A Systematic Review of Nanomedicine in Glioblastoma Treatment: Clinical Efficacy, Safety, and Future Directions
Abstract
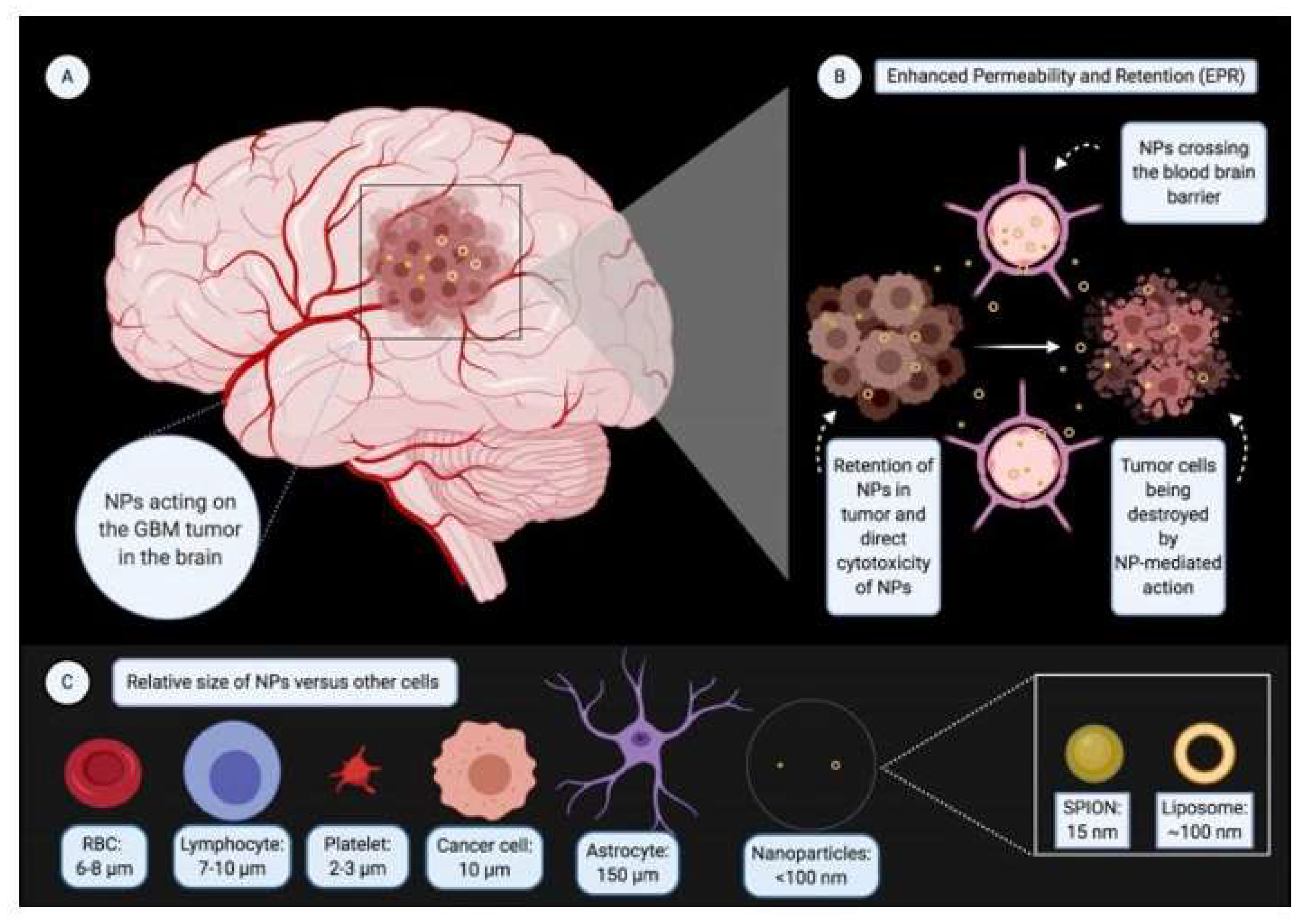
1. Introduction
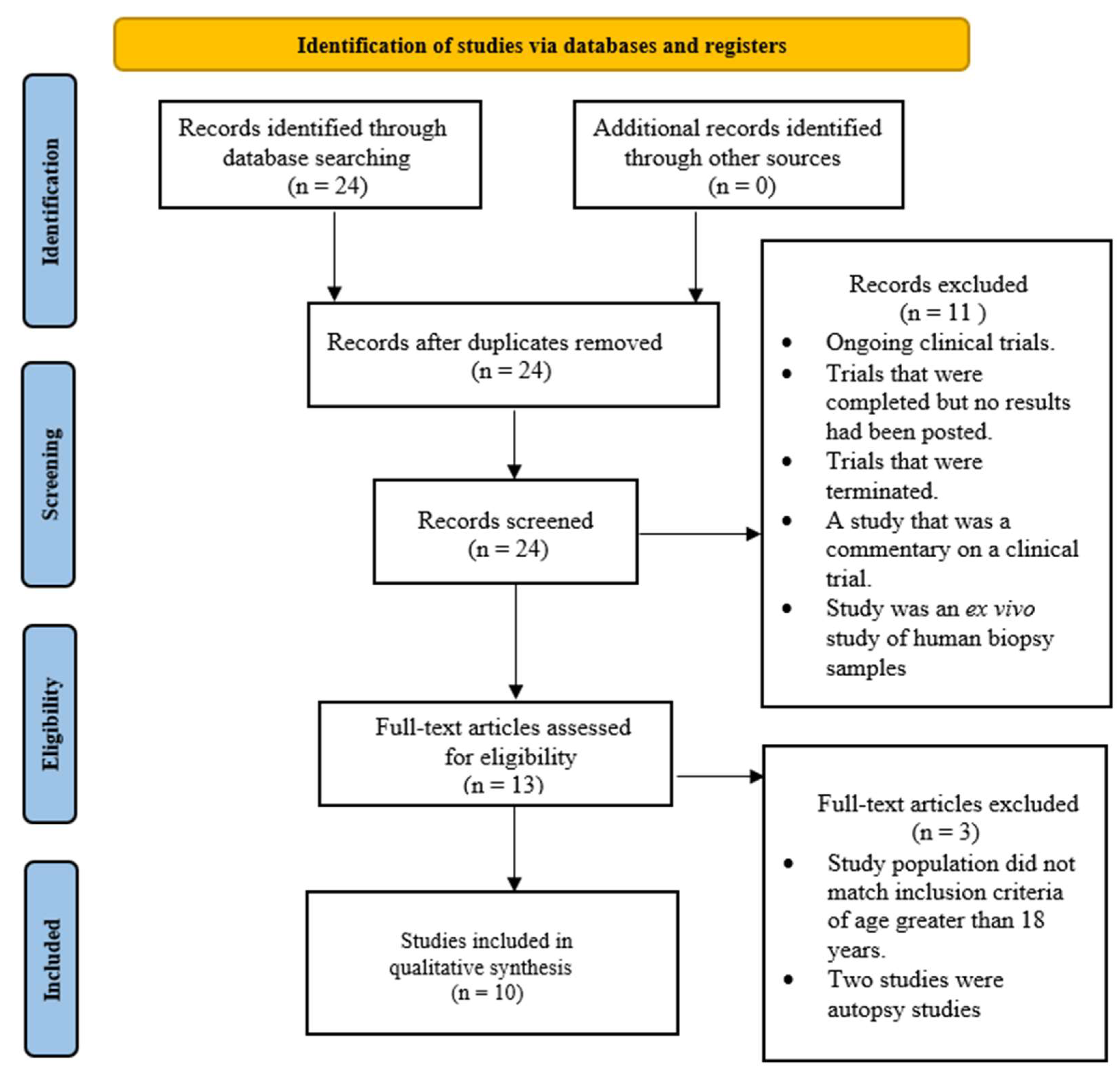
2. Materials and Methods
Quality Assessment
3. Results
3.1. Baseline Characteristics of Patients
3.1.1. Primary GBM
3.1.2. Recurrent GBM
3.2. Clinical Outcomes
3.2.1. Primary GBM
3.2.2. Recurrent GBM
3.3. Side Effects Profile
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Perry, J.R.; Laperriere, N.; O’Callaghan, C.J.; Brandes, A.A.; Menten, J.; Phillips, C.; Fay, M.; Nishikawa, R.; Cairncross, J.G.; Roa, W.; et al. Short-Course Radiation plus Temozolomide in Elderly Patients with Glioblastoma. N. Engl. J. Med. 2017, 376, 1027–1037. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316, Erratum in JAMA 2018, 319, 1824. [Google Scholar] [CrossRef]
- Tamimi, A.F.; Juweid, M. Epidemiology and Outcome of Glioblastoma. In Glioblastoma; De Vleeschouwer, S., Ed.; Exon Publications: Brisbane, QLD, Australia, 2017; Volume 20, pp. 143–153. [Google Scholar]
- Skog, J.; Würdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Sena-Esteves, M.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
- Al-Nedawi, K.; Meehan, B.; Micallef, J.; Lhotak, V.; May, L.; Guha, A.; Rak, J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008, 10, 619–624, Erratum in Nat Cell Biol. 2008, 10, 752. [Google Scholar] [CrossRef]
- Setti, M.; Osti, D.; Richichi, C.; Ortensi, B.; Del Bene, M.; Fornasari, L.; Beznoussenko, G.; Mironov, A.; Rappa, G.; Cuomo, A.; et al. Extracellular vesicle-mediated transfer of CLIC1 protein is a novel mechanism for the regulation of glioblastoma growth. Oncotarget 2015, 6, 31413–31427. [Google Scholar] [CrossRef]
- Arscott, W.T.; Tandle, A.T.; Zhao, S.; Shabason, J.E.; Gordon, I.K.; Schlaff, C.D.; Zhang, G.; Tofilon, P.J.; Camphausen, K.A. Ionizing radiation and glioblastoma exosomes: Implications in tumor biology and cell migration. Transl. Oncol. 2013, 6, 638–648. [Google Scholar] [CrossRef]
- Svensson, K.J.; Kucharzewska, P.; Christianson, H.C.; Sköld, S.; Löfstedt, T.; Johansson, M.C.; Mörgelin, M.; Bengzon, J.; Ruf, W.; Belting, M. Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells. Proc. Natl. Acad. Sci. USA 2011, 108, 13147–13152. [Google Scholar] [CrossRef]
- Kucharzewska, P.; Christianson, H.C.; Welch, J.E.; Svensson, K.J.; Fredlund, E.; Ringnér, M.; Mörgelin, M.; Bourseau-Guilmain, E.; Bengzon, J.; Belting, M. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc. Natl. Acad. Sci. USA 2013, 110, 7312–7317. [Google Scholar] [CrossRef]
- van der Vos, K.E.; Abels, E.R.; Zhang, X.; Lai, C.; Carrizosa, E.; Oakley, D.; Prabhakar, S.; Mardini, O.; Crommentuijn, M.H.; Skog, J.; et al. Directly visualized glioblastoma-derived extracellular vesicles transfer RNA to microglia/macrophages in the brain. Neuro Oncol. 2016, 18, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, A.M.; Hervey-Jumper, S.; Morshed, R.A.; Young, J.; Han, S.J.; Chunduru, P.; Zhang, Y.; Phillips, J.J.; Shai, A.; Lafontaine, M.; et al. Association of Maximal Extent of Resection of Contrast-Enhanced and Non-Contrast-Enhanced Tumor With Survival Within Molecular Subgroups of Patients With Newly Diagnosed Glioblastoma. JAMA Oncol. 2020, 6, 495–503, Erratum in JAMA Oncol. 2020, 6, 444. [Google Scholar] [CrossRef]
- Alexander, B.M.; Cloughesy, T.F. Adult Glioblastoma. J. Clin. Oncol. 2017, 35, 2402–2409. [Google Scholar] [CrossRef]
- Fabian, D.; Guillermo Prieto Eibl, M.D.P.; Alnahhas, I.; Sebastian, N.; Giglio, P.; Puduvalli, V.; Gonzalez, J.; Palmer, J.D. Treatment of Glioblastoma (GBM) with the Addition of Tumor-Treating Fields (TTF): A Review. Cancers 2019, 11, 174. [Google Scholar] [CrossRef]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef]
- Wu, W.; Pu, Y.; Shi, J. Nanomedicine-enabled chemotherapy-based synergetic cancer treatments. J. Nanobiotechnol. 2022, 4, 4. [Google Scholar] [CrossRef]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Alharbi, K.K.; Al-sheikh, Y.A. Role and implications of nanodiagnostics in the changing trends of clinical diagnosis. Saudi J. Biol. Sci. 2014, 21, 109–117. [Google Scholar] [CrossRef]
- Abd Ellah, N.H.; Gad, S.F.; Muhammad, K.; EBatiha, G.; Hetta, H.F. Nanomedicine as a promising approach for diagnosis, treatment and prophylaxis against COVID-19. Nanomedicine 2020, 15, 2085–2102. [Google Scholar] [CrossRef]
- Chen, Z.; Krishnamachary, B.; Pachecho-Torres, J.; Penet, M.; Bhujwalla, Z.M. Theranostic small interfering RNA nanoparticles in cancer precision nanomedicine. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1595. [Google Scholar] [CrossRef]
- Noble, C.O.; Krauze, M.T.; Drummond, D.C.; Yamashita, Y.; Saito, R.; Berger, M.S.; Kirpotin, D.B.; Bankiewicz, K.S.; Park, J.W. Novel nanoliposomal CPT-11 infused by convection-enhanced delivery in intracranial tumors: Pharmacology and efficacy. Cancer Res. 2006, 66, 2801–2806. [Google Scholar] [CrossRef]
- Lin, T.; Zhao, P.; Jiang, Y.; Tang, Y.; Jin, H.; Pan, Z.; He, H.; Yang, V.C.; Huang, Y. Blood-Brain-Barrier-Penetrating Albumin Nanoparticles for Biomimetic Drug Delivery via Albumin-Binding Protein Pathways for Antiglioma Therapy. ACS Nano 2016, 10, 9999–10012. [Google Scholar] [CrossRef]
- Inoue, T.; Yamashita, Y.; Nishihara, M.; Sugiyama, S.; Sonoda, Y.; Kumabe, T.; Yokoyama, M.; Tominaga, T. Therapeutic efficacy of a polymeric micellar doxorubicin infused by convection-enhanced delivery against intracranial 9L brain tumor models. Neuro-Oncology 2009, 11, 151–157. [Google Scholar] [CrossRef]
- Shirvalilou, S.; Khoei, S.; Esfahani, A.J.; Kamali, M.; Shirvaliloo, M.; Sheervalilou, R.; Mirzaghavami, P. Magnetic Hyperthermia as an adjuvant cancer therapy in combination with radiotherapy versus radiotherapy alone for recurrent/progressive glioblastoma: A systematic review. J. Neuro-Oncol. 2021, 152, 419–428. [Google Scholar] [CrossRef]
- Alavian, F.; Ghasemi, S. The Effectiveness of Nanoparticles on Gene Therapy for Glioblastoma Cells Apoptosis: A Systematic Review. Curr. Gene Ther. 2021, 21, 230–245. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Moola, S.; Munn, Z.; Sears, K.; Sfetcu, R.; Currie, M.; Lisy, K.; Tufanaru, C.; Qureshi, R.; Mattis, P.; Mu, P. Conducting systematic reviews of association (etiology): The Joanna Briggs Institute’s approach. Int. J. Evid.-Based Healthc. 2015, 13, 163–169. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Rothe, R.; Scholz, R.; Gneveckow, U.; Wust, P.; Thiesen, B.; Feussner, A.; von Deimling, A.; Waldoefner, N.; Felix, R.; et al. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: Results of a feasibility study on patients with glioblastoma multiforme. J. Neurooncol. 2007, 81, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neurooncol. 2011, 103, 317–324. [Google Scholar] [CrossRef]
- Grauer, O.; Jaber, M.; Hess, K.; Weckesser, M.; Schwindt, W.; Maring, S.; Wölfer, J.; Stummer, W. Combined intracavitary thermotherapy with iron oxide nanoparticles and radiotherapy as local treatment modality in recurrent glioblastoma patients. J. Neurooncol. 2019, 141, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Beier, C.P.; Schmid, C.; Gorlia, T.; Kleinletzenberger, C.; Beier, D.; Grauer, O.; Steinbrecher, A.; Hirschmann, B.; Brawanski, A.; Dietmaier, C.; et al. RNOP-09: Pegylated liposomal doxorubicine and prolonged temozolomide in addition to radiotherapy in newly diagnosed glioblastoma—A phase II study. BMC Cancer 2009, 9, 308. [Google Scholar] [CrossRef] [PubMed]
- Glas, M.; Koch, H.; Hirschmann, B.; Jauch, T.; Steinbrecher, A.; Herrlinger, U.; Bogdahn, U.; Hau, P. Pegylated Liposomal Doxorubicin in Recurrent Malignant Glioma: Analysis of a Case Series. Oncology 2007, 72, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Hau, P.; Fabel, K.; Baumgart, U.; Rümmele, P.; Grauer, O.; Bock, A.; Dietmaier, C.; Dietmaier, W.; Dietrich, J.; Dudel, C.; et al. Pegylated liposomal doxorubicin-efficacy in patients with recurrent high-grade glioma. Cancer 2004, 100, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Fabel, K.; Dietrich, J.; Hau, P.; Wismeth, C.; Winner, B.; Przywara, S.; Steinbrecher, A.; Ullrich, W.; Bogdahn, U. Long-term stabilization in patients with malignant glioma after treatment with liposomal doxorubicin. Cancer 2001, 92, 1936–1942. [Google Scholar] [CrossRef] [PubMed]
- Frankel, B.M.; Cachia, D.; Patel, S.J.; Das, A. Targeting Subventricular Zone Progenitor Cells with Intraventricular Liposomal Encapsulated Cytarabine in Patients with Secondary Glioblastoma: A Report of Two Cases. SN Compr. Clin. Med. 2020, 2, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, J.; Mizuno, M.; Fujii, M.; Kajita, Y.; Nakahara, N.; Hatano, M.; Saito, R.; Nobayashi, M.; Wakabayashi, T. Human gene therapy for malignant gliomas (glioblastoma multiforme and anaplastic astrocytoma) by in vivo transduction with human interferon beta gene using cationic liposomes. Hum. Gene Ther. 2004, 15, 77–86. [Google Scholar] [CrossRef]
- Whittle, J.R.; Lickliter, J.D.; Gan, H.K.; Scott, A.M.; Simes, J.; Solomon, B.J.; MacDiarmid, J.A.; Brahmbhatt, H.; Rosenthal, M.A. First in human nanotechnology doxorubicin delivery system to target epidermal growth factor receptors in recurrent glioblastoma. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2015, 22, 1889–1894. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef]
- Zhang, T.T.; Li, W.; Meng, G.; Wang, P.; Liao, W. Strategies for transporting nanoparticles across the blood-brain barrier. Biomater. Sci. 2016, 4, 219–229. [Google Scholar] [CrossRef]
- Buerki, R.A.; Chheda, Z.S.; Okada, H. Immunotherapy of primary brain tumors: Facts and hopes. Clin. Cancer Res. 2018, 24, 5198–5205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, S.; Luo, S.; Tang, P.; Wan, M.; Wu, D.; Gao, W. Ultrasound-assisted brain delivery of nanomedicines for brain tumor therapy: Advance and prospect. J. Nanobiotechnol. 2022, 20, 287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Dmello, C.; Chen, L.; Arrieta, V.A.; Gonzalez-Buendia, E.; Kane, J.R.; Magnusson, L.P.; Baran, A.; James, C.D.; Horbinski, C.; et al. Ultrasound-mediated Delivery of Paclitaxel for Glioma: A Comparative Study of Distribution, Toxicity, and Efficacy of Albumin-bound Versus Cremophor Formulations. Clin. Cancer Res. 2020, 26, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Reilly, R.M.; Pezo, R.C.; Trudeau, M.; Sahgal, A.; Singnurkar, A.; Perry, J.; Myrehaug, S.; People, C.B.; Davidson, B.; et al. MR-guided focused ultrasound enhances delivery of trastuzumab to Her2-positive brain metastases. Sci. Transl. Med. 2021, 13, eabj4011. [Google Scholar] [CrossRef] [PubMed]
- Noroozian, Z.; Xhima, K.; Huang, Y.; Kaspar, B.K.; Kügler, S.; Hynynen, K.; Aubert, I. MRI-Guided Focused Ultrasound for Targeted Delivery of rAAV to the Brain. Methods Mol. Biol. 2019, 1950, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Morse, S.V.; Mishra, A.; Chan, T.G.; de Rosales, R.T.M.; Choi, J.J. Liposome delivery to the brain with rapid short-pulses of focused ultrasound and microbubbles. J. Control. Release 2022, 341, 605–615. [Google Scholar] [CrossRef]
- Bajracharya, R.; Cruz, E.; Götz, J.; Nisbet, R.M. Ultrasound-mediated delivery of novel tau-specific monoclonal antibody enhances brain uptake but not therapeutic efficacy. J. Control. Release 2022, 349, 634–648. [Google Scholar] [CrossRef]
- Wei, K.C.; Chu, P.C.; Wang, H.Y.; Huang, C.Y.; Chen, P.Y.; Tsai, H.C.; Lu, Y.J.; Lee, P.Y.; Tseng, I.C.; Feng, L.Y.; et al. Focused ultrasound-induced blood-brain barrier opening to enhance temozolomide delivery for glioblastoma treatment: A preclinical study. PLoS ONE. 2013, 8, e58995. [Google Scholar] [CrossRef]
- Treat, L.H.; McDannold, N.; Vykhodtseva, N.; Zhang, Y.; Tam, K.; Hynynen, K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int. J. Cancer 2007, 121, 901–907. [Google Scholar] [CrossRef]
- Coluccia, D.; Figueiredo, C.A.; Wu, M.Y.; Riemenschneider, A.N.; Diaz, R.; Luck, A.; Smith, C.; Das, S.; Ackerley, C.; O’Reilly, M.; et al. Enhancing glioblastoma treatment using cisplatin-gold-nanoparticle conjugates and targeted delivery with magnetic resonance-guided focused ultrasound. Nanomedicine 2018, 14, 1137–1148. [Google Scholar] [CrossRef]
- Liu, H.L.; Hua, M.Y.; Chen, P.Y.; Chu, P.C.; Pan, C.H.; Yang, H.W.; Huang, C.Y.; Wang, J.J.; Yen, T.C.; Wei, K.C. Blood-brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment. Radiology 2010, 255, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Papachristodoulou, A.; Signorell, R.D.; Werner, B.; Brambilla, D.; Luciani, P.; Cavusoglu, M.; Grandjean, J.; Silginer, M.; Rudin, M.; Martin, E.; et al. Chemotherapy sensitization of glioblastoma by focused ultrasound-mediated delivery of therapeutic liposomes. J. Control. Release 2019, 295, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Kong, C.; Cho, J.S.; Lee, J.; Koh, C.S.; Yoon, M.S.; Na, Y.C.; Chang, W.S.; Chang, J.W. Focused ultrasound-mediated noninvasive blood-brain barrier modulation: Preclinical examination of efficacy and safety in various sonication parameters. Neurosurg. Focus. 2018, 44, E15. [Google Scholar] [CrossRef] [PubMed]
- Beccaria, K.; Canney, M.; Goldwirt, L.; Fernandez, C.; Adam, C.; Piquet, J.; Autret, G.; Clément, O.; Lafon, C.; Chapelon, J.Y.; et al. Opening of the blood-brain barrier with an unfocused ultrasound device in rabbits. J. Neurosurg. 2013, 119, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Downs, M.E.; Buch, A.; Sierra, C.; Karakatsani, M.E.; Teichert, T.; Chen, S.; Konofagou, E.E.; Ferrera, V.P. Long-Term Safety of Repeated Blood-Brain Barrier Opening via Focused Ultrasound with Microbubbles in Non-Human Primates Performing a Cognitive Task. PLoS ONE 2015, 10, e0125911, Erratum in PLoS ONE 2015, 10, e0130860. [Google Scholar] [CrossRef]
- McMahon, D.; Hynynen, K. Acute Inflammatory Response Following Increased Blood-Brain Barrier Permeability Induced by Focused Ultrasound is Dependent on Microbubble Dose. Theranostics 2017, 7, 3989–4000. [Google Scholar] [CrossRef]
- Kovacs, Z.I.; Kim, S.; Jikaria, N.; Qureshi, F.; Milo, B.; Lewis, B.K.; Bresler, M.; Burks, S.R.; Frank, J.A. Disrupting the blood-brain barrier by focused ultrasound induces sterile inflammation. Proc. Natl. Acad. Sci. USA 2017, 114, E75–E84. [Google Scholar] [CrossRef]
- Hynynen, K.; McDannold, N.; Sheikov, N.A.; Jolesz, F.A.; Vykhodtseva, N. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage 2005, 24, 12–20. [Google Scholar] [CrossRef]
- Pandit, R.; Chen, L.; Götz, J. The blood-brain barrier: Physiology and strategies for drug delivery. Adv. Drug Deliv. Rev. 2020, 165–166, 1–14. [Google Scholar] [CrossRef]
- Raposo, C.D.; Conceição, C.A.; Barros, M.T. Nanoparticles Based on Novel Carbohydrate-Functionalized Polymers. Molecules 2020, 25, 1744. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Ribovski, L.; Hamelmann, N.M.; Paulusse, J.M.J. Polymeric Nanoparticles Properties and Brain Delivery. Pharmaceutics 2021, 13, 2045. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Yi, X.; Hou, F.; Wibowo, D.; Zhang, F.; Zhao, D.; Gao, H.; Zhao, C.X. Role of Nanoparticle Mechanical Properties in Cancer Drug Delivery. ACS Nano 2019, 13, 7410–7424. [Google Scholar] [CrossRef] [PubMed]
- Silant’ev, V.E.; Shmelev, M.E.; Belousov, A.S.; Patlay, A.A.; Shatilov, R.A.; Farniev, V.M.; Kumeiko, V.V. How to Develop Drug Delivery System Based on Carbohydrate Nanoparticles Targeted to Brain Tumors. Polymers 2023, 15, 2516. [Google Scholar] [CrossRef] [PubMed]
- Di Tacchio, M.; Macas, J.; Weissenberger, J.; Sommer, K.; Bähr, O.; Steinbach, J.P.; Senft, C.; Seifert, V.; Glas, M.; Herrlinger, U.; et al. Tumor Vessel Normalization, Immunostimulatory Reprogramming, and Improved Survival in Glioblastoma with Combined Inhibition of PD-1, Angiopoietin-2, and VEGF. Cancer Immunol. Res. 2019, 7, 1910–1927. [Google Scholar] [CrossRef] [PubMed]
- Pfirschke, C.; Engblom, C.; Rickelt, S.; Cortez-Retamozo, V.; Garris, C.; Pucci, F.; Yamazaki, T.; Poirier-Colame, V.; Newton, A.; Redouane, Y.; et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity 2016, 44, 343–354. [Google Scholar] [CrossRef]
- Kaur, P.; Asea, A. Radiation-induced effects and the immune system in cancer. Front. Oncol. 2012, 2, 191. [Google Scholar] [CrossRef]
- Kim, S.S.; Harford, J.B.; Moghe, M.; Slaughter, T.; Doherty, C.; Chang, E.H. A tumor-targeting nanomedicine carrying the p53 gene crosses the blood-brain barrier and enhances anti-PD-1 immunotherapy in mouse models of glioblastoma. Int. J. Cancer 2019, 145, 2535–2546. [Google Scholar] [CrossRef]
- Kim, S.S.; Rait, A.; Kim, E.; Pirollo, K.F.; Nishida, M.; Farkas, N.; Dagata, J.A.; Chang, E.H. A nanoparticle carrying the p53 gene targets tumors including cancer stem cells, sensitizes glioblastoma to chemotherapy and improves survival. ACS Nano 2014, 8, 5494–5514. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Burkhart, A.; Melander, F.; Kempen, P.J.; Vejlebo, J.B.; Siupka, P.; Nielsen, M.S.; Andresen, T.L.; Moos, T. Targeting transferrin receptors at the blood-brain barrier improves the uptake of immunoliposomes and subsequent cargo transport into the brain parenchyma. Sci. Rep. 2017, 7, 10396. [Google Scholar] [CrossRef]
- Pulicherla, K.K.; Verma, M.K. Targeting therapeutics across the blood brain barrier (BBB), prerequisite towards thrombolytic therapy for cerebrovascular disorders-an overview and advancements. AAPS PharmSciTech. 2015, 16, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Voth, B.; Nagasawa, D.T.; Pelargos, P.E.; Chung, L.K.; Ung, N.; Gopen, Q.; Tenn, S.; Kamei, D.T.; Yang, I. Transferrin receptors and glioblastoma multiforme: Current findings and potential for treatment. J. Clin. Neurosci. 2015, 22, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yang, Z.; Zhang, S.; Cao, S.; Pang, Z.; Yang, X.; Jiang, X. Glioma-homing peptide with a cell-penetrating effect for targeting delivery with enhanced glioma localization, penetration and suppression of glioma growth. J. Control. Release 2013, 172, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Friden, P.M.; Walus, L.R.; Musso, G.F.; Taylor, M.A.; Malfroy, B.; Starzyk, R.M. Anti-transferrin receptor antibody and antibody-drug conjugates cross the blood-brain barrier. Proc. Natl. Acad. Sci. USA 1991, 88, 4771–4775. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Jiang, M.; Jiang, D.; Feng, X.; Yao, J.; Song, Q.; Chen, H.; Gao, X.; Chen, J. Enhancing Glioblastoma-Specific Penetration by Functionalization of Nanoparticles with an Iron-Mimic Peptide Targeting Transferrin/Transferrin Receptor Complex. Mol. Pharm. 2015, 12, 2947–2961. [Google Scholar] [CrossRef] [PubMed]
- Karthic, A.; Roy, A.; Lakkakula, J.; Alghamdi, S.; Shakoori, A.; Babalghith, A.O.; Emran, T.B.; Sharma, R.; Lima, C.M.G.; Kim, B.; et al. Cyclodextrin nanoparticles for diagnosis and potential cancer therapy: A systematic review. Front. Cell Dev. Biol. 2022, 10, 984311. [Google Scholar] [CrossRef] [PubMed]
- Ombredane, A.S.; Silva, V.R.P.; Andrade, L.R.; Pinheiro, W.O.; Simonelly, M.; Oliveira, J.V.; Pinheiro, A.C.; Gonçalves, G.F.; Felice, G.J.; Garcia, M.P.; et al. In Vivo Efficacy and Toxicity of Curcumin Nanoparticles in Breast Cancer Treatment: A Systematic Review. Front. Oncol. 2021, 11, 612903. [Google Scholar] [CrossRef]
- Wong, E.T.; Gautam, S.; Malchow, C.; Lun, M.; Pan, E.; Brem, S. Bevacizumab for recurrent glioblastoma multiforme: A meta-analysis. J. Natl. Compr. Cancer Netw. JNCCN 2011, 9, 403–407. [Google Scholar] [CrossRef]
- Kumthekar, P.U.; Rademaker, A.W.; Ko, C.H.; Dixit, K.S.; Schwartz, M.A.; Sonabend, A.M.; Sharp, L.; Lukas, R.V.; Stupp, R.; Horbinski, C.M.; et al. A phase 0 first-in-human study using NU-0129: A gold base spherical nucleic acid (SNA) nanoconjugate targeting BCL2L12 in recurrent glioblastoma patients. J. Clin. Oncol. 2019, 37, 3012. [Google Scholar] [CrossRef]
- SynerGene Therapeutics, Inc. Phase II Study of Combined Temozolomide and Targeted P53 Gene Therapy (SGT-53) for Treatment of Patients with Recurrent Glioblastoma [clinicaltrials.gov; 2020]. Available online: https://clinicaltrials.gov/ct2/show/NCT02340156 (accessed on 22 June 2020).
- BBB-Therapeutics, B.V. An Open-label, Phase I/IIa, Dose Escalating Study of 2B3-101 in Patients with Solid Tumors and Brain Metastases or Recurrent Malignant Glioma. [clinicaltrials.gov; 2015]. Available online: https://clinicaltrials.gov/ct2/show/NCT01386580 (accessed on 22 June 2020).
- BrUOG 329 GBM Onyvide With TMZ—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03119064 (accessed on 24 June 2020).
- Filon, O.; Krivorotko, P.; Kobyakov, G.; Razjivina, V.; Maximenko, O.; Gelperina, S.; Kreuter, J. A phase I study of safety and pharmacokinetics of NanoBB- 1-Dox in patients with advanced solid tumors. J. Clin. Oncol. 2017, 35, e13537. [Google Scholar] [CrossRef]
- van Landeghem, F.K.; Maier-Hauff, K.; Jordan, A.; Hoffmann, K.-T.; Gneveckow, U.; Scholz, R.; Thiesen, B.; Brück, W.; von Deimling, A. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials 2009, 30, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Chastagner, P.; Devictor, B.; Geoerger, B.; Aerts, I.; Leblond, P.; Frappaz, D.; Gentet, J.C.; Bracard, S.; André, N. Phase I study of non-pegylated liposomal doxorubicin in children with recurrent/refractory high-grade glioma. Cancer Chemother. Pharmacol. 2015, 76, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Doxorubicin-Loaded Anti-EGFR-Immunoliposomes (C225-ILs-dox) in High-Grade Gliomas—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03603379 (accessed on 24 June 2020).
- Hegi, M.E.; Liu, L.; Herman, J.G.; Stupp, R.; Wick, W.; Weller, M.; Mehta, M.P.; Gilbert, M.R. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 4189–4199. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.; Madhwani, K.; Chaurasia, B. Stroke and immunotherapy: Potential mechanisms and its implications as immune-therapeutics. Eur. J. Neurosci. 2021, 54, 4338–4357. [Google Scholar] [CrossRef] [PubMed]
- Kedia, S.; Banson, M.; Cheserem, B.; Chaurasia, B.; Karekezi, C.; Uche, E.; Apuahe, E.; Balogun, J.A.; Lucena, L.L.; Mbaye, M.; et al. Brain Tumor Programs in Asia and Africa: Current Status, Challenges, and Future Perspectives. World Neurosurg. 2023, 175, e1041–e1048. [Google Scholar] [CrossRef]
- Ahmed, N.; Ferini, G.; Barua, K.K.; Halder, R.; Barua, S.; Priola, S.; Tomasi, O.; Umana, G.E.; Shlobin, N.A.; Scalia, G.; et al. Adult-Onset Pilocytic Astrocytoma Predilecting Temporal Lobe: A Brief Review. Life 2022, 12, 931. [Google Scholar] [CrossRef]
- Aquino, A.A.; Ramirez, M.D.; Bozkurt, I.; González, J.A.; Goncharov, E.; Caballero, A.D.; Nurmukhametov, R.; Montemurro, N.; Chaurasia, B.; Goncharov, E. Treatment of Intracranial Tumors With Stereotactic Radiosurgery: Short-Term Results From Cuba. Cureus 2022, 14, e29955. [Google Scholar]
- Shahid, A.H.; Tripathi, M.; Batish, A.; Parth, J.; Bhatta, R.K.; Chaurasia, B.; Marcel, E.I.; Bal, A.; Dutta, P.; Mohindra, S.; et al. Letter to the Editor Regarding “Small Cell Glioblastoma of the Sella Turcica Region: Case Report and Review of the Literature”. World Neurosurg. 2023, 171, 185–189. [Google Scholar] [CrossRef]
| No. of Patients | 63 | 1 | 2 | Total = 66 |
| Male | 40 | 0 | 2 | Total = 42 |
| Female | 23 | 1 | 0 | Total = 24 |
| Median Age (Range) (In years) | 54 (30–73) | 31 | 55 (35–73) | Final Median Age = 54 |
| Molecular Markers | MGMT methylation | TNF-α, IL-1, 1β, IFN-β, IL-6 | N/A | N/A |
| Induction of Therapy from Diagnosis | Within 4 weeks | 16 months | N/A | N/A |
| Prior Treatment | Surgery | Surgery, radiotherapy, chemotherapy, and immunotherapy | Surgery, radiation, chemotherapy | N/A |
| KPS before Treatment | 90 | 50 | 70 | Median = 90 |
| Type of NP | Pegylated Liposome | Cationic liposome | SPIONs | N/A |
| Drug Encapsulation/Mode of Treatment | Doxorubicin encapsulation (Caelyx TM, PEG-Dox) | In vivo transduction with human interferon β-gene (gene delivery) | Intracranial thermotherapy by amino silane-coated iron oxide NPs | N/A |
| Simultaneous Standard Therapy | Prolonged temozolomide chemotherapy and radiotherapy | Surgery | External beam radiotherapy | N/A |
| References | [33] | [38] | [30] | N/A |
| No. of Patients | 28 | 31 | 7 | 12 | 59 | 6 | 14 | 2 | Total = 159 |
| Male | 20 | 18 | 5 | 7 | 32 | N/A | 7 | 1 | Total = 90 |
| Female | 8 | 13 | 2 | 5 | 27 | N/A | 7 | 1 | Total = 63 |
| Median Age (Range) (in years) | 53 (27–68) | 50 (21–70) | 43 (26–65) | 55 (35–73) | 55.7 | 60 (42–75) | 2 | 38.5 (30–47) | Final Median Age = 52.5 |
| Molecular Markers | Multidrug resistance protein 1 (MDR-1) and Multiple resistance protein (MRP) | N/A | N/A | N/A | N/A | MGMT methylation | EGFR expression | Nestin; GFAP; Ki-67; CD133; CD140; TUJ-1 | N/A |
| Induction of Therapy from Diagnosis | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 54 weeks for patient 1; 24 weeks for patient 2 | N/A |
| Prior Treatment | Surgery; radiation; chemotherapy | Surgery; radiation; chemotherapy | Surgery; radiation; chemotherapy | Surgery; radiation; chemotherapy | Surgery; radiation; chemotherapy | Not reported | Chemotherapy | Surgery; radiation; chemotherapy | N/A |
| KPS before Treatment | 80 | 80 | 80 | 70 | 90 | N/A | N/A | 100 | Median = 80 |
| Type of NP | Pegylated Liposome | Pegylated Liposome | Liposome | SPIONs | SPIONs | SPIONs | Minicell (VED Vox) (EnGeneIC) | Liposome (DepCyt) | N/A |
| Drug Encapsulation/Mode of Treatment | Doxorubicin encapsulation (Caelyx TM, PEG-Dox) | Doxorubicin encapsulation (Caelyx TM, PEG-Dox) | Doxorubicin encapsulation (Caelyx TM) | Intracranial thermotherapy by aminosilane-coated iron oxide NPs | Intratumoral thermotherapy by aminosilane-coated iron oxide NPs | Intracavitary thermotherapy by aminosilane-coated iron oxide NPs | Doxorubicin encapsulation; EGFR targeting via Vectibix | Cytarabine encapsulation-Intraventicular administration | N/A |
| Simultaneous Standard Therapy | Alone or in combination with tamoxifen | Alone or in combination with temozolomide | None | External beam radiotherapy | External beam radiotherapy | Concurrent, fractionated radiotherapy | None | None | N/A |
| References | [35] | [34] | [36] | [30] | [31] | [32] | [39] | [37] |
| Type of NP | Pegylated Liposome | Cationic Liposome | SPIONs | |
|---|---|---|---|---|
| Drug Encapsulation/Mode of Treatment | Doxorubicin encapsulation (Caely TM, x PEG-Dox) | In vivo transduction with human interferon β-gene (gene delivery) | Intracranial thermotherapy by amino silane-coated iron oxide NPs | |
| Simultaneous standard therapy | Prolonged temozolomide chemotherapy and radiotherapy | Surgery | External beam radiotherapy | |
| Duration of Treatment | 8 weeks | 28 days = 4 weeks | N/A | |
| Follow-up period | 20 weeks | 3 years until death | 3-monthly | |
| Patients lost to follow-up | 1 (included in statistical analysis) | N/A | N/A | Total = 1 |
| Mos in months | mOS = 17.6; [OS-24 = 35.3%] | 22 weeks (5.1 months approx.) | OS-1 = 3 months (Patient 1); 8.4 months (Patient 2) | Median mOS = 6.75 months |
| PFS rate | PFS = 12 = 30.2% | 10 weeks = 2.3 months | TTP 1 = 4.5 TTP 2§ = 5.9 months | Mean PFS-12 = 30.2%; Mean PFS = 2.3 months |
| KPS after treatment | 85 | 70 | N/A | Median = 80 |
| Macdonald criteria | 2 CR; 3 PR; 41 SD | 1 SD | N/A | Total = 2 CR, 3 PR, 42 SD |
| Type of Trial | Phase-I/II trial; non-randomized; non-controlled; multi-center trial; non-randomized; non-controlled; multi-center | Non-randomized; non-controlled; single-arm | Non-randomized; non-controlled; single-arm | |
| Level of Evidence | 4 | 4 | 4 | |
| Reference | [33] | [38] | [30] |
| Type of NP | Pegylated Liposome | Pegylated Liposome | Liposome | SPIONs | SPIONs | SPIONS | Minicell (VEDVDox) (EnGeneIC) | Liposome (DepoCyt) | |
|---|---|---|---|---|---|---|---|---|---|
| Drug encapsulation/Mode of Treatment | Doxorubicin encapsulation (Caely TM, x PEG-Dox) | Doxorubicin encapsulation (Caely TM, x PEG-Dox) | Doxorubicin encapsulation (Caelyx TM) | Intracranial thermotherapy by aminosaline-coated iron oxide NPs | Intratumoral thermotherapy by aminosaline-coated iron oxide NPs | Intracavitary thermotherapy by aminosaline-coated iron oxide NPs | Doxorubicin encapsulation; EGFR targeting via Vectibix | Cytarabine encapsulation—Intraventricular administration | |
| Simultaneous standard therapy | Alone or in combination with tamoxifen | Alone or in combination with temozolomide | None | External beam radiotherapy | External beam radiotherapy | Concurrent, fractionated radiotherapy | None | None | |
| Duration of Treatment | 8 weeks | N/A (duration of study—5 years) | 7 weeks (median calculated) | N/A | N/A | N/A | 8 weeks/until disease progression | 6 months (24 weeks) | |
| Follow-up period | 3 years | N/A (Until death) | 20 months | 3-monthly | 3-month intervals | 3-monthly basis (mean = 11.8 ± 9.3 months) | until death | until death | |
| Patients lost to follow-up | 0 | 0 | 0 | N/A | 1 | N/A | N/A | N/A | Total = 1 |
| mOS in months | 26 weeks (6 months approx.) | 7 months | 37 weeks (8.5 months approx.) | OS-1 = 14.5 months; OS-2 = 7.6 months | OS-1 = 23.2 months; OS-2 = 13.4 months | 8.15 months in general; mOS at first recurrence = 23.9 months; mOS at second recurrence = 7.1 months | 9.7 (2.1–23.6) months | 18 months | Median mOS = 9.7 months |
| PFS rate | PFS-6 = 15; PFS-12 = 7.5% | PFS-6 = 23; PFS-12 = 6% | PFS-12 = 15% | TTP-1 = 4.5; TTP-2 = 5.9 months | TTP-1 = 8 months | Median PFS = 6.25 months | 1.6 months (0.7–11.3); PFS-6 = 2 months | N/A | Mean PFS-12 = 8.2%; Mean PFS = 3.92 months |
| KPS after treatment | N/A | N/A | 85 | N/A | N/A | N/A | N/A | 70 | Median = 90 |
| Macdonald criteria | 1 CR; 1 PR; 9 SD | 2 PR | 5 PD; 2 SD | N/A | N/A | N/A | 4 SD | PR | Total = 1 CR, 9 PR, 15 SD |
| Type of Trial | Phase II trial; non-randomized; non-controlled; multi-arm | Non-randomized; non-controlled; multi-arm | Non-randomized; non-controlled; single-arm | Non-randomized; non-controlled; single-arm | Phase II; Non-randomized; non-controlled; single-arm | Non-randomized; non-controlled; single-arm | Phase I; Non-randomized; non-controlled; single-arm | case report; non-randomized; non-controlled; single-arm | |
| Level of Evidence | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | |
| Reference | [35] | [34] | [36] | [30] | [31] | [32] | [39] | [37] |
| Major Type | Severe Adverse Events/Side Effects | Primary GBM (n = 66) | Secondary GBM (n = 159) | Pooled (n = 225) |
|---|---|---|---|---|
| Gastrointestinal | Vomiting/nausea | 4 (6.06%) | 31 (19.5%) | 35 (15.56%) |
| Stomatitis | 2 (3.03%) | 0 | 2 (0.89%) | |
| Gastritis | 2 (3.03%) | 0 | 2 (0.89%) | |
| Diarrhea | 3 (4.54%) | 0 | 3 (1.33%) | |
| Myelotoxicity | Leukopenia, lymphopenia, thrombocytopenia, neutropenia, anemia | 55 (83.33%) | 18 (11.32%) | 73 (32.44%) |
| Thromboembolic | Deep vein thrombosis | 2 (3.03%) | 1 (0.63%) | 3 (1.33%) |
| Pulmonary embolism | 1 (1.52%) | 0 | 1 (0.44%) |
| Nanotechnology | Type of Intervention | Reference |
|---|---|---|
| NU-0129: Spherical Nucleic Acid (SNA) platform consisting of nucleic acids arranged on the surface of a small spherical gold nanoparticle that targets cancer cells, via the BBB, to inhibit the activity of the Bcl2L12 gene to induce apoptosis | Therapeutic—siRNA delivery | [80] |
| SGT-53: complex of cationic liposome encapsulating a normal human wild-type p53 DNA sequence in a plasmid backbone for delivery to tumor cells via the BBB. | Therapeutic—drug (Temozolomide) and gene Delivery | [81] |
| 2B3- 101: Glutathione pegylated liposomal doxorubicin hydrochloride | Therapeutic—drug delivery | [82] |
| BrUOG 329 (Onivyde): Nanoliposomal Irinotecan with enhanced ability to cross the BBB | Therapeutic—drug delivery | [83] |
| NanoBB- 1-Dox: nanoparticle-based formulation of doxorubicin, which enables passage of the drug across the BBB and delivery to the tumor inside the brain | Therapeutic—drug delivery | [84] |
| NanoTherm®: superparamagnetic iron oxide nanoparticles (SPIONS) | Therapeutic—Hyperthermia | [30,31,32,85] |
| EnGeneIC delivery vehicle (EDV): Novel nanoparticle (minicell) made from Salmonella typhi that encapsulates doxorubicin and targets Epithelial growth factor receptor (EGFR) by Vectibix | Therapeutic—drug delivery | [39] |
| Pegylated liposomal doxorubicin (Caelyx™, PEG-Dox) | Therapeutic—drug delivery | [33,34,35] |
| Myocet®: a non-pegylated liposomal doxorubicin | Therapeutic—drug delivery | [86] |
| Doxorubicin-loaded Anti-EGFR-immunoliposomes (C225-ILs- dox) in High-grade Gliomas (GBM-LIPO) | Therapeutic—drug delivery | [87] |
| Type of Therapy | Nanotherapy | Bevacizumab | ||||
|---|---|---|---|---|---|---|
| Type of GB | Primary | Recurrent | Overall | Primary | Recurrent | Overall |
| No. of Patients | 66 | 159 | 225 | 637 | 548 | 1185 |
| mOS | 6.75 months | 9.7 months | 8.2 months | 15.7 months | 9.3 months | 12.5 months |
| Mean PFS | 2.3 months | 3.92 months | 3.11 months | 10.7 months | - | - |
| Macdonald criteria | CR = 3.03%, PR = 4.54%, SD = 63.64% | CR = 0.63%, PR = 5.66%, SD = 9.43% | CR = 1.33%, PR = 5.33%, SD = 25.33% | - | CR = 6.02%, PR = 49.09%, SD = 29.02% | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, M.; Scalia, G.; Umana, G.E.; Parekh, U.A.; Naeem, F.; Abid, S.F.; Khan, M.H.; Zahra, S.G.; Sarkar, H.P.; Chaurasia, B. A Systematic Review of Nanomedicine in Glioblastoma Treatment: Clinical Efficacy, Safety, and Future Directions. Brain Sci. 2023, 13, 1727. https://doi.org/10.3390/brainsci13121727
Farooq M, Scalia G, Umana GE, Parekh UA, Naeem F, Abid SF, Khan MH, Zahra SG, Sarkar HP, Chaurasia B. A Systematic Review of Nanomedicine in Glioblastoma Treatment: Clinical Efficacy, Safety, and Future Directions. Brain Sciences. 2023; 13(12):1727. https://doi.org/10.3390/brainsci13121727
Chicago/Turabian StyleFarooq, Minaam, Gianluca Scalia, Giuseppe E. Umana, Urja A. Parekh, Faiza Naeem, Sayeda Fatima Abid, Muhammad Hammad Khan, Shah Gul Zahra, Hrishikesh P. Sarkar, and Bipin Chaurasia. 2023. "A Systematic Review of Nanomedicine in Glioblastoma Treatment: Clinical Efficacy, Safety, and Future Directions" Brain Sciences 13, no. 12: 1727. https://doi.org/10.3390/brainsci13121727
APA StyleFarooq, M., Scalia, G., Umana, G. E., Parekh, U. A., Naeem, F., Abid, S. F., Khan, M. H., Zahra, S. G., Sarkar, H. P., & Chaurasia, B. (2023). A Systematic Review of Nanomedicine in Glioblastoma Treatment: Clinical Efficacy, Safety, and Future Directions. Brain Sciences, 13(12), 1727. https://doi.org/10.3390/brainsci13121727










