Abstract
This meta-analysis investigates auditory steady-state responses (ASSRs) as potential biomarkers of schizophrenia, focusing on previously unexplored clinical populations, frequencies, and variables. We examined 37 studies, encompassing a diverse cohort of 1788 patients with schizophrenia, including 208 patients with first-episode psychosis, 281 at-risk individuals, and 1603 healthy controls. The results indicate moderate reductions in 40 Hz ASSRs in schizophrenia patients, with significantly greater reductions in first-episode psychosis patients and minimal changes in at-risk individuals. These results call into question the expected progression of ASSR alterations across all stages of schizophrenia. The analysis also revealed the sensitivity of ASSR alterations at 40 Hz to various factors, including stimulus type, level of analysis, and attentional focus. In conclusion, our research highlights ASSRs, particularly at 40 Hz, as potential biomarkers of schizophrenia, revealing varied implications across different stages of the disorder. This study enriches our understanding of ASSRs in schizophrenia, highlighting their potential diagnostic and therapeutic relevance, particularly in the early stages of the disease.
1. Introduction
Auditory hallucinations are prevalent symptoms of schizophrenia, reported in 60 to 80% of patients [1]. These hallucinations are hypothesized to be closely related to a neural imbalance between excitation and inhibition, particularly involving synaptic interactions between parvalbumin interneurons and pyramidal neurons [2]. Emerging evidence suggests that this excitation–inhibition imbalance is not merely the result of GABAergic dysfunction but also involves deficits in glutamatergic NMDA receptors on parvalbumin-positive GABAergic interneurons, leading to alterations in gamma oscillations [3,4]. Moreover, these dysfunctional gamma-band oscillations, specifically in the 30–100 Hz range, have been linked to cognitive deficits such as impaired information processing speed and working memory, commonly observed in schizophrenia [5,6]. Therefore, the role of gamma-band oscillations becomes a central focus in understanding the pathophysiology of schizophrenia.
Auditory Steady-State Responses (ASSRs) are electrophysiological markers of auditory system activity, often observed in the gamma frequency range (30–100 Hz) with a robust response at 40 Hz [7]. Generated in response to periodic auditory stimuli, such as click trains or amplitude-modulated tones, ASSRs primarily consists of two components: power and phase. Power provides insights into the intensity of neural oscillatory activity, while phase gives information about the timing of these oscillations relative to the auditory stimulus. These components can be measured using non-invasive electrophysiological tools such as electroencephalography (EEG) and magnetoencephalography (MEG) [8]. Neural sources of ASSRs have been mainly located in brain regions such as the medial primary auditory cortex, the superior temporal gyrus, and Heschl’s gyrus—areas frequently implicated in auditory processing [9,10,11,12,13].
The 40 Hz ASSR, with its capacity to quantitatively measure gamma-band oscillations, has been increasingly acknowledged as a possible biomarker for evaluating the underlying neural mechanisms of excitation/inhibition imbalance in schizophrenia [14,15]. This result has been supported by a meta-analysis by Thuné et al. [16], which synthesized outcomes from 20 EEG and/or MEG studies, demonstrating reduced power and phase in response to 40 Hz ASSRs among schizophrenia patients relative to healthy controls.
Numerous new studies have been published since that first meta-analysis [16]. This surge in available data offers an opportunity for enhanced analysis. While their work made valuable contributions by highlighting the reduced power and phase of 40 Hz ASSRs in schizophrenia, it also included some exploratory research on beta oscillations at 20 Hz. However, a more in-depth study on 20 Hz and any investigation of 30 Hz were notably absent, likely due to insufficient data focusing on these frequencies. The investigation of 20 Hz is of methodological interest, as it may allow us to determine the specificity of gamma oscillations (30–100 Hz range) compared to beta oscillations (20 Hz). Additionally, the available data offer an opportunity to look at specific subgroups, such as those with first-episode psychosis (FEP) and at-risk populations. Recent studies have shown deficits in ASSRs in these populations [14]. It remains to be determined, however, whether the amplitude of ASSR alterations exhibit a gradient effect (SCZ > FEP > at risk), similar to what has been observed in the case of other potential biomarkers of schizophrenia [17,18].
Expanding on the previous meta-analysis, we aim to investigate the same variables further: stimulus duration, stimulus type (click vs. amplitude tones), and analysis level (sensor vs. source). The distinction between sensor and source and between EEG and MEG is important. Edgar et al. [19] demonstrated that sensor-based evaluations have limitations, like the inability to discern hemisphere variations in 40 Hz responses, whereas source-based assessments in regions like the STG provide more precise and region-specific insights. In addition, we planned to look at variables that have not been fully explored before, including the effects of symptom severity, medication dosage, and attention load during stimuli presentation. Understanding the role of these factors is crucial. If the ASSRs are reduced in SZ, regardless of where the person’s attention is, it suggests that ASSRs might indicate a basic sensory deficit in psychosis rather than a problem with adjusting neuromodulation based on the context [20,21].
Therefore, our study aims to offer a comprehensive and updated meta-analysis that synthesizes these new data, enhancing our understanding of the utility of ASSRs as a biomarker for schizophrenia while paying detailed attention to the clinical and methodological factors that could contribute to variability in results across studies.
2. Materials and Methods
2.1. Literature Search Strategy
This meta-analysis followed the Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [22] (Supplementary Figure S1). Four databases were searched: MedLine, Scopus, Embase, and the Web of Science. To build upon the work of the last comprehensive meta-analysis by Thuné et al. [16], which ceased its literature search in March 2016, we conducted an updated search extending from March 2016 to June 2023. The combination of keywords included auditory (or gamma or 40 Hz) steady-state response and schizophrenia (or psychosis or psychoses or psychotic disorder or schizophrenic disorder).
2.2. Study Selection
The initial inclusion criteria were also based on the previous meta-analysis [16]: (1) articles written in French or English, (2) human studies, (3) original research articles with new data, (4) studied that used EEG or MEG to measure ASSRs, (5) studies with at least one sample of patients with schizophrenia or an at-risk population and one sample of healthy controls, (6) studies containing measures of spectral evoked power and/or intertrial phase coherence (ITPC, also known as phase-lock factor and phase synchronization), and (7) studies that included sufficient statistical information (sample sizes and mean values and/or raw data and/or p-values and/or effect sizes). Exclusion criteria were as follows: (1) published before March 2016; (2) full-text not available; and (3) duplicates. The selection of studies was based on a consensual decision by IZ and SP.
The collected articles were imported into the EndNote 20 software, and throughout the selection process, which followed the PRISMA model, the two investigators engaged in ongoing consultation to make their decisions.
2.3. Data Extraction
The two investigators decided on the charting form as follows: authors, publication year, sample size for each population, percentage of males within each population, age, illness duration (mean, sd), chlorpromazine dosage in mg/day (mean, sd), PANS scores or scores converted from SANS or SAPS to PANSS via an open-access website (http://converteasy.org, accessed on 15 August 2023), image analysis level (sensor and/or source), stimulus type (click or amplitude), duration of stimulus presentation (brief ≤ 500 ms or long ≥ 1000 ms), attention load (characterized as passive, active, or involving distraction), and analyzed frequencies (40, 30 or 20 Hz). The populations include Healthy Controls (HC), individuals with Schizophrenia (SC), individuals with First Episode Psychosis (FEP), and At-Risk populations (AR), which consisted of both clinical at-risk individuals and relatives of the patients.
2.4. Statistical Analysis
Comprehensive Meta-Analysis Version-2 was used to calculate effect size estimates of the differences in ASSRs between schizophrenia patients (and at-risk individuals) and healthy volunteers [23]. The effect size estimates were calculated using Cohen’s d [24]. The direction of the effect size was considered positive if the ASSRs were reduced in schizophrenia patients (and at-risk individuals) relative to healthy individuals. Effect size estimates were calculated based on oscillation frequencies (40 Hz, 30 Hz, and 20 Hz) and ASSR components (power vs. phase). Following the convention of Cohen [24], effect size estimates of 0.2, 0.5, and 0.8 were considered small, medium, and large, respectively.
Aggregating effect size estimates across studies is more legitimate when estimates are homogeneous. Heterogeneity among effect size estimates was assessed with the Q statistics [25], with the magnitude of heterogeneity being evaluated with the I2 index [26]. The level of significance was set at p < 0.05. Given the heterogeneity of the meta-analysis (see below), a random-effects model was used for study aggregation. Relative to fixed-effects models, random-effects models take between-study variability into account and allow for population-level inferences [27].
To determine the potential impact on the results of methodological parameters, we performed sub-analyses on analysis type (sensor vs. source), stimulus types (click vs. amplitude), stimuli duration (brief vs. long), and attention load (active, distraction, and passive). Given the absence of an effect for beta oscillations at 20 Hz and the limited studies focusing on gamma oscillations at 30 Hz, the sub-analyses were confined to studies that examined 40 Hz gamma oscillations. To estimate the effect of sociodemographic variables [e.g., age of participants (in years) and sex ratio (% of males)], antipsychotic dosage (e.g., mean chlorpromazine equivalents) and psychiatric (positive and negative) symptoms on results, we performed meta-regression analyses. Finally, the possibility of publication bias was examined with Egger’s regression test [28], Begg and Mazumdar’s rank correlation test [29], and a visual inspection of the funnel plot. As per guidelines from Cochrane (https://cochrane.org, accessed on 15 August 2023), we performed the sub-analyses, meta-regression, and publication bias analyses only for outcomes comprising at least 10 study arms.
Some studies reported negative findings without providing values (t-, p-, means and SD, etc.), allowing us to calculate effect size estimates. Based on an approach described in Appendix A, the effect size estimates for these studies were imputed. As per guidelines from Cochrane (https://cochrane.org), we aggregated results across studies with and without the imputed effect size estimates.
3. Results
3.1. Sample
A total of 37 publications were included in the meta-analysis (Supplementary Figure S1). (n = 480) Based on our criteria, 70 articles were excluded (healthy controls only or patients only [n = 29], data re-analyzed in later studies [n = 9], full text not available [n = 28] and no power or phase results [n = 5]). In cases where datasets from the earlier studies had either been reused or developed in more recent studies, we retained the datasets with the largest sample sizes for a more robust analysis. This strategy was notably true for the dataset initially presented by Tada et al. [30], which was subsequently expanded by Koshiyama et al. [31], for nine recent studies that have since been updated with larger sample sizes.
The total sample consists of 1788 individuals diagnosed with schizophrenia, with 208 of them experiencing their first episode of psychosis,281 individuals identified as at-risk (either relatives or clinical high-risk individuals), along with 1603 healthy controls (Supplementary Table S1). Each included study provided data on ASSR power and/or phase, recorded at the sensor or source level. The stimuli used were either click or amplitude-modulated tones, and the studies varied in attention load and stimulus duration. Notably, results for 40 Hz oscillations were reported in all included studies (see Table 1).

Table 1.
Methodological characteristics of the selected studies.
3.2. Differences in ASSRs between Groups
3.2.1. Gamma Oscillations at 40 Hz
For gamma oscillations, the ASSRs were reduced in schizophrenia patients relative to controls, and the magnitude of this reduction was in the moderate range (Table 2). This was the case for analyses based on both power (28 study arms) and phase (23 study arms) components of ASSRs. For both power and phase, there was evidence of publication bias, as determined by Egger’s test (power: t = 2.0; p = 0.057; phase: t = 3.1; p = 0.005; Supplementary Figure S2) and the Begg and Mazumbar’s test (power: Kendall’s tau = 0.392; p = 0.003; phase: Kendall’s tau = 0.454; p = 0.002; Supplementary Figure S3). With the addition of the imputed value of negative findings, the estimates remained moderate in both cases (power and phase) for comparing schizophrenia patients and controls (Table 2).

Table 2.
Main results.
In individuals with a first episode of psychosis, moderate-to-large effects were observed for the power and phase components of the ASSRs (8 and 4 study arms), respectively (Table 2). In individuals at risk for psychosis, a non-significant and small effect was observed for the power component of the ASSRs (6 study arms), while a small-to-moderate reduction was observed in the case of the phase component of the ASSRs (4 study arms) (Table 2).
3.2.2. Gamma Oscillations at 30 Hz
For gamma oscillations (30 Hz), small-to-moderate reductions in the ASSRs were observed in schizophrenia patients relative to controls for both power (7 study arms) and phase (5 study arms) (Table 2).
3.2.3. Beta Oscillations at 20 Hz
For beta oscillations, no differences in the ASSRs were observed between schizophrenia patients and healthy volunteers, regardless of power and phase (Table 2). There was no evidence of publication bias (power; 11 study arms) using both Egger’s test (t = 1.6; p = 0.153) and Begg and Mazumbar’s test (Kendall’s tau = −0.127; p = 0.586) (Supplementary Figure S4).
3.3. Sub-Analyses
Due to a lack of effect for beta oscillations at 20 Hz and a few studies examining gamma oscillations at 30 Hz, sub-analyses were only performed with studies examining 40 Hz gamma oscillations. Overall, for the power component of ASSRs, image analysis type, stimulus type, stimuli duration, and attention load had small effects on the results (Table 3). The reduction in ASSRs in schizophrenia patients was larger in studies using source analyses than analyses of sensors. The reduction in ASSRs was more prominent in studies using click trains instead of amplitude-modulated tones. The reduction in ASSRs was more significant in studies using brief rather than long stimuli. Finally, it is important to note that the reduction in ASSRs in schizophrenia patients was more pronounced in studies that used a distracting attention load. However, this difference was observed based on only 2 study arms.

Table 3.
Sub-analyses of gamma oscillations (40 Hz) for both the power and phase components of the auditory steady-state response.
As for the phase component of the ASSRs, there were no significant effects of analysis type, stimulus type, or the duration of stimuli on gamma oscillation results (Table 3). The reduction in ASSRs in schizophrenia patients was larger in studies that used a distracting attention load; however, this difference was observed based on only 2 study arms.
3.4. Meta-Regression Analyses
Due to a lack of effect for beta oscillations at 20 Hz and a small number of studies examining gamma oscillations at 30 Hz, sub-analyses were only performed with studies examining 40 Hz gamma oscillations. For the power component of ASSRs, there were no significant associations between age, sex ratio, positive symptoms, negative symptoms, chlorpromazine equivalents, or the magnitude of ASSR reduction observed in schizophrenia (Table 4).

Table 4.
Meta-regression analyses for studies assessing gamma oscillations (40 Hz).
For the phase component, a significant and negative association was observed between patients’ age and the magnitude of the ASSR reduction, such that the reduction in gamma oscillations was more robust in younger patients (Table 4; Figure 1). None of the other clinical variables were significantly associated with the magnitude of the reduction in ASSRs observed in schizophrenia (Table 4).
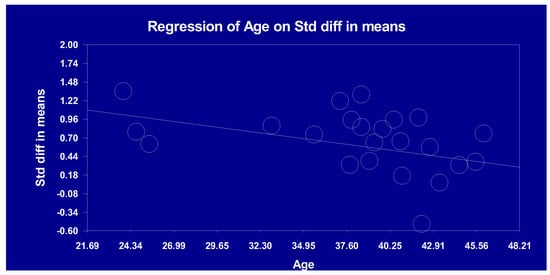
Figure 1.
Linear association between age and auditory steady-state response (40 Hz; phase).
4. Discussion
Our updated meta-analysis aimed to provide an overview of ASSRs as potential biomarkers of schizophrenia by synthesizing previous data with recent ones, focusing on previously unexplored clinical populations, frequencies, and variables. Our main findings include moderate ASSR reductions in gamma oscillations at 40 Hz, a subtle effect at 30 Hz in schizophrenia patients, no discernible difference in beta oscillations at 20 Hz, and distinct associations based on attentional load and age.
4.1. ASSRs in Different Clinical Stages of Schizophrenia
In patients with schizophrenia, a significant effect in the gamma range at 40 Hz was observed, shedding light on the role of gamma-band abnormalities in the pathophysiology of schizophrenia, potentially indicating deficits in the integration of cortical activity. The modest effect at 30 Hz and not at 20 Hz suggests a possible frequency-specific nature of ASSR deficiencies in schizophrenia, which needs further exploration. A publication bias was found when examining auditory steady-state responses (ASSRs) in gamma oscillations’ power and phase components at 40 Hz.
In individuals at risk for psychosis, only a small effect was seen in the power component, while the phase component showed a small-to-moderate reduction. Conversely, in the context of patients experiencing their first episode of psychosis, the observed moderate-to-large effects on both the power and phase components of the 40 Hz ASSR suggest a significant reduction in auditory processing capabilities during these early stages of the disorder. This pattern contrasts with the anticipated gradient of ASSR alterations (SCZ > FEP > at risk), typically seen in other potential biomarkers of schizophrenia, such as neurobiological and neurocognitive markers [18].
Gamma oscillations are known to be dependent on glutamatergic mechanisms [3,4]. Interestingly, a meta-analysis from Nakahara et al. [67] revealed elevated levels of glutamate in limbic and subcortical brain regions that were particularly prominent in individuals with early stage schizophrenia. In theory, it can be hypothesized that deficits in gamma oscillations are related to the pronounced glutamatergic alterations observed in the early stages of psychosis [68,69]. However, this hypothesis would need to be tested. Alternatively, our results could be explained by the effect of antipsychotics. Indeed, the pronounced ASSR deficits observed in FEP patients could be due to a relative normalization of gamma oscillations with long-term exposure to antipsychotics in chronic patients. This remains speculative, however, as the literature on the effect of antipsychotics on gamma oscillations is still in its infancy [49].
4.2. Sub-Analyses
Regarding 40 Hz-gamma oscillations, variations in analysis types, stimulus types, and durations were observed, with more significant reductions in ASSR in certain conditions, notably source analyses, click trains, and studies based on distraction. The 40 Hz ASSR power decrease in schizophrenia patients was more pronounced in studies using source analyses than in sensor analyses. Sensor-based analyses, mainly using EEG electrodes at Cz and Fz, give a broader view of neuronal activity, reflecting signals dispersed over the skull. While effective in registering ASSRs due to their fronto-central location, these sensors might miss subtleties from specific regions like the auditory cortex. Importantly, ASSR sources are not limited to the auditory cortex but also encompass areas like the cerebellum, frontal, and parietal lobes [70,71]. Conversely, source-based analyses target auditory regions, offering a detailed and localized perspective. Edgar et al. [19] point out that this approach can discern complex hemispheric response variations at 40 Hz, offering more region-specific insights. Therefore, the pronounced reduction observed in ASSR power from source-level analyses can be attributed to its precision in targeting ASSR origins, whereas sensor-level analysis, capturing a broader, averaged view, may not reflect the reduction to the same extent. It must be noted, however, that the difference between the source and sensor was small (power: d = 0.663 vs. d = 0.483; phase: d = 0.746 vs. d = 0.553), but it was observed in the case of both the power and phase components of the ASSR.
Studies employing click trains over amplitude-modulated tones saw a more extensive 40 Hz ASSR reduction in the case of the power component. According to Griskova-Bulanova et al. [52], click stimuli generated more powerful and better-coordinated ASSRs. The underlying reason could be the nature of the stimuli: clicks, characterized by a brief broadband noise with rapid transitions, engage larger regions of the auditory cortex. This broad engagement, combined with the abruptness of clicks, results in more robust ASSR responses. By contrast, amplitude-modulated tones have slower transitions and target a narrower frequency range, which could lead to milder ASSR results [72].
Regarding the influence of stimulus duration on 40 Hz ASSR effects (power and phase components), our findings indicate a trend where the reduction in ASSRs was more pronounced in studies utilizing brief stimuli compared to those with longer durations. Hamm et al. [20] reported that decreases in 40 Hz training in schizophrenia were more noticeable with 500 ms stimulus trains than with 1500 ms trains. This further underlines the possible influence of stimulus duration on the effects of ASSRs, though the overall difference was relatively small (d ≈ 0.15).
A significant observation was made regarding attention load. Studies focusing on distraction showed a more pronounced reduction in ASSRs in power and phase components. While this effect appears to be primarily attributed to distraction, it is essential to note that this observation is derived from only two studies. Given this limited dataset, we should interpret these findings with caution. This underscores the need for additional research to validate these results.
4.3. Meta-Regression Analysis
Our meta-regression analysis did not reveal significant associations between ASSR power reduction in schizophrenia patients and variables such as age, sex ratio, types of symptoms, or medication dosages. Interestingly, our data indicate that younger schizophrenia patients exhibit a more pronounced 40 Hz ASSR phase. This finding seems at odds with research suggesting an age-related neurobiological deterioration in schizophrenia [73]. Yet, it aligns with our observation of a greater ASSR reduction in First Episode Psychosis (FEP) patients than in chronic cases. Such results underscore the need for more extensive research across various age groups and stages of schizophrenia. As such, our results extend those from the previous meta-analysis by Thuné et al. [16], which also pinpointed the significant impact of age, with younger patients (≤39.8 years) showing a trend toward more robust ASSR reductions compared to older ones (>39.8 years). Given that only three studies drove the association between age and the 40 Hz ASSR phase, there is a need for more research across varied age groups and stages of schizophrenia.
4.4. Strengths and Limitations
Building on prior work by Thuné et al. [16], our meta-analysis significantly expands the scope of the research by incorporating a more extensive and diverse set of clinical populations, including recent data on first-episode psychosis and at-risk populations. It offers a comprehensive update, examining stimulus duration, type, and level of analysis, and uniquely addresses under-explored factors such as symptom severity, medication dosage, and attentional focus, advancing our understanding of ASSRs in schizophrenia. Despite these strengths, the current meta-analysis has a few limitations that need to be acknowledged. First, the insufficiency of studies, particularly in 30 Hz and at-risk populations, is a limitation that prevents the validation of the observed trends. Furthermore, the paucity of research on different methodologies limited our ability to investigate the effects of right vs. left hemisphere involvement and distinctions between time response segments when comparing ASSRs across groups. In addition, publication bias suggests a predisposition toward publishing studies with significant effects, which could distort the results. We incorporated the non-significant results into our models using our custom imputation technique to address this issue. Even with the inclusion of these imputed negative results, our overall results did not change. Finally, the lack of measurement of specific positive symptoms, such as auditory hallucinations, may have masked potential associations between ASSRs and symptoms. Current bottom-up models have proposed that abnormalities in the early processing of auditory stimuli could be involved in the emergence of auditory hallucinations [2]. Only one study has examined ASSRs in schizophrenia patients who experience auditory hallucinations and those who do not [48], but it unexpectedly failed to show differences between these two subgroups.
5. Conclusions
Our meta-analysis, encompassing recent and diverse data, reinforces the potential of auditory steady-state responses (ASSRs) as biomarkers of schizophrenia. Key findings include moderate reductions in gamma oscillations at 40 Hz and subtle effects at 30 Hz, with no significant changes at 20 Hz. In particular, patients suffering from a first episode of psychosis showed pronounced reductions in ASSRs, contrasting with the less severe changes observed in at-risk individuals. Furthermore, variations in ASSRs related to analysis type, stimulus, duration, and attention load highlight the complex influence of methodological factors on capturing auditory processing abnormalities in schizophrenia. Future research directions should focus on expanding the scope of the study to incorporate and differentiate clinical populations, such as at-risk individuals and those with first-episode psychosis. Also, more studies at frequencies other than 40 HZ could help confirm the specificity of ASSR impairment at 40 Hz in schizophrenia. There is also a need for a detailed exploration of specific positive symptoms, particularly auditory hallucinations. In addition, future studies could focus more on exploring conditions of attention and distraction, given their potential notable impact on ASSRs, as suggested in this research. As more pronounced effects were observed with source analysis, click and brief stimuli, future research should consider using these methods to obtain more conclusive and significant results.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/brainsci13121722/s1, Figure S1: Selection of articles following the PRISMA method; Figure S2: Funnel plot of the studies on gamma oscillations (40 Hz) for the power component of auditory steady-state response; Figure S3: Funnel of the studies on gamma oscillations (40 Hz) for the phase component of auditory steady-state response; Figure S4: funnel plot of standard error (vertical axis) vs. standardized difference in means (horizontal axis) for studies on the power component of beta oscillations in auditory steady-state responses; Table S1: Participants characteristics across studies.
Author Contributions
Conceptualization, I.Z. and S.P.; methodology, S.P.; formal analysis, S.P.; data curation, I.Z. and M.E.L.; writing—original draft preparation, I.Z. and S.P.; writing—review and editing, A.D. and M.E.L.; supervision, S.P. and A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
S.P. is holder of the Eli Lilly Canada Chair on schizophrenia research. A.D. is the holder of a Senior salary award from the Fonds de Recherche du Québec en Santé.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Some studies reported negative findings without providing values (t-, p-, means, and SD, etc.) that would have been required in order to calculate effect size estimates. Since the exclusion of those negative findings would likely produce an over-estimation of the composite effect size estimate, the effect size estimates for these studies were therefore imputed. Since there is no consensus on the best imputation method, we based the imputation on the studies included in the meta-analysis that failed to identify significant differences between groups (p > 0.05), but nevertheless reported values (t-, p-, means, and SD, etc.) that allow for calculating effect size estimates. For the power component of the ASSR (40 Hz), we identified six studies that did so [33,34,43,57,61]. In the case of the phase component of the ASSR (40 Hz), two studies were identified [32,66]. The aggregation of these studies produced a composite effect size estimate of d = 0.277, using a random-effect model. As an imputation method, the missing negative findings were replaced by this effect size estimate as a proxy of a representative negative finding. As per guidelines from Cochrane (https://cochrane.org), we aggregated results across studies with and without the imputed effect size estimates.
References
- Waters, F.; Collerton, D.; Ffytche, D.H.; Jardri, R.; Pins, D.; Dudley, R.; Blom, J.D.; Mosimann, U.P.; Eperjesi, F.; Ford, S.; et al. Visual Hallucinations in the Psychosis Spectrum and Comparative Information From Neurodegenerative Disorders and Eye Disease. Schizophr. Bull. 2014, 40 (Suppl. 4), S233–S245. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ouyang, P.; Zheng, Y.; Mi, L.; Zhao, J.; Ning, Y.; Guo, W. A Selective Review of the Excitatory-Inhibitory Imbalance in Schizophrenia: Underlying Biology, Genetics, Microcircuits, and Symptoms. Front. Cell Dev. Biol. 2021, 9, 664535. [Google Scholar] [CrossRef] [PubMed]
- Carlén, M.; Meletis, K.; Siegle, J.H.; Cardin, J.A.; Futai, K.; Vierling-Claassen, D.; Rühlmann, C.; Jones, S.R.; Deisseroth, K.; Sheng, M.; et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol. Psychiatry 2012, 17, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Nakao, K.; Nakazawa, K. Brain state-dependent abnormal LFP activity in the auditory cortex of a schizophrenia mouse model. Front. Neurosci. 2014, 8, 168. [Google Scholar] [CrossRef] [PubMed]
- Bora, E.; Yücel, M.; Pantelis, C. Cognitive impairment in schizophrenia and affective psychoses: Implications for DSM-V criteria and beyond. Schizophr. Bull. 2010, 36, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Bosman, C.A.; Lansink, C.S.; Pennartz, C.M. Functions of gamma-band synchronization in cognition: From single circuits to functional diversity across cortical and subcortical systems. Eur. J. Neurosci. 2014, 39, 1982–1999. [Google Scholar] [CrossRef] [PubMed]
- Picton, T.W.; John, M.S.; Dimitrijevic, A.; Purcell, D. Human auditory steady-state responses. Int. J. Audiol. 2003, 42, 177–219. [Google Scholar] [CrossRef]
- Legget, K.T.; Hild, A.K.; Steinmetz, S.E.; Simon, S.T.; Rojas, D.C. MEG and EEG demonstrate similar test-retest reliability of the 40 Hz auditory steady-state response. Int. J. Psychophysiol. 2017, 114, 16–23. [Google Scholar] [CrossRef]
- Gutschalk, A.; Mase, R.; Roth, R.; Ille, N.; Rupp, A.; Hähnel, S.; Picton, T.W.; Scherg, M. Deconvolution of 40 Hz steady-state fields reveals two overlapping source activities of the human auditory cortex. Clin. Neurophysiol. 1999, 110, 856–868. [Google Scholar] [CrossRef]
- Herdman, A.T.; Lins, O.; Van Roon, P.; Stapells, D.R.; Scherg, M.; Picton, T.W. Intracerebral sources of human auditory steady-state responses. Brain Topogr. 2002, 15, 69–86. [Google Scholar] [CrossRef]
- Pantev, C.; Roberts, L.E.; Elbert, T.; Ross, B.; Wienbruch, C. Tonotopic organization of the sources of human auditory steady-state responses. Hear. Res. 1996, 101, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Reyes, S.A.; Lockwood, A.H.; Salvi, R.J.; Coad, M.L.; Wack, D.S.; Burkard, R.F. Mapping the 40-Hz auditory steady-state response using current density reconstructions. Hear. Res. 2005, 204, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, I.; Gutschalk, A. Potential fMRI correlates of 40-Hz phase locking in primary auditory cortex, thalamus and midbrain. Neuroimage 2011, 54, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Onitsuka, T.; Tsuchimoto, R.; Oribe, N.; Spencer, K.M.; Hirano, Y. Neuronal imbalance of excitation and inhibition in schizophrenia: A scoping review of gamma-band ASSR findings. Psychiatry Clin. Neurosci. 2022, 76, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Tada, M.; Kirihara, K.; Koshiyama, D.; Fujioka, M.; Usui, K.; Uka, T.; Komatsu, M.; Kunii, N.; Araki, T.; Kasai, K. Gamma-Band Auditory Steady-State Response as a Neurophysiological Marker for Excitation and Inhibition Balance: A Review for Understanding Schizophrenia and Other Neuropsychiatric Disorders. Clin. EEG Neurosci. 2020, 51, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Thuné, H.; Recasens, M.; Uhlhaas, P.J. The 40-Hz Auditory Steady-State Response in Patients With Schizophrenia: A Meta-analysis. JAMA Psychiatry 2016, 73, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Cannon, T.D. Brain Biomarkers of Vulnerability and Progression to Psychosis. Schizophr. Bull. 2015, 42 (Suppl. S1), S127–S132. [Google Scholar] [CrossRef]
- McGorry, P.; Keshavan, M.; Goldstone, S.; Amminger, P.; Allott, K.; Berk, M.; Lavoie, S.; Pantelis, C.; Yung, A.; Wood, S.; et al. Biomarkers and clinical staging in psychiatry. World Psychiatry 2014, 13, 211–223. [Google Scholar] [CrossRef]
- Edgar, J.C.; Fisk, C.I.; Chen, Y.H.; Stone-Howell, B.; Hunter, M.A.; Huang, M.; Bustillo, J.R.; Cañive, J.M.; Miller, G.A. By our bootstraps: Comparing methods for measuring auditory 40 Hz steady-state neural activity. Psychophysiology 2017, 54, 1110–1127. [Google Scholar] [CrossRef]
- Hamm, J.P.; Bobilev, A.M.; Hayrynen, L.K.; Hudgens-Haney, M.E.; Oliver, W.T.; Parker, D.A.; McDowell, J.E.; Buckley, P.A.; Clementz, B.A. Stimulus train duration but not attention moderates gamma-band entrainment abnormalities in schizophrenia. Schizophr. Res. 2015, 165, 97–102. [Google Scholar] [CrossRef]
- Javitt, D.C. When doors of perception close: Bottom-up models of disrupted cognition in schizophrenia. Annu. Rev. Clin. Psychol. 2009, 5, 249–275. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Rothstein, H. Comprehensive Meta-Analysis; Biostat: Addison, TX, USA, 1999. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; L. Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Paulson, J.F.; Bazemore, S.D. Prenatal and postpartum depression in fathers and its association with maternal depression: A meta-analysis. JAMA 2010, 303, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Lipsey, M.W.; Wilson, D.B. Practical Meta-Analysis; Sage Publications, Inc.: Thousand Oaks, CA, USA, 2001. [Google Scholar]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Tada, M.; Nagai, T.; Kirihara, K.; Koike, S.; Suga, M.; Araki, T.; Kobayashi, T.; Kasai, K. Differential Alterations of Auditory Gamma Oscillatory Responses Between Pre-Onset High-Risk Individuals and First-Episode Schizophrenia. Cereb. Cortex 2016, 26, 1027–1035. [Google Scholar] [CrossRef]
- Koshiyama, D.; Kirihara, K.; Tada, M.; Nagai, T.; Fujioka, M.; Ichikawa, E.; Ohta, K.; Tani, M.; Tsuchiya, M.; Kanehara, A.; et al. Auditory gamma oscillations predict global symptomatic outcome in the early stages of psychosis: A longitudinal investigation. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2018, 129, 2268–2275. [Google Scholar] [CrossRef]
- Kwon, J.S.; O’Donnell, B.F.; Wallenstein, G.V.; Greene, R.W.; Hirayasu, Y.; Nestor, P.G.; Hasselmo, M.E.; Potts, G.F.; Shenton, M.E.; McCarley, R.W. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch. Gen. Psychiatry 1999, 56, 1001–1005. [Google Scholar] [CrossRef]
- Hong, L.E.; Summerfelt, A.; McMahon, R.; Adami, H.; Francis, G.; Elliott, A.; Buchanan, R.W.; Thaker, G.K. Evoked gamma band synchronization and the liability for schizophrenia. Schizophr. Res. 2004, 70, 293–302. [Google Scholar] [CrossRef]
- Spencer, K.M.; Salisbury, D.F.; Shenton, M.E.; McCarley, R.W. gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol. Psychiatry 2008, 64, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Vierling-Claassen, D.; Siekmeier, P.; Stufflebeam, S.; Kopell, N. Modeling GABA alterations in schizophrenia: A link between impaired inhibition and altered gamma and beta range auditory entrainment. J. Neurophysiol. 2008, 99, 2656–2671. [Google Scholar] [CrossRef] [PubMed]
- Teale, P.; Collins, D.; Maharajh, K.; Rojas, D.C.; Kronberg, E.; Reite, M. Cortical source estimates of gamma band amplitude and phase are different in schizophrenia. Neuroimage 2008, 42, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.W.; Hernandez, O.O.; Asherin, R.M.; Teale, P.D.; Reite, M.L.; Rojas, D.C. Cortical gamma generators suggest abnormal auditory circuitry in early-onset psychosis. Cereb. Cortex 2008, 18, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.M.; Niznikiewicz, M.A.; Nestor, P.G.; Shenton, M.E.; McCarley, R.W. Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC Neurosci. 2009, 10, 85. [Google Scholar] [CrossRef]
- Hamm, J.P.; Gilmore, C.S.; Picchetti, N.A.M.; Sponheim, S.R.; Clementz, B.A. Abnormalities of Neuronal Oscillations and Temporal Integration to Low- and High-Frequency Auditory Stimulation in Schizophrenia. Biol. Psychiatry 2011, 69, 989–996. [Google Scholar] [CrossRef]
- Tsuchimoto, R.; Kanba, S.; Hirano, S.; Oribe, N.; Ueno, T.; Hirano, Y.; Nakamura, I.; Oda, Y.; Miura, T.; Onitsuka, T. Reduced high and low frequency gamma synchronization in patients with chronic schizophrenia. Schizophr. Res. 2011, 133, 99–105. [Google Scholar] [CrossRef]
- Hamm, J.P.; Gilmore, C.S.; Clementz, B.A. Augmented gamma band auditory steady-state responses: Support for NMDA hypofunction in schizophrenia. Schizophr. Res. 2012, 138, 1–7. [Google Scholar] [CrossRef]
- Komek, K.; Ermentrout, G.B.; Walker, C.P.; Cho, R.Y. Dopamine and gamma band synchrony in schizophrenia—Insights from computational and empirical studies. Eur. J. Neurosci. 2012, 36, 2146–2155. [Google Scholar] [CrossRef]
- Rass, O.; Forsyth, J.K.; Krishnan, G.P.; Hetrick, W.P.; Klaunig, M.J.; Breier, A.; O’Donnell, B.F.; Brenner, C.A. Auditory steady state response in the schizophrenia, first-degree relatives, and schizotypal personality disorder. Schizophr. Res. 2012, 136, 143–149. [Google Scholar] [CrossRef]
- Kirihara, K.; Rissling, A.J.; Swerdlow, N.R.; Braff, D.L.; Light, G.A. Hierarchical Organization of Gamma and Theta Oscillatory Dynamics in Schizophrenia. Biol. Psychiatry 2012, 71, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Roach, B.J.; Ford, J.M.; Hoffman, R.E.; Mathalon, D.H. Converging evidence for gamma synchrony deficits in schizophrenia. Suppl. Clin. Neurophysiol. 2013, 62, 163–180. [Google Scholar] [PubMed]
- Edgar, J.C.; Chen, Y.H.; Lanza, M.; Howell, B.; Chow, V.Y.; Heiken, K.; Liu, S.; Wootton, C.; Hunter, M.A.; Huang, M.X.; et al. Cortical thickness as a contributor to abnormal oscillations in schizophrenia? NeuroImage-Clin. 2014, 4, 122–129. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hirano, Y. Spontaneous Gamma Activity in Schizophrenia. JAMA Psychiatry 2015, 72, 297. [Google Scholar] [CrossRef] [PubMed]
- Griskova-Bulanova, I.; Hubl, D.; van Swam, C.; Dierks, T.; Koenig, T. Early- and late-latency gamma auditory steady-state response in schizophrenia during closed eyes: Does hallucination status matter? Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2016, 127, 2214–2221. [Google Scholar] [CrossRef]
- Alegre, M.; Molero, P.; Valencia, M.; Mayner, G.; Ortuño, F.; Artieda, J. Atypical antipsychotics normalize low-gamma evoked oscillations in patients with schizophrenia. Psychiatry Res. 2017, 247, 214–221. [Google Scholar] [CrossRef]
- Light, G.A.; Zhang, W.; Joshi, Y.B.; Bhakta, S.; Talledo, J.A.; Swerdlow, N.R. Single-Dose Memantine Improves Cortical Oscillatory Response Dynamics in Patients with Schizophrenia. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2017, 42, 2633–2639. [Google Scholar] [CrossRef]
- Edgar, J.C.; Fisk, C.L.; Chen, Y.H.; Stone-Howell, B.; Liu, S.; Hunter, M.A.; Huang, M.; Bustillo, J.; Canive, J.M.; Miller, G.A. Identifying auditory cortex encoding abnormalities in schizophrenia: The utility of low-frequency versus 40 Hz steady-state measures. Psychophysiology 2018, 55, e13074. [Google Scholar] [CrossRef]
- Griskova-Bulanova, I.; Dapsys, K.; Melynyte, S.; Voicikas, A.; Maciulis, V.; Andruskevicius, S.; Korostenskaja, M. 40 Hz auditory steady-state response in schizophrenia: Sensitivity to stimulation type (clicks versus flutter amplitude-modulated tones). Neurosci. Lett. 2018, 662, 152–157. [Google Scholar] [CrossRef]
- Puvvada, K.C.; Summerfelt, A.; Du, X.; Krishna, N.; Kochunov, P.; Rowland, L.M.; Simon, J.Z.; Hong, L.E. Delta vs. Gamma Auditory Steady State Synchrony in Schizophrenia. Schizophr. Bull. 2018, 44, 378–387. [Google Scholar] [CrossRef]
- Sun, C.; Zhou, P.; Wang, C.; Fan, Y.; Tian, Q.; Dong, F.; Zhou, F.; Wang, C. Defects of Gamma Oscillations in Auditory Steady-State Evoked Potential of Schizophrenia. Shanghai Arch. Psychiatry 2018, 30, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, Y.; Curtin, A.; Chan, R.C.K.; Wang, Y.; Li, H.; Zhang, T.; Qian, Z.; Guo, Q.; Li, Y.; et al. Abnormal auditory-evoked gamma band oscillations in first-episode schizophrenia during both eye open and eye close states. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 86, 279–286. [Google Scholar] [CrossRef]
- Zhou, T.-H.; Mueller, N.E.; Spencer, K.M.; Mallya, S.G.; Lewandowski, K.E.; Norris, L.A.; Levy, D.L.; Cohen, B.M.; Öngür, D.; Hall, M.-H. Auditory steady state response deficits are associated with symptom severity and poor functioning in patients with psychotic disorder. Schizophr. Res. 2018, 201, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Bartolomeo, L.A.; Wright, A.M.; Ma, R.E.; Hummer, T.A.; Francis, M.M.; Visco, A.C.; Mehdiyoun, N.F.; Bolbecker, A.R.; Hetrick, W.P.; Dydak, U.; et al. Relationship of auditory electrophysiological responses to magnetic resonance spectroscopy metabolites in Early Phase Psychosis. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2019, 145, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jang, S.-K.; Kim, D.-W.; Shim, M.; Kim, Y.-W.; Im, C.-H.; Lee, S.-H. Cortical volume and 40-Hz auditory-steady-state responses in patients with schizophrenia and healthy controls. NeuroImage Clin. 2019, 22, 101732. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.A.; Hamm, J.P.; McDowell, J.E.; Keedy, S.K.; Gershon, E.S.; Ivleva, E.I.; Pearlson, G.D.; Keshavan, M.S.; Tamminga, C.A.; Sweeney, J.A.; et al. Auditory steady-state EEG response across the schizo-bipolar spectrum. Schizophr. Res. 2019, 209, 218–226. [Google Scholar] [CrossRef]
- Lepock, J.R.; Ahmed, S.; Mizrahi, R.; Gerritsen, C.J.; Maheandiran, M.; Drvaric, L.; Bagby, R.M.; Korostil, M.; Light, G.A.; Kiang, M. Relationships between cognitive event-related brain potential measures in patients at clinical high risk for psychosis. Schizophr. Res. 2020, 226, 84–94. [Google Scholar] [CrossRef]
- Murphy, N.; Ramakrishnan, N.; Walker, C.P.; Polizzotto, N.R.; Cho, R. Intact Auditory Cortical Cross-Frequency Coupling in Early and Chronic Schizophrenia. Biol. Psychiatry 2020, 87 (Suppl. S9), S188. [Google Scholar] [CrossRef]
- Grent-’t-Jong, T.; Gajwani, R.; Gross, J.; Gumley, A.I.; Krishnadas, R.; Lawrie, S.M.; Schwannauer, M.; Schultze-Lutter, F.; Uhlhaas, P.J. 40-Hz Auditory Steady-State Responses Characterize Circuit Dysfunctions and Predict Clinical Outcomes in Clinical High-Risk for Psychosis Participants: A Magnetoencephalography Study. Biol. Psychiatry 2021, 90, 419–429. [Google Scholar] [CrossRef]
- Koshiyama, D.; Thomas, M.L.; Miyakoshi, M.; Joshi, Y.B.; Molina, J.L.; Tanaka-Koshiyama, K.; Sprock, J.; Braff, D.L.; Swerdlow, N.R.; Light, G.A. Hierarchical Pathways from Sensory Processing to Cognitive, Clinical, and Functional Impairments in Schizophrenia. Schizophr. Bull. 2020, 28, 373–385. [Google Scholar] [CrossRef]
- Coffman, B.A.; Ren, X.; Longenecker, J.; Torrence, N.; Fishel, V.; Seebold, D.; Wang, Y.; Curtis, M.; Salisbury, D.F. Aberrant attentional modulation of the auditory steady state response (ASSR) is related to auditory hallucination severity in the first-episode schizophrenia-spectrum. J. Psychiatr. Res. 2022, 151, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Hare, S.; Summerfelt, A.; Adhikari, B.M.; Garcia, L.; Marshall, W.; Zan, P.; Kvarta, M.; Goldwaser, E.; Bruce, H.; et al. Cortical connectomic mediations on gamma band synchronization in schizophrenia. Transl. Psychiatry 2023, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Ogyu, K.; Matsushita, K.; Honda, S.; Wada, M.; Tamura, S.; Takenouchi, K.; Tobari, Y.; Kusudo, K.; Kato, H.; Koizumi, T.; et al. Decrease in gamma-band auditory steady-state response in patients with treatment-resistant schizophrenia. Schizophr. Res. 2023, 252, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Tsugawa, S.; Noda, Y.; Ueno, F.; Honda, S.; Kinjo, M.; Segawa, H.; Hondo, N.; Mori, Y.; Watanabe, H.; et al. Glutamatergic and GABAergic metabolite levels in schizophrenia-spectrum disorders: A meta-analysis of 1H-magnetic resonance spectroscopy studies. Mol. Psychiatry 2022, 27, 744–757. [Google Scholar] [CrossRef] [PubMed]
- Anticevic, A.; Corlett, P.R.; Cole, M.W.; Savic, A.; Gancsos, M.; Tang, Y.; Repovs, G.; Murray, J.D.; Driesen, N.R.; Morgan, P.T.; et al. N-Methyl-D-Aspartate Receptor Antagonist Effects on Prefrontal Cortical Connectivity Better Model Early Than Chronic Schizophrenia. Biol. Psychiatry 2015, 77, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Grent-’t-Jong, T.; Gross, J.; Goense, J.; Wibral, M.; Gajwani, R.; Gumley, A.I.; Lawrie, S.M.; Schwannauer, M.; Schultze-Lutter, F.; Navarro Schröder, T.; et al. Resting-state gamma-band power alterations in schizophrenia reveal E/I-balance abnormalities across illness-stages. eLife 2018, 7, e37799. [Google Scholar] [CrossRef] [PubMed]
- Farahani, E.D.; Goossens, T.; Wouters, J.; van Wieringen, A. Spatiotemporal reconstruction of auditory steady-state responses to acoustic amplitude modulations: Potential sources beyond the auditory pathway. NeuroImage 2017, 148, 240–253. [Google Scholar] [CrossRef]
- Tada, M.; Kirihara, K.; Ishishita, Y.; Takasago, M.; Kunii, N.; Uka, T.; Shimada, S.; Ibayashi, K.; Kawai, K.; Saito, N.; et al. Global and Parallel Cortical Processing Based on Auditory Gamma Oscillatory Responses in Humans. Cereb. Cortex 2021, 31, 4518–4532. [Google Scholar] [CrossRef]
- O’Donnell, B.F.; Vohs, J.L.; Krishnan, G.P.; Rass, O.; Hetrick, W.P.; Morzorati, S.L. The auditory steady-state response (ASSR): A translational biomarker for schizophrenia. Suppl. Clin. Neurophysiol. 2013, 62, 101–112. [Google Scholar] [CrossRef]
- Harvey, P.D.; Rosenthal, J.B. Cognitive and functional deficits in people with schizophrenia: Evidence for accelerated or exaggerated aging? Schizophr. Res. 2018, 196, 14–21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).