On the Relationship between Value- and Threat-Driven Attentional Capture and Approach-Avoidance Biases
Abstract
:1. Introduction
2. Experiment 1
2.1. Methods
2.1.1. Participants
2.1.2. Apparatus
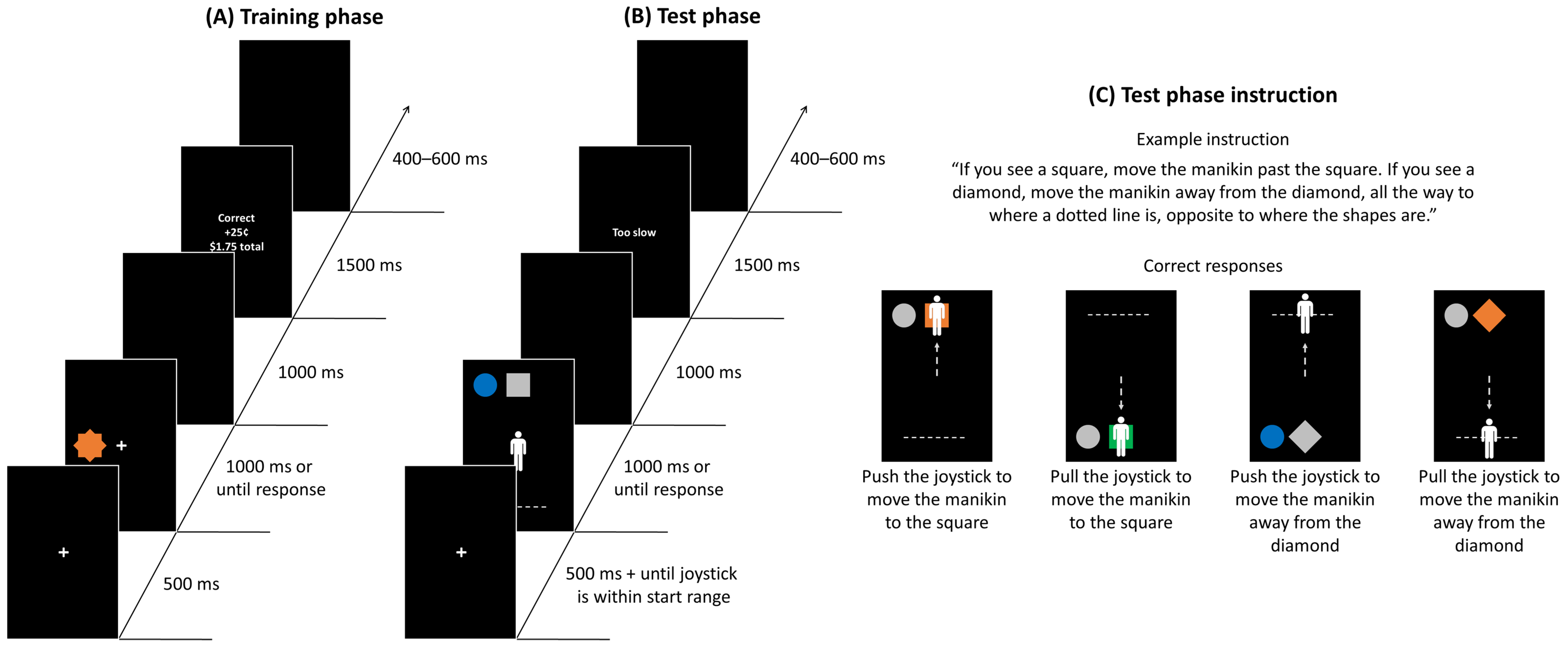
2.1.3. Procedure
Shock Calibration
Training Phase
Test Phase
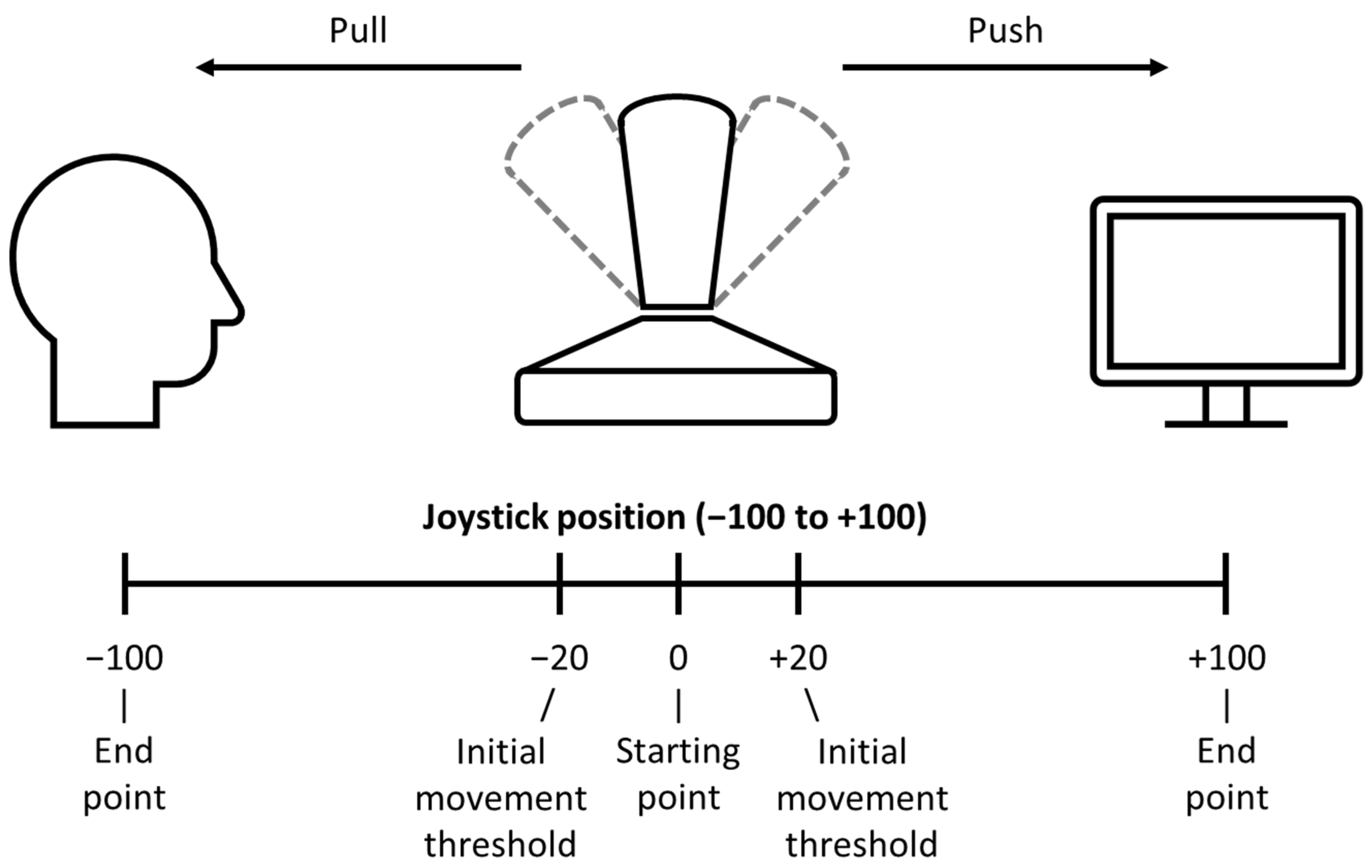
2.2. Data Analysis
2.3. Results
2.3.1. Training Phase
2.3.2. Test Phase
2.4. Discussion
3. Experiment 2
3.1. Methods
3.1.1. Participants
3.1.2. Apparatus
3.1.3. Procedure
Shock Calibration
Training Phase
Test Phase
3.2. Data Analysis
3.3. Results
3.3.1. Training Phase
3.3.2. Test Phase
3.4. Discussion
4. General Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, B.A.; Kim, H.; Kim, A.J.; Liao, M.-R.; Mrkonja, L.; Clement, A.; Grégoire, L. The past, present, and future of selection history. Neurosci. Biobehav. Rev. 2021, 130, 326–350. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.A.; Laurent, P.A.; Yantis, S. Value-driven attentional capture. Proc. Natl. Acad. Sci. USA 2011, 108, 10367–10371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, L.J.; Belopolsky, A.V.; Theeuwes, J. Attentional capture by signals of threat. Cogn. Emot. 2015, 29, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Bucker, B.; Theeuwes, J. Pavlovian reward learning underlies value driven attentional capture. Atten. Percept. Psychophys. 2017, 79, 415–428. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Anderson, B.A. Dissociable Components of Experience-Driven Attention. Curr. Biol. 2019, 29, 841–845.e842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Anderson, B.A. How does the attention system learn from aversive outcomes? Emotion 2021, 21, 898–903. [Google Scholar] [CrossRef]
- Le Pelley, M.E.; Pearson, D.; Griffiths, O.; Beesley, T. When goals conflict with values: Counterproductive attentional and oculomotor capture by reward-related stimuli. J. Exp. Psychol. Gen. 2015, 144, 158–171. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Nanavaty, N.; Ahmed, H.; Mathur, V.A.; Anderson, B.A. Motivational Salience Guides Attention to Valuable and Threatening Stimuli: Evidence from Behavior and Functional Magnetic Resonance Imaging. J. Cogn. Neurosci. 2021, 33, 2440–2460. [Google Scholar] [CrossRef]
- Gable, P.; Harmon-Jones, E. The motivational dimensional model of affect: Implications for breadth of attention, memory, and cognitive categorisation. Cogn. Emot. 2010, 24, 322–337. [Google Scholar] [CrossRef]
- Brosch, T.; Sander, D.; Pourtois, G.; Scherer, K.R. Beyond Fear: Rapid Spatial Orienting Toward Positive Emotional Stimuli. Psychol. Sci. 2008, 19, 362–370. [Google Scholar] [CrossRef]
- Vuilleumier, P. How brains beware: Neural mechanisms of emotional attention. Trends Cogn. Sci. 2005, 9, 585–594. [Google Scholar] [CrossRef]
- Lang, P.J.; Bradley, M.M.; Cuthbert, B.N. Motivated attention: Affect, activation, and action. In Attention and Orienting: Sensory and Motivational Processes; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 1997; pp. 97–135. [Google Scholar]
- Elliot, A.J.; Covington, M.V. Approach and Avoidance Motivation. Educ. Psychol. Rev. 2001, 13, 73–92. [Google Scholar] [CrossRef]
- Anderson, B.A. Going for It: The Economics of Automaticity in Perception and Action. Curr. Dir. Psychol. Sci. 2017, 26, 140–145. [Google Scholar] [CrossRef]
- Meyer, K.N.; Sheridan, M.A.; Hopfinger, J.B. Reward history impacts attentional orienting and inhibitory control on untrained tasks. Atten. Percept. Psychophys. 2020, 82, 3842–3862. [Google Scholar] [CrossRef]
- Moher, J.; Anderson, B.A.; Song, J.-H. Dissociable Effects of Salience on Attention and Goal-Directed Action. Curr. Biol. 2015, 25, 2040–2046. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Anderson, B.A. Neural evidence for automatic value-modulated approach behaviour. NeuroImage 2019, 189, 150–158. [Google Scholar] [CrossRef]
- van Wouwe, N.C.; van den Wildenberg, W.P.M.; Ridderinkhof, K.R.; Claassen, D.O.; Neimat, J.S.; Wylie, S.A. Easy to learn, hard to suppress: The impact of learned stimulus–outcome associations on subsequent action control. Brain Cogn. 2015, 101, 17–34. [Google Scholar] [CrossRef] [Green Version]
- Anderson, B.A.; Folk, C.L.; Garrison, R.; Rogers, L. Mechanisms of habitual approach: Failure to suppress irrelevant responses evoked by previously reward-associated stimuli. J. Exp. Psychol. Gen. 2016, 145, 796–805. [Google Scholar] [CrossRef]
- Chen, M.; Bargh, J.A. Consequences of Automatic Evaluation: Immediate Behavioral Predispositions to Approach or Avoid the Stimulus. Personal. Soc. Psychol. Bull. 1999, 25, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Krieglmeyer, R.; Deutsch, R.; De Houwer, J.; De Raedt, R. Being Moved: Valence Activates Approach-Avoidance Behavior Independently of Evaluation and Approach-Avoidance Intentions. Psychol. Sci. 2010, 21, 607–613. [Google Scholar] [CrossRef]
- Guitart-Masip, M.; Huys, Q.J.M.; Fuentemilla, L.; Dayan, P.; Duzel, E.; Dolan, R.J. Go and no-go learning in reward and punishment: Interactions between affect and effect. NeuroImage 2012, 62, 154–166. [Google Scholar] [CrossRef] [Green Version]
- Krieglmeyer, R.; De Houwer, J.; Deutsch, R. On the Nature of Automatically Triggered Approach–Avoidance Behavior. Emot. Rev. 2013, 5, 280–284. [Google Scholar] [CrossRef]
- Jones, I.F.; Young, S.G.; Claypool, H.M. Approaching the familiar: On the ability of mere exposure to direct approach and avoidance behavior. Motiv. Emot. 2011, 35, 383–392. [Google Scholar] [CrossRef]
- Kim, H.; Anderson, B.A. Dissociable neural mechanisms underlie value-driven and selection-driven attentional capture. Brain Res. 2019, 1708, 109–115. [Google Scholar] [CrossRef]
- Anderson, B.A.; Laurent, P.A.; Yantis, S. Value-driven attentional priority signals in human basal ganglia and visual cortex. Brain Res. 2014, 1587, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Anderson, B.A.; Kuwabara, H.; Wong, D.F.; Gean, E.G.; Rahmim, A.; Brašić, J.R.; George, N.; Frolov, B.; Courtney, S.M.; Yantis, S. The Role of Dopamine in Value-Based Attentional Orienting. Curr. Biol. 2016, 26, 550–555. [Google Scholar] [CrossRef] [Green Version]
- Anderson, B.A.; Kuwabara, H.; Wong, D.F.; Roberts, J.; Rahmim, A.; Brašić, J.R.; Courtney, S.M. Linking dopaminergic reward signals to the development of attentional bias: A positron emission tomographic study. NeuroImage 2017, 157, 27–33. [Google Scholar] [CrossRef]
- Kim, H.F.; Ghazizadeh, A.; Hikosaka, O. Dopamine Neurons Encoding Long-Term Memory of Object Value for Habitual Behavior. Cell 2015, 163, 1165–1175. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.F.; Hikosaka, O. Distinct Basal Ganglia Circuits Controlling Behaviors Guided by Flexible and Stable Values. Neuron 2013, 79, 1001–1010. [Google Scholar] [CrossRef] [Green Version]
- Hikosaka, O.; Kim, H.F.; Yasuda, M.; Yamamoto, S. Basal Ganglia Circuits for Reward Value–Guided Behavior. Annu. Rev. Neurosci. 2014, 37, 289–306. [Google Scholar] [CrossRef]
- Ghazizadeh, A.; Griggs, W.; Hikosaka, O. Ecological Origins of Object Salience: Reward, Uncertainty, Aversiveness, and Novelty. Front. Neurosci. 2016, 10, 378. [Google Scholar] [CrossRef] [Green Version]
- Albin, R.L.; Young, A.B.; Penney, J.B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989, 12, 366–375. [Google Scholar] [CrossRef]
- Soares-Cunha, C.; Coimbra, B.; Sousa, N.; Rodrigues, A.J. Reappraising striatal D1- and D2-neurons in reward and aversion. Neurosci. Biobehav. Rev. 2016, 68, 370–386. [Google Scholar] [CrossRef]
- Gerfen, C.R.; Surmeier, D.J. Modulation of Striatal Projection Systems by Dopamine. Annu. Rev. Neurosci. 2011, 34, 441–466. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.; Shin, E.Y.; Kim, S. Spatiotemporal dissociation of fMRI activity in the caudate nucleus underlies human de novo motor skill learning. Proc. Natl. Acad. Sci. USA 2020, 117, 23886–23897. [Google Scholar] [CrossRef]
- LeDoux, J.E. The Emotional Brain: The Mysterious Underpinnings of Emotional Life; Simon & Schuster: New York, NY, USA, 1996; p. 384. [Google Scholar]
- Öhman, A.; Mineka, S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychol. Rev. 2001, 108, 483–522. [Google Scholar] [CrossRef] [Green Version]
- Baxter, M.G.; Murray, E.A. The amygdala and reward. Nat. Rev. Neurosci. 2002, 3, 563–573. [Google Scholar] [CrossRef]
- Peck, C.J.; Lau, B.; Salzman, C.D. The primate amygdala combines information about space and value. Nat. Neurosci. 2013, 16, 340–348. [Google Scholar] [CrossRef] [Green Version]
- Peck, C.J.; Salzman, C.D. The Amygdala and Basal Forebrain as a Pathway for Motivationally Guided Attention. J. Neurosci. 2014, 34, 13757. [Google Scholar] [CrossRef] [Green Version]
- Peck, C.J.; Salzman, C.D. Amygdala neural activity reflects spatial attention towards stimuli promising reward or threatening punishment. eLife 2014, 3, e04478. [Google Scholar] [CrossRef]
- Ousdal, O.T.; Specht, K.; Server, A.; Andreassen, O.A.; Dolan, R.J.; Jensen, J. The human amygdala encodes value and space during decision making. NeuroImage 2014, 101, 712–719. [Google Scholar] [CrossRef] [Green Version]
- Maeda, K.; Inoue, K.-i.; Kunimatsu, J.; Takada, M.; Hikosaka, O. Primate Amygdalo-Nigral Pathway for Boosting Oculomotor Action in Motivating Situations. iScience 2020, 23, 101194. [Google Scholar] [CrossRef]
- Kim, J.; Zhang, X.; Muralidhar, S.; LeBlanc, S.A.; Tonegawa, S. Basolateral to Central Amygdala Neural Circuits for Appetitive Behaviors. Neuron 2017, 93, 1464–1479.e1465. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Pignatelli, M.; Xu, S.; Itohara, S.; Tonegawa, S. Antagonistic negative and positive neurons of the basolateral amygdala. Nat. Neurosci. 2016, 19, 1636–1646. [Google Scholar] [CrossRef] [Green Version]
- Terburg, D.; Scheggia, D.; Triana Del Rio, R.; Klumpers, F.; Ciobanu, A.C.; Morgan, B.; Montoya, E.R.; Bos, P.A.; Giobellina, G.; van den Burg, E.H.; et al. The Basolateral Amygdala Is Essential for Rapid Escape: A Human and Rodent Study. Cell 2018, 175, 723–735.e716. [Google Scholar] [CrossRef] [Green Version]
- Mobbs, D.; Petrovic, P.; Marchant, J.L.; Hassabis, D.; Weiskopf, N.; Seymour, B.; Dolan, R.J.; Frith, C.D. When Fear Is Near: Threat Imminence Elicits Prefrontal-Periaqueductal Gray Shifts in Humans. Science 2007, 317, 1079–1083. [Google Scholar] [CrossRef] [Green Version]
- Rinck, M.; Becker, E.S. Approach and avoidance in fear of spiders. J. Behav. Ther. Exp. Psychiatry 2007, 38, 105–120. [Google Scholar] [CrossRef]
- Brainard, D.H. The Psychophysics Toolbox. Spat. Vis. 1997, 10, 433–436. [Google Scholar] [CrossRef] [Green Version]
- Suh, J.; Abrams, R.A. Approach and avoidance movements modulate value-driven attentional capture. J. Exp. Psychol. Hum. Percept. Perform. 2020, 46, 105–123. [Google Scholar] [CrossRef]
- Lavender, T.; Hommel, B. Affect and action: Towards an event-coding account. Cogn. Emot. 2007, 21, 1270–1296. [Google Scholar] [CrossRef]
- Neumann, R.; Förster, J.; Strack, F. Motor compatibility: The bidirectional link between behavior and evaluation. In The Psychology of Evaluation: Affective Processes in Cognition and Emotion; Musch, J., Klauer, K.C., Eds.; Lawrence Erlbaum Associates, Inc.: Mahwah, NJ, USA, 2003; pp. 371–391. [Google Scholar]
- Phaf, R.H.; Mohr, S.E.; Rotteveel, M.; Wicherts, J.M. Approach, avoidance, and affect: A meta-analysis of approach-avoidance tendencies in manual reaction time tasks. Front. Psychol. 2014, 5, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wentura, D.; Rothermund, K.; Bak, P. Automatic vigilance: The attention-grabbing power of approach- and avoidance-related social information. J. Personal. Soc. Psychol. 2000, 78, 1024–1037. [Google Scholar] [CrossRef] [PubMed]
- van Dantzig, S.; Pecher, D.; Zwaan, R.A. Approach and avoidance as action effects. Q. J. Exp. Psychol. 2008, 61, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, A.C.; Schueuer, F.; Bestmann, S. Emotional valence and contextual affordances flexibly shape approach-avoidance movements. Front. Psychol. 2013, 4, 933. [Google Scholar] [CrossRef] [PubMed]
- Krieglmeyer, R.; Deutsch, R. Comparing measures of approach–avoidance behaviour: The manikin task vs. two versions of the joystick task. Cogn. Emot. 2010, 24, 810–828. [Google Scholar] [CrossRef]
- Hoofs, V.; Carsten, T.; Boehler, C.N.; Krebs, R.M. Interactions between incentive valence and action information in a cued approach–avoidance task. Psychol. Res. 2019, 83, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Shimojo, S.; O’Doherty, J.P. Is Avoiding an Aversive Outcome Rewarding? Neural Substrates of Avoidance Learning in the Human Brain. PLOS Biol. 2006, 4, e233. [Google Scholar] [CrossRef] [Green Version]
- Rotteveel, M.; Phaf, R.H. Automatic Affective Evaluation Does Not Automatically Predispose for Arm Flexion and Extension. Emotion 2004, 4, 156–172. [Google Scholar] [CrossRef]
- Marsh, A.A.; Ambady, N.; Kleck, R.E. The Effects of Fear and Anger Facial Expressions on Approach- and Avoidance-Related Behaviors. Emotion 2005, 5, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Seidel, E.-M.; Habel, U.; Kirschner, M.; Gur, R.C.; Derntl, B. The impact of facial emotional expressions on behavioral tendencies in women and men. J. Exp. Psychol. Hum. Percept. Perform. 2010, 36, 500–507. [Google Scholar] [CrossRef]
- Aulbach, M.B.; Knittle, K.; Haukkala, A. Implicit process interventions in eating behaviour: A meta-analysis examining mediators and moderators. Health Psychol. Rev. 2019, 13, 179–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veling, H.; Verpaalen, I.A.M.; Liu, H.; Mosannenzadeh, F.; Becker, D.; Holland, R.W. How can food choice best be trained? Approach-avoidance versus go/no-go training. Appetite 2021, 163, 105226. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Anderson, B.A. Primary rewards and aversive outcomes have comparable effects on attentional bias. Behav. Neurosci. 2022. [Google Scholar] [CrossRef] [PubMed]

| Start RT (ms) | End RT (ms) | Accuracy (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Reward | Shock | Neutral | Reward | Shock | Neutral | Reward | Shock | Neutral | |
| Pull/ approach | 601.9 (92) | 609.4 (98.1) | 611.8 (94.1) | 736 (120.6) | 744.7 (127.5) | 746.8 (122.8) | 99 (1.7) | 97.7 (3.9) | 98.4 (2.4) |
| Push/ withdraw | 602.8 (98) | 604.6 (111.5) | 601.9 (99.3) | 766.8 (140.5) | 780.4 (165.9) | 775.8 (153.1) | 98.2 (2.9) | 97 (3.5) | 98.2 (2.3) |
| Start RT (ms) | End RT (ms) | Accuracy (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Reward | Shock | Neutral | Reward | Shock | Neutral | Reward | Shock | Neutral | |
| Pull/ approach | 644.9 (85.4) | 642.7 (96) | 649.5 (96.8) | 753 (108) | 751.5 (119.6) | 757.3 (119.7) | 98.5 (2.5) | 99 (2.8) | 98.3 (2.3) |
| Push/ withdraw | 642.7 (103.3) | 632.3 (100.1) | 641.5 (103.5) | 785.5 (138.7) | 774.1 (134.5) | 781.9 (139.6) | 97.7 (3) | 98.5 (1.9) | 98.3 (2.1) |
| Reward | Shock | Neutral | |
|---|---|---|---|
| RT (ms) | 318.3 (42.4) | 322.9 (54) | 316.9 (43.7) |
| Accuracy (%) | 99.7 (0.6) | 98.9 (2.1) | 99.7 (0.6) |
| Valent Target | |||||||||
| Start RT (ms) | End RT (ms) | Accuracy (%) | |||||||
| Reward | Shock | Neutral | Reward | Shock | Neutral | Reward | Shock | Neutral | |
| Approach | 607.1 (79.7) | 611.7 (77.4) | 610.8 (84.5) | 758.1 (101.4) | 753.5 (90) | 753.8 (94.3) | 98 (3.5) | 98.1 (3) | 98.7 (2.6) |
| Withdraw | 638.4 (97.1) | 642 (88.9) | 641.7 (95.8) | 780.3 (107.8) | 784.7 (95.4) | 785.8 (106.9) | 99.1 (2.2) | 98.5 (2.9) | 99.2 (2.3) |
| Valent Distractor | |||||||||
| Start RT (ms) | End RT (ms) | Accuracy (%) | |||||||
| Reward | Shock | Neutral | Reward | Shock | Neutral | Reward | Shock | Neutral | |
| Approach | 619.8 (83.4) | 611.7 (80) | 607.9 (79.9) | 769.6 (97.3) | 758.6 (95.8) | 751.4 (91.5) | 98.3 (3) | 98.4 (3.5) | 99.1 (2.4) |
| Withdraw | 649.8 (97.7) | 645.4 (86.3) | 636.7 (92.3) | 800 (111.1) | 797.5 (102.3) | 776.6 (102.2) | 99.1 (2.3) | 99 (2.2) | 98.6 (2.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Anderson, B.A. On the Relationship between Value- and Threat-Driven Attentional Capture and Approach-Avoidance Biases. Brain Sci. 2023, 13, 158. https://doi.org/10.3390/brainsci13020158
Kim H, Anderson BA. On the Relationship between Value- and Threat-Driven Attentional Capture and Approach-Avoidance Biases. Brain Sciences. 2023; 13(2):158. https://doi.org/10.3390/brainsci13020158
Chicago/Turabian StyleKim, Haena, and Brian A. Anderson. 2023. "On the Relationship between Value- and Threat-Driven Attentional Capture and Approach-Avoidance Biases" Brain Sciences 13, no. 2: 158. https://doi.org/10.3390/brainsci13020158





