Spatial Frequency Tuning of Body Inversion Effects
Abstract
:1. Introduction
2. Methods and Materials
2.1. Participants
2.2. Stimuli
2.3. Procedure
2.4. Data Handling
3. Results
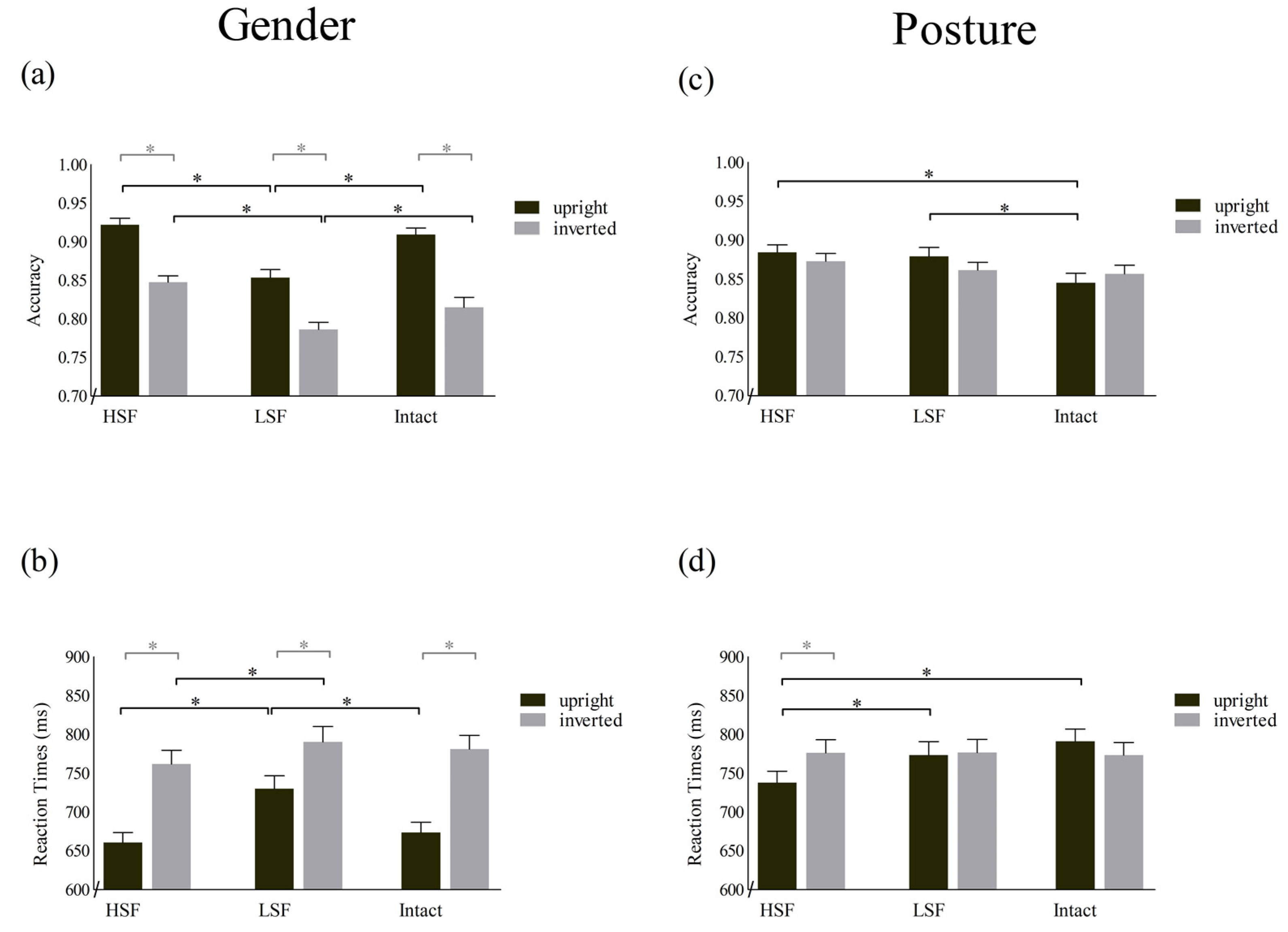
3.1. Accuracy
3.2. Reaction Times (RTs)
3.3. Correlations
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SF | Spatial Frequency |
| HSF | High Spatial Frequency |
| LSF | Low Spatial Frequency |
| BIE | Body Inversion Effect |
| BIEI | Body Inversion Effect Index |
| EBA | Extrastriate Body Area |
| AQ | Autistic Quotient |
References
- Goodale, M.A.; Milner, A.D.; Jakobson, L.S.; Carey, D.P. A neurological dissociation between perceiving objects and grasping them. Nature 1991, 349, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Goodale, M.A.; Meenan, J.P.; Bülthoff, H.H.; Nicolle, D.A.; Murphy, K.J.; Racicot, C.I. Separate neural pathways for the visual analysis of object shape in perception and prehension. Curr. Biol. 1994, 4, 604–610. [Google Scholar] [CrossRef]
- Denison, R.N.; Vu, A.T.; Yacoub, E.; Feinberg, D.A.; Silver, M.A. Functional mapping of the magnocellular and parvocellular subdivisions of human LGN. Neuroimage 2014, 102, 358–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosslyn, S.M.; Chabris, C.F.; Marsolek, C.J.; Koenig, O. Categorical Versus Coordinate Spatial Relations: Computational Analyses and Computer Simulations. J. Exp. Psychol. Hum. Percept. Perform. 1992, 18, 562. [Google Scholar] [CrossRef] [PubMed]
- Saneyoshi, A.; Michimata, C. Categorical and coordinate processing in object recognition depends on different spatial frequencies. Cogn. Process. 2015, 16, 27–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michimata, C.; Saneyoshi, A.; Okubo, M.; Laeng, B. Effects of the global and local attention on the processing of categorical and coordinate spatial relations. Brain Cogn. 2011, 77, 292–297. [Google Scholar] [CrossRef]
- Kauffmann, L.; Ramanoël, S.; Peyrin, C. The neural bases of spatial frequency processing during scene perception. Front. Integr. Neurosci. 2014, 8, 37. [Google Scholar] [CrossRef] [Green Version]
- Goffaux, V.; Peters, J.; Haubrechts, J.; Schiltz, C.; Jansma, B.; Goebel, R. From coarse to fine? spatial and temporal dynamics of cortical face processing. Cereb. Cortex 2011, 21, 467–476. [Google Scholar] [CrossRef] [Green Version]
- Dailey, M.N.; Cottrell, G.W. Organization of face and object recognition in modular neural network models. Neural Netw. 1999, 12, 1053–1074. [Google Scholar] [CrossRef] [Green Version]
- Minnebusch, D.A.; Suchan, B.; Daum, I. Losing your head: Behavioral and electrophysiological effects of body inversion. J. Cogn. Neurosci. 2009, 21, 865–874. [Google Scholar] [CrossRef]
- Sergent, J. An investigation into component and configural processes underlying face perception. Br. J. Psychol. 1984, 75, 221–242. [Google Scholar] [CrossRef] [PubMed]
- Young, A.W.; Hellawell, D.; Hay, D.C. Configurational information in face perception. Perception 1987, 42, 1166–1178. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.W.; Farah, M.J. Parts and Wholes in Face Recognition. Q. J. Exp. Psychol. 1993, 46, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.K. Looking at upide-down faces. J. Exp. Psychol. 1969, 81, 141. [Google Scholar] [CrossRef]
- Reed, C.L.; Stone, V.E.; Bozova, S.; Tanaka, J. The body-inversion effect. Psychol. Sci. 2003, 14, 302–308. [Google Scholar] [CrossRef]
- Reed, C.L.; Stone, V.E.; Grubb, J.D.; McGoldrick, J.E. Turning configural processing upside down: Part and whole body postures. J. Exp. Psychol. Hum. Percept. Perform. 2006, 32, 73–87. [Google Scholar] [CrossRef] [Green Version]
- Bullier, J. Integrated model of visual processing. Brain Res. Rev. 2001, 36, 96–107. [Google Scholar] [CrossRef]
- Flevaris, A.V.; Robertson, L.C. Attention to hierarchical level influences attentional selection of spatial scale. J. Exp. Psychol. 2011, 37, 12–22. [Google Scholar] [CrossRef]
- Watt, R.J. Scanning from coarse to fine spatial scales in the human visual system after the onset of a stimulus. J. Opt. Soc. Am. A. 1987, 4, 2006–2021. [Google Scholar] [CrossRef]
- Hegdé, J. Time course of visual perception: Coarse-to-fine processing and beyond. Prog. Neurobiol. 2008, 84, 405–439. [Google Scholar] [CrossRef]
- Goffaux, V. Spatial interactions in upright and inverted faces: Re-exploration of spatial scale influence. Vis. Res. 2009, 49, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Goffaux, V.; Rossion, B. Faces are ‘spatial’--holistic face perception is supported by low spatial frequencies. J. Exp. Psychol. Hum. Percept. Perform. 2006, 32, 1023–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, O.S.; Richler, J.J.; Palmeri, T.J.; Gauthier, I. Revisiting the Role of Spatial Frequencies in the Holistic Processing of Faces. J. Exp. Psychol. Hum. Percept. Perform. 2008, 34, 1327–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boutet, I.; Collin, C.; Faubert, J. Configural face encoding and spatial frequency information. Percept. Psychophys. 2003, 65, 1078–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaspar, C.; Sekuler, A.B.; Bennett, P.J. Spatial frequency tuning of upright and inverted face identification. Vis. Res. 2008, 48, 2817–2826. [Google Scholar] [CrossRef] [Green Version]
- Flevaris, A.V.; Robertson, L.C.; Bentin, S. Using spatial frequency scales for processing face features and face configuration: An ERP analysis. Brain Res. 2008, 1194, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Goffaux, V.; Gauthier, I.; Rossion, B. Spatial scale contribution to early visual differences between face and object processing. Brain Res. Cogn. Brain Res. 2003, 16, 416–424. [Google Scholar] [CrossRef]
- Halit, H.; de Haan, M.; Schyns, P.G.; Johnson, M.H. Is high-spatial frequency information used in the early stages of face detection? Brain Res. 2006, 1117, 154–161. [Google Scholar] [CrossRef]
- Canário, N.; Jorge, L.; Loureiro Silva, M.F.; Alberto Soares, M.; Castelo-Branco, M. Distinct preference for spatial frequency content in ventral stream regions underlying the recognition of scenes, faces, bodies and other objects. Neuropsychologia 2016, 87, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.C.; Wiggett, A.J.; Downing, P.E. Functional MRI analysis of body and body part representations in the extrastriate and fusiform body areas. J. Neurophysiol. 2007, 98, 1626–1633. [Google Scholar] [CrossRef]
- Brandman, T.; Yovel, G. Bodies are Represented as Wholes Rather Than Their Sum of Parts in the Occipital-Temporal Cortex. Cereb. Cortex 2016, 26, 530–543. [Google Scholar] [CrossRef] [Green Version]
- Urgesi, C.; Candidi, M.; Ionta, S.; Aglioti, S.M. Representation of body identity and body actions in extrastriate body area and ventral premotor cortex. Nat. Neurosci. 2007, 10, 30–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandolfo, M.; Downing, P.E. Asymmetric visual representation of sex from human body shape. Cognition 2020, 205, 104436. [Google Scholar] [CrossRef] [PubMed]
- Giese, M.A.; Poggio, T. Cognitive neuroscience: Neural mechanisms for the recognition of biological movements. Nat. Rev. Neurosci. 2003, 4, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Urgesi, C.; Calvo-Merino, B.; Haggard, P.; Aglioti, S.M. Transcranial magnetic stimulation reveals two cortical pathways for visual body processing. J. Neurosci. 2007, 27, 8023–8030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schyns, P.G.; Oliva, A. Flexible, diagnosticity-driven, rather than fixed, perceptually determined scale selection in scene and face recognition. Perception 1997, 26, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Goffaux, V.; Hault, B.; Michel, C.; Vuong, Q.C.; Rossion, B. The respective role of low and high spatial frequencies in supporting configural and featural processing of faces. Perception 2005, 34, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Rosenblum, M.; Yacoob, Y.; Davis, L.S. Human expression recognition from motion using a radial basis function network architecture. IEEE Trans. Neural Netw. 1996, 7, 1121–1138. [Google Scholar] [CrossRef]
- Deruelle, C.; Fagot, J. Categorizing facial identities, emotions, and genders: Attention to high- and low-spatial frequencies by children and adults. J. Exp. Child Psychol. 2005, 90, 172–184. [Google Scholar] [CrossRef]
- Koyama, S.; Gu, J.; Hibino, H. Perception of race and sex differently depends on the low and high spatial frequency channels. J. Vis. 2010, 10, 704. [Google Scholar] [CrossRef]
- Jeantet, C.; Laprevote, V.; Schwan, R.; Schwitzer, T.; Maillard, L.; Lighezzolo-Alnot, J.; Caharel, S. Time course of spatial frequency integration in face perception: An ERP study. Int. J. Psychophysiol. 2019, 143, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Schyns, P.G.; Oliva, A. Dr. Angry and Mr. Smile: When categorization flexibly modifies the perception of faces in rapid visual presentations. Cognition 1999, 69, 243–265. [Google Scholar] [CrossRef]
- Vuilleumier, P.; Armony, J.L.; Driver, J.; Dolan, R.J. Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nat. Neurosci. 2003, 6, 624–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, C.L. Brief report: Perception of body posture—What individuals with autism spectrum disorder might be missing. J. Autism Dev. Disord. 2007, 37, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Happé, F.G.E.; Booth, R.D.L. The power of the positive: Revisiting weak coherence in autism spectrum disorders. Q. J. Exp. Psychol. 2008, 61, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Booth, R.D.L.; Happé, F.G.E. Evidence of Reduced Global Processing in Autism Spectrum Disorder. J. Autism Dev. Disord. 2018, 48, 1397–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A. Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- D’Argenio, G.; Finisguerra, A.; Urgesi, C. Motion and Gender-Typing Features Interact in the Perception of Human Bodies. Front. Neurosci. 2020, 14, 277. [Google Scholar] [CrossRef] [Green Version]
- Baron-Cohen, S.; Wheelwright, S.; Skinner, R.; Martin, J.; Clubley, E. The Autism-Spectrum Quotient (AQ): Evidence from Asperger Syndrome/High-Functioning Autism, Males and Females, Scientists and Mathematicians. J. Autism Dev. Disord. 2001, 31, 5–17. [Google Scholar] [CrossRef]
- Ruzich, E.; Allison, C.; Smith, P.; Watson, P.; Auyeung, B.; Ring, H.; Baron-Cohen, S. Measuring autistic traits in the general population: A systematic review of the Autism-Spectrum Quotient (AQ) in a nonclinical population sample of 6,900 typical adult males and females. Mol. Autism 2015, 6, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, K.L.; Lurye, L.E.; Tassinary, L.G. Sex categorization among preschool children: Increasing utilization of sexually dimorphic cues. Child Dev. 2010, 81, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.L.; Tassinary, L.G. Perceiving sex directly and indirectly: Meaning in motion and morphology. Psychol. Sci. 2005, 16, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.M.; Gallup, G.G. Sex differences in morphological predictors of sexual behavior. Shoulder to hip and waist to hip ratios. Evol. Hum. Behav. 2003, 24, 173–178. [Google Scholar] [CrossRef]
- Gandolfo, M.; Downing, P.E. Perceiving emotion and sex from the body: Evidence from the Garner task for independent processes. Cogn. Emot. 2020, 34, 427–437. [Google Scholar]
- Skomina, Z.; Verdenik, M.; Hren, N.I. Effect of aging and body characteristics on facial sexual dimorphism in the Caucasian Population. PLoS ONE 2020, 15, e0231983. [Google Scholar] [CrossRef]
- Downing, P.E.; Peelen, M.V. The role of occipitotemporal body-selective regions in person perception. Cogn. Neurosci. 2011, 2, 186–203. [Google Scholar] [CrossRef]
- Morrison, D.J.; Schyns, P.G. Usage of spatial scales for the categorization of faces, objects, and scenes. Psychon. Bull. Rev. 2001, 8, 454–469. [Google Scholar] [PubMed]
- Bar, M. A cortical mechanism for triggering top-down facilitation in visual object recognition. J. Cogn. Neurosci. 2003, 15, 600–609. [Google Scholar] [CrossRef]
- Bar, M.; Kassam, K.S.; Ghuman, A.S.; Boshyan, J.; Schmid, A.M.; Dale, A.M.; Hämäläinen, M.S.; Marinkovic, K.; Schacter, D.L.; Rosen, B.R.; et al. Top-down facilitation of visual recognition. Proc. Natl. Acad. Sci. USA 2006, 103, 449–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amoruso, L.; Finisguerra, A.; Urgesi, C. Spatial frequency tuning of motor responses reveals differential contribution of dorsal and ventral systems to action comprehension. Proc. Natl. Acad. Sci. USA 2020, 117, 13151–13161. [Google Scholar] [CrossRef] [PubMed]
- Collin, C.A.; Therrien, M.; Martin, C.; Rainville, S. Spatial frequency thresholds for face recognition when comparison faces are filtered and unfiltered. Percept. Psychophys. 2006, 68, 879–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kornowski, J.A.; Petersik, J.T. Effects on face recognition of spatial-frequency information contained in inspection and test stimuli. J. Gen. Psychol. 2003, 130, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Minnebusch, D.A.; Daum, I. Neuropsychological mechanisms of visual face and body perception. Neurosci. Biobehav. Rev. 2009, 33, 1133–1144. [Google Scholar] [CrossRef]
- Bauser, D.A.S.; Suchan, B.; Daum, I. Differences between perception of human faces and body shapes: Evidence from the composite illusion. Vis. Res. 2011, 51, 195–202. [Google Scholar]
- Harris, A.; Vyas, D.B.; Reed, C.L. Holistic processing for bodies and body parts: New evidence from stereoscopic depth manipulations. Psychon. Bull. Rev. 2016, 23, 1513–1519. [Google Scholar] [CrossRef] [Green Version]
- Blake, R.; Turner, L.M.; Smoski, M.J.; Pozdol, S.L.; Stone, W.L. Visual recognition of biological motion is impaired in children with autism. Psychol. Sci. 2003, 14, 151–157. [Google Scholar] [CrossRef]
- Maurer, D.; Le Grand, R.; Mondloch, C.J. The many faces of configural processing. Trends Cogn. Sci. 2002, 6, 255–260. [Google Scholar] [CrossRef]
- Cogoni, C.; Carnaghi, A.; Mitrovic, A.; Leder, H.; Fantoni, C.; Silani, G. Understanding the mechanisms behind the sexualized-body inversion hypothesis: The role of asymmetry and attention biases. PLoS ONE 2018, 13, e0193944. [Google Scholar] [CrossRef]
- Sincich, L.C.; Horton, J.C. The circuitry of V1 and V2: Integration of color, form, and motion. Annu. Rev. Neurosci. 2005, 28, 303–326. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Argenio, G.; Finisguerra, A.; Urgesi, C. Spatial Frequency Tuning of Body Inversion Effects. Brain Sci. 2023, 13, 190. https://doi.org/10.3390/brainsci13020190
D’Argenio G, Finisguerra A, Urgesi C. Spatial Frequency Tuning of Body Inversion Effects. Brain Sciences. 2023; 13(2):190. https://doi.org/10.3390/brainsci13020190
Chicago/Turabian StyleD’Argenio, Giulia, Alessandra Finisguerra, and Cosimo Urgesi. 2023. "Spatial Frequency Tuning of Body Inversion Effects" Brain Sciences 13, no. 2: 190. https://doi.org/10.3390/brainsci13020190





