaite, D.; Oernbo, E.K.; Wardman, J.H.; Toft-Bertelsen, T.L.; Conti, E.; Andreassen, S.N.; Gerkau, N.J.; Rose, C.R.; MacAulay, N. Acetazolamide modulates intracranial pressure directly by its action on the cerebrospinal fluid secretion apparatus. Fluids Barriers CNS 2022, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Williamson, M.R.; Wilkinson, C.M.; Dietrich, K.; Colbourne, F. Acetazolamide Mitigates Intracranial Pressure Spikes Without Affecting Functional Outcome After Experimental Hemorrhagic Stroke. Transl. Stroke Res. 2019, 10, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Heuser, D.; Astrup, J.; Lassen, N.A.; Betz, E. Brain carbonic acid acidosis after acetazolamide. Acta Physiol. Scand. 1975, 93, 385–390. [Google Scholar] [CrossRef]
- Severinghaus, J.W.; Cotev, S. Carbonic acidosis and cerebral vasodilation after diamox. Scand. J. Clin. Lab. Investig. Suppl. 1968, 21, IE. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.E. Functional TCD: Regulation of cerebral hemodynamics-cerebral autoregulation, vasomotor reactivity, and neurovascular coupling. Front Transl. Neurosonol. 2015, 36, 40–56. [Google Scholar]
- Laux, B.E.; Raichle, M.E. The effect of acetazolamide on cerebral blood flow and oxygen utilization in the rhesus monkey. J. Clin. Investig. 1978, 62, 585–592. [Google Scholar] [CrossRef]
- Zirak, P.; Delgado-Mederos, R.; Martí-Fàbregas, J.; Durduran, T. Effects of acetazolamide on the micro- and macro-vascular cerebral hemodynamics: A diffuse optical and transcranial doppler ultrasound study. Biomed. Opt. Express 2010, 1, 1443–1459. [Google Scholar] [CrossRef]
- Vorstrup, S.; Henriksen, L.; Paulson, O.B. Effect of acetazolamide on cerebral blood flow and cerebral metabolic rate for oxygen. J. Clin. Investig. 1984, 74, 1634–1639. [Google Scholar] [CrossRef]
- Rostami, E.; Engquist, H.; Enblad, P. Imaging of cerebral blood flow in patients with severe traumatic brain injury in the neurointensive care. Front. Neurol. 2014, 5, 114. [Google Scholar] [CrossRef] [PubMed]
- Kaminogo, M.; Ichikura, A.; Shibata, S.; Toba, T.; Yonekura, M. Effect of acetazolamide on regional cerebral oxygen saturation and regional cerebral blood flow. Stroke 1995, 26, 2358–2360. [Google Scholar] [CrossRef] [PubMed]
- Robba, C.; Taccone, F.S.; Citerio, G. Monitoring cerebral oxygenation in acute brain-injured patients. Intensiv. Care Med. 2022, 48, 1463–1466. [Google Scholar] [CrossRef] [PubMed]
- Oddo, M.; Bösel, J.; Participants in the International Multidisciplinary Consensus Conference on Multimodality Monitoring. Monitoring of brain and systemic oxygenation in neurocritical care patients. Neurocritical Care 2014, 21 (Suppl. S2), 103–120. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Prisma-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (Minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Kitahara, T.; Yamashita, T.; Kashiwagi, S.; Kawakami, N.; Ishihara, H.; Ito, H. Hemodynamics of hypertensive putaminal hemorrhage evaluated by Xenon-enhanced computed tomography and acetazolamide test. Acta Neurol. Scand. Suppl. 1996, 93, 139–143. [Google Scholar] [CrossRef]
- Tanaka, A.; Yoshinaga, S.; Nakayama, Y.; Kimura, M.; Tomonaga, M. Cerebral blood flow and clinical outcome in patients with thalamic hemorrhages: A comparison with putaminal hemorrhages. J. Neurol. Sci. 1996, 144, 191–197. [Google Scholar] [CrossRef]
- Nogami, K.; Fujii, M.; Kato, S.; Nishizaki, T.; Suzuki, M.; Yamashita, S.; Oda, Y.; Sadamitsu, D.; Maekawa, T. Analysis of magnetic resonance imaging (MRI) morphometry and cerebral blood flow in patients with hypoxic-ischemic encephalopathy. J. Clin. Neurosci. 2004, 11, 376–380. [Google Scholar] [CrossRef]
- Chang, C.-C.; Kuwana, N.; Ito, S.; Yokoyama, T.; Kanno, H.; Yamamoto, I. Cerebral haemodynamics in patients with hydrocephalus after subarachnoid haemorrhage due to ruptured aneurysm. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 123–126. [Google Scholar] [CrossRef]
- Bøthun, M.L.; Haaland, A.; Moen, G.; Logallo, N.; Svendsen, F.; Thomassen, L.; Helland, C.A. Impaired cerebrovascular reactivity may predict delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. J. Neurol. Sci. 2019, 407, 116539. [Google Scholar] [CrossRef]
- Shinoda, J.; Kimura, T.; Funakoshi, T.; Araki, Y.; Imao, Y. Acetazolamide reactivity on cerebral blood flow in patients with subarachnoid haemorrhage. Acta Neurochir. 1991, 109, 102–108. [Google Scholar] [CrossRef]
- Kimura, T.; Shinoda, J.; Funakoshi, T. Prediction of cerebral infarction due to vasospasm following aneurysmal subarachnoid haemorrhage using acetazolamide-activated123I-IMP SPECT. Acta Neurochir. 1993, 123, 125–128. [Google Scholar] [CrossRef]
- Yoshida, K.; Nakamura, S.; Watanabe, H.; Kinoshita, K. Early cerebral blood flow and vascular reactivity to acetazolamide in predicting the outcome after ruptured cerebral aneurysm. Acta Neurol. Scand. Suppl. 1996, 93, 131–134. [Google Scholar] [CrossRef]
- Szabo, S.; Sheth, R.N.; Novak, L.; Rozsa, L.; Ficzere, A. Cerebrovascular reserve capacity many years after vasospasm due to aneurysmal subarachnoid hemorrhage: A transcranial Doppler study with acetazolamide test. Stroke 1997, 28, 2479–2482. [Google Scholar] [CrossRef]
- Tanaka, A.; Yoshinaga, S.; Nakayama, Y.; Tomonaga, M. Cerebral blood flow and the response to acetazolamide during the acute, subacute, and chronic stages of aneurysmal subarachnoid hemorrhage. Neurol. Med. Chir. 1998, 38, 623–632. [Google Scholar] [CrossRef]
- Jarus-Dziedzic, K.; Głowacki, M.; Warzecha, A.; Jurkiewicz, J.; Czernicki, Z.; Fersten, E. Cerebrovascular reactivity evaluated by transcranial doppler sonography in patients after aneurysmal subarachnoid haemorrhage treated with microsurgical clipping or endovascular coiling technique. Neurol. Res. 2011, 33, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrase inhibitors. Bioorganic Med. Chem. Lett. 2010, 20, 3467–3474. [Google Scholar] [CrossRef] [PubMed]
- Mokri, B. The Monro-Kellie hypothesis: Applications in CSF volume depletion. Neurology 2001, 56, 1746–1748. [Google Scholar] [CrossRef] [PubMed]
- Radolovich, D.K.; Czosnyka, M.; Timofeev, I.; Lavinio, A.; Kim, D.-J.; Jaeger, M.; Hutchinson, P.; Gupta, A.; Pickard, J.D.; Smielewski, P. Transient changes in brain tissue oxygen in response to modifications of cerebral perfusion pressure: An observational study. Anesth. Analg. 2010, 110, 165–173. [Google Scholar] [CrossRef]
- Megjhani, M.; Weiss, M.; Ford, J.; Terilli, K.; Kastenholz, N.; Nametz, D.; Bin Kwon, S.; Velazquez, A.; Agarwal, S.; Roh, D.J.; et al. Optimal cerebral perfusion pressure and brain tissue oxygen in aneurysmal subarachnoid hemorrhage. Stroke 2023, 54, 189–197. [Google Scholar] [CrossRef]
- Dias, C.; Maia, I.; Cerejo, A.; Varsos, G.; Smielewski, P.; Paiva, J.-A.; Czosnyka, M. Pressures, flow, and brain oxygenation during plateau waves of intracranial pressure. Neurocritical Care 2013, 21, 124–132. [Google Scholar] [CrossRef]
- Scotton, W.J.; Botfield, H.F.; Westgate, C.S.; Mitchell, J.L.; Yiangou, A.; Uldall, M.S.; Jensen, R.H.; Sinclair, A.J. Topiramate is more effective than acetazolamide at lowering intracranial pressure. Cephalalgia 2019, 39, 209–218. [Google Scholar] [CrossRef]
- Osgood, M.L. Aneurysmal Subarachnoid Hemorrhage: Review of the Pathophysiology and Management Strategies. Curr. Neurol. Neurosci. Rep. 2021, 21, 50. [Google Scholar] [CrossRef]
- Naraoka, M.; Matsuda, N.; Shimamura, N.; Asano, K.; Ohkuma, H. the role of arterioles and the microcirculation in the development of vasospasm after aneurysmal SAH. BioMed. Res. Int. 2014, 2014, 253746. [Google Scholar] [CrossRef]
- Balbi, M.; Koide, M.; Wellman, G.C.; Plesnila, N. Inversion of neurovascular coupling after subarachnoid hemorrhage in vivo. J. Cereb. Blood Flow Metab. 2017, 37, 3625–3634. [Google Scholar] [CrossRef]
- Suzuki, H.; Kanamaru, H.; Kawakita, F.; Asada, R.; Fujimoto, M.; Shiba, M. Cerebrovascular pathophysiology of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Histol. Histopathol. 2020, 36, 143–158. [Google Scholar] [PubMed]
- Ling, G.S.F.; Neal, C.J. Maintaining cerebral perfusion pressure is a worthy clinical goal. Neurocritical Care 2005, 2, 075–082. [Google Scholar] [CrossRef] [PubMed]
- Kealy, J.; Bennett, R.; Lowry, J.P. Simultaneous recording of hippocampal oxygen and glucose in real time using constant potential amperometry in the freely-moving rat. J. Neurosci. Methods 2013, 215, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Bickler, P.E.; Litt, L.; Severinghaus, J.W. Effects of acetazolamide on cerebrocortical NADH and blood volume. J. Appl. Physiol. 1988, 65, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Buch, S.; Ye, Y.; Haacke, E.M. Quantifying the changes in oxygen extraction fraction and cerebral activity caused by caffeine and acetazolamide. J. Cereb. Blood Flow Metab. 2017, 37, 825–836. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stella, C.; Hachlouf, A.; Calabrò, L.; Cavalli, I.; Schuind, S.; Gouvea Bogossian, E.; Taccone, F.S. The Effects of Acetazolamide on Cerebral Hemodynamics in Adult Patients with an Acute Brain Injury: A Systematic Review. Brain Sci. 2023, 13, 1678. https://doi.org/10.3390/brainsci13121678
Stella C, Hachlouf A, Calabrò L, Cavalli I, Schuind S, Gouvea Bogossian E, Taccone FS. The Effects of Acetazolamide on Cerebral Hemodynamics in Adult Patients with an Acute Brain Injury: A Systematic Review. Brain Sciences. 2023; 13(12):1678. https://doi.org/10.3390/brainsci13121678
Chicago/Turabian StyleStella, Claudia, Anas Hachlouf, Lorenzo Calabrò, Irene Cavalli, Sophie Schuind, Elisa Gouvea Bogossian, and Fabio Silvio Taccone. 2023. "The Effects of Acetazolamide on Cerebral Hemodynamics in Adult Patients with an Acute Brain Injury: A Systematic Review" Brain Sciences 13, no. 12: 1678. https://doi.org/10.3390/brainsci13121678






