Genetic and Epigenetic Sexual Dimorphism of Brain Cells during Aging
Abstract
:1. Introduction
2. Genetic Effects of Sex Chromosomes in the Brain
2.1. X-Linked Genes
2.2. Y-Linked Genes
2.3. Paralogous Genes of Sex Chromosomes
| Gene | Localization | Function | References |
|---|---|---|---|
| DMD | Xp21.2-p21.1 | Encodes an actin-binding cytoskeletal protein. Distal DMD mutations are linked to cognitive impairment. The risk and severity of cognitive disability are associated with a cumulative loss of distal DMD. | [16,60,61] |
| SYP | Xp11.22-p11.23 | SYP is an integral membrane protein of synaptic vesicles. | [62,63] |
| HSD17B10 | Xp11.2 | Affects the cognitive functions of the brain through a change in the vulnerability of synaptic mitochondria to estrogen. | [19,64] |
| STS | Xp22.31 | A key role in regulating the formation of biologically active steroids, it is also associated with attention deficit disorder and aggressive behavior. | [21,22,23,65] |
| PTCHD1 | Xp22.11 | PTCHD1 is associated with sleep, sensorimotor processing, and attention. PTCHD1 is predicted to be a transmembrane protein that encodes the 12 transmembrane helices that form two modules. A distinct pattern of membrane localization within dendritic spines. In addition, a portion of the intracellular C-terminal tail encoded appears to be essential for dendritic and synaptic targeting. | [26,27,66] |
| CASK | Xp11.4 | A role in a wide variety of cellular functions including transcription regulation, insulin signaling, and secretion. MICPCH is also considered a neurodevelopmental disorder that occurs due to heterozygous mutations in gene CASK in girls. Some missense CASK mutations in boys are milder and are usually found in cases of X-linked mental retardation in normocephalic boys. | [33,67,68,69] |
| SRY | Yp11.2 1 | The Sry transcript can function as a regulator for non-coding RNA (ncRNA). As is known, not only are ncRNAs involved in the spatial and temporal control of mRNA translation, which is necessary for functionally separated neurons, but they are also associated with brain development, neuronal differentiation, and complex functions such as learning and memory. | [39,43,44] |
| Usp9x/y | Xp11.4/Yq11.221 1 | Usp9x encodes a ubiquitin protease implicated in synaptic development, to be significantly higher in adult female mouse brains than in male brains. | [70] |
| UTX/Y | Xp11.3/Yq11.221 1 | Utx is involved in regulating HOX genes. Utx was expressed mainly in the amygdala, and Uty was expressed in the paraventricular nucleus of the hypothalamus. | [52,71] |
| PCDH11X/Y | Xq21.3/Yp11.2 1 | The protein plays a fundamental role in cell–cell recognition essential for the segmental development and function of the central nervous system. | [72] |
| NLGN4X/Y and RPS4X/Y | Xp22.3/Yq11.2 (NLGN4X/Y) and Xq21.3/Yp11.2 (PCDH11X/Y) | NLGN4X/Y genes encoding the synaptic cell adhesion molecules neuroligins. NLGN4 has a trafficking deficit that hinders its ability to induce synapses, due to its inability to move to the surface. | [53,55,73] |
| RPS4X/Y | Xq13.1/p11.31 | The RPS4 gene codifies for ribosomal protein S4. | [74,75] |
| DDX3X/Y | Xp11.3–11.23/AZFa region on the Y-chr | DDX3X enhances transcription by interacting with transcription factors. DDX3Y is expressed more broadly in tissues across the human body. DDX3Y is assumed to be one of the candidates for physiological changes in the development of PD. | [57,58] |
| TBL1X/Y | Xp22.31-p22.2/Yp11.2 1 | One of the variants of the TBL1Y gene is considered to be a potential cause of hereditary hearing loss. | [59] |
3. Expression of Non-Sex Chromosome Genes in the Brain
3.1. Changes in the Expression of Genes Associated with the Signaling Pathways of Insulin and Insulin-like Growth Factor-I (IGF-I), Collectively Called IIS (Insulin/IGF Signaling)
3.2. Changes in the Expression of Genes Responsible for the mTOR Signaling Pathway That Activate the Downstream Effector Kinase 1 of Ribosomal Protein S6 (S6K1)
3.3. Changes in the Expression of Regulatory Genes
3.4. Changes in the Expression of Sirtuins (SIRT6)
3.5. Heterozygosity of GIT2
4. Microglia as the Main Cellular Source of Sexual Dimorphism in the Brain
4.1. Pathogen-Associated Inflammation
4.2. Traumatic Brain Injury (TBI) and Obesity
4.3. Proteinopathy
4.4. Hypoxia/Ischemia
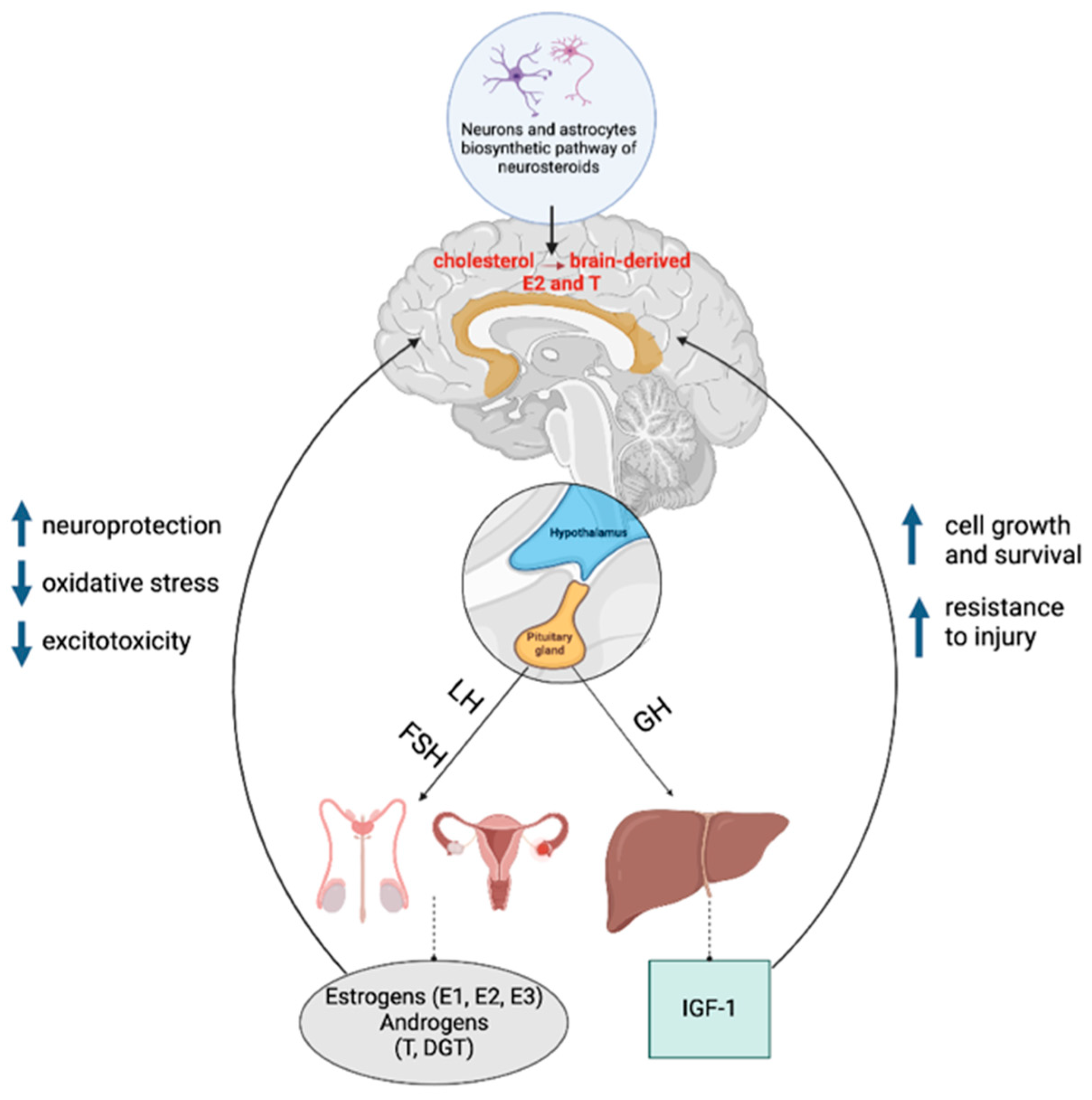
5. Sex-Dependent Neuroendocrine Aspects of Aging
5.1. Estrogens (E)
5.2. Sexual Dimorphism of the Dopaminergic System
5.3. Testosterone (T)
5.4. The Interaction of Sex Steroids with the Growth Hormone (GH)/Insulin Growth Factor 1 (IGF-1) System during Aging
5.5. The Influence of Sex Hormones on Epigenetic Processes
6. Sex-Dependent Regulation of the Mitochondrial Genome of the Brain
7. Sex-Dependent Epigenetic Regulation of the Brain during Aging
7.1. Role of Histone Modifications
7.2. Role of DNA Methylation
7.3. Role of miRs
7.4. Role of lncRNA
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Austad, S.N. Sex differences in health and aging: A dialog between the brain and gonad? Geroscience 2019, 41, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Clayton, J.A. Studying both sexes: A guiding principle for biomedicine. FASEB J. 2016, 30, 519–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauvais-Jarvis, F.; Bairey Merz, N.; Barnes, P.J.; Brinton, R.D.; Carrero, J.-J.; DeMeo, D.L.; de Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and gender: Modifiers of health, disease, and medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef]
- Bacon, E.R.; Brinton, R.D. Epigenetics of the developing and aging brain: Mechanisms that regulate onset and outcomes of brain reorganization. Neurosci. Biobehav. Rev. 2021, 125, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Qu, J.; Ma, H. Recent developments in understanding brain aging: Sex differences, mechanisms, and implications in diseases. Ageing Neurodegener. Dis. 2022, 2, 3. [Google Scholar] [CrossRef]
- DeCasien, A.R.; Guma, E.; Liu, S.; Raznahan, A. Sex differences in the human brain: A roadmap for more careful analysis and interpretation of a biological reality. Biol. Sex Differ. 2022, 13, 43. [Google Scholar] [CrossRef]
- Williamson, J.; Yabluchanskiy, A.; Mukli, P.; Wu, D.H.; Sonntag, W.; Ciro, C.; Yang, Y. Sex differences in brain functional connectivity of hippocampus in mild cognitive impairment. Front. Aging Neurosci. 2022, 14, 959394. [Google Scholar] [CrossRef]
- Sanfilippo, C.; Castrogiovanni, P.; Imbesi, R.; Tibullo, D.; Li Volti, G.; Barbagallo, I.; Vicario, N.; Musumeci, G.; Di Rosa, M. Middle-aged healthy women and Alzheimer’s disease patients present an overlapping of brain cell transcriptional profile. Neuroscience 2019, 406, 333–344. [Google Scholar] [CrossRef]
- Brooks, L.R.K.; Mias, G.I. Data-Driven Analysis of Age, Sex, and Tissue Effects on Gene Expression Variability in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 392. [Google Scholar] [CrossRef] [Green Version]
- Seney, M.L.; Huo, Z.; Cahill, K.; French, L.; Puralewski, R.; Zhang, J.; Logan, R.W.; Tseng, G.; Lewis, D.A.; Sibille, E. Opposite Molecular Signatures of Depression in Men and Women. Biol. Psychiatry 2018, 84, 18–27. [Google Scholar] [CrossRef]
- López-Cerdán, A.; Andreu, Z.; Hidalgo, M.R.; Grillo-Risco, R.; Català-Senent, J.F.; Soler-Sáez, I.; Neva-Alejo, A.; Gordillo, F.; de La Iglesia-Vayá, M.; García-García, F. Unveiling sex-based differences in Parkinson’s disease: A comprehensive meta-analysis of transcriptomic studies. Biol. Sex Differ. 2022, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Pinares-Garcia, P.; Stratikopoulos, M.; Zagato, A.; Loke, H.; Lee, J. Sex: A Significant Risk Factor for Neurodevelopmental and Neurodegenerative Disorders. Brain Sci. 2018, 8, 154. [Google Scholar] [CrossRef] [Green Version]
- Furman, B.L.S.; Metzger, D.C.H.; Darolti, I.; Wright, A.E.; Sandkam, B.A.; Almeida, P.; Shu, J.J.; Mank, J.E. Sex Chromosome Evolution: So Many Exceptions to the Rules. Genome Biol. Evol. 2020, 12, 750–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellott, D.W.; Page, D.C. Dosage-sensitive functions in embryonic development drove the survival of genes on sex-specific chromosomes in snakes, birds, and mammals. Genome Res. 2021, 31, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Pallier, P.N.; Ferrara, M.; Romagnolo, F.; Ferretti, M.T.; Soreq, H.; Cerase, A. Chromosomal and environmental contributions to sex differences in the vulnerability to neurological and neuropsychiatric disorders: Implications for therapeutic interventions. Prog. Neurobiol. 2022, 219, 102353. [Google Scholar] [CrossRef]
- Waite, A.; Brown, S.C.; Blake, D.J. The dystrophin-glycoprotein complex in brain development and disease. Trends Neurosci. 2012, 35, 487–496. [Google Scholar] [CrossRef]
- Tarpey, P.S.; Smith, R.; Pleasance, E.; Whibley, A.; Edkins, S.; Hardy, C.; O’Meara, S.; Latimer, C.; Dicks, E.; Menzies, A.; et al. A systematic, large-scale resequencing screen of X-chromosome coding exons in mental retardation. Nat. Genet. 2009, 41, 535–543. [Google Scholar] [CrossRef]
- Head, E.; Corrada, M.M.; Kahle-Wrobleski, K.; Kim, R.C.; Sarsoza, F.; Goodus, M.; Kawas, C.H. Synaptic proteins, neuropathology and cognitive status in the oldest-old. Neurobiol. Aging 2009, 30, 1125–1134. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.-Y.; He, X.-Y.; Miller, D. HSD17B10: A gene involved in cognitive function through metabolism of isoleucine and neuroactive steroids. Mol. Genet. Metab. 2007, 92, 36–42. [Google Scholar] [CrossRef]
- Brookes, K.J.; Hawi, Z.; Park, J.; Scott, S.; Gill, M.; Kent, L. Polymorphisms of the steroid sulfatase (STS) gene are associated with attention deficit hyperactivity disorder and influence brain tissue mRNA expression. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2010, 153, 1417–1424. [Google Scholar] [CrossRef] [Green Version]
- Reed, M.J.; Purohit, A.; Woo, L.W.L.; Newman, S.P.; Potter, B.V.L. Steroid sulfatase: Molecular biology, regulation, and inhibition. Endocr. Rev. 2005, 26, 171–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.-J.; Chan, W.-C.; Chou, M.-C.; Chou, W.-J.; Lee, M.-J.; Lee, S.-Y.; Lin, P.-Y.; Yang, Y.-H.; Yen, C.-F. Polymorphisms of STS gene and SULT2A1 gene and neurosteroid levels in Han Chinese boys with attention-deficit/hyperactivity disorder: An exploratory investigation. Sci. Rep. 2017, 7, 45595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortaud, S.; Nicolas, L.; Pinoteau, W.; Tordjman, S.; Carlier, M.; Roubertoux, P.L. Brain pathways mediating the pro-aggressive effect of the steroid sulfatase (Sts) gene. Behav. Genet. 2010, 40, 211–219. [Google Scholar] [CrossRef]
- Hawkinson, J.E.; Kimbrough, C.L.; McCauley, L.D.; Bolger, M.B.; Lan, N.C.; Gee, K.W. The neuroactive steroid 3 alpha-hydroxy-5 beta-pregnan-20-one is a two-component modulator of ligand binding to the GABAA receptor. Eur. J. Pharmacol. 1994, 269, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Guarneri, P.; Russo, D.; Cascio, C.; de Leo, G.; Piccoli, T.; Sciuto, V.; Piccoli, F.; Guarneri, R. Pregnenolone sulfate modulates NMDA receptors, inducing and potentiating acute excitotoxicity in isolated retina. J. Neurosci. Res. 1998, 54, 787–797. [Google Scholar] [CrossRef]
- Pastore, S.F.; Ko, S.Y.; Frankland, P.W.; Hamel, P.A.; Vincent, J.B. PTCHD1: Identification and Neurodevelopmental Contributions of an Autism Spectrum Disorder and Intellectual Disability Susceptibility Gene. Genes 2022, 13, 527. [Google Scholar] [CrossRef]
- Wells, M.F.; Wimmer, R.D.; Schmitt, L.I.; Feng, G.; Halassa, M.M. Thalamic reticular impairment underlies attention deficit in Ptchd1(Y/-) mice. Nature 2016, 532, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Noor, A.; Whibley, A.; Marshall, C.R.; Gianakopoulos, P.J.; Piton, A.; Carson, A.R.; Orlic-Milacic, M.; Lionel, A.C.; Sato, D.; Pinto, D.; et al. Disruption at the PTCHD1 Locus on Xp22.11 in Autism spectrum disorder and intellectual disability. Sci. Transl. Med. 2010, 2, 49ra68. [Google Scholar] [CrossRef] [Green Version]
- Ung, D.C.; Iacono, G.; Méziane, H.; Blanchard, E.; Papon, M.-A.; Selten, M.; van Rhijn, J.-R.; Montjean, R.; Rucci, J.; Martin, S.; et al. Ptchd1 deficiency induces excitatory synaptic and cognitive dysfunctions in mouse. Mol. Psychiatry 2018, 23, 1356–1367. [Google Scholar] [CrossRef] [Green Version]
- Fortunato, F.; Farnè, M.; Ferlini, A. The DMD gene and therapeutic approaches to restore dystrophin. Neuromuscul. Disord. 2021, 31, 1013–1020. [Google Scholar] [CrossRef]
- Waite, A.; Tinsley, C.L.; Locke, M.; Blake, D.J. The neurobiology of the dystrophin-associated glycoprotein complex. Ann. Med. 2009, 41, 344–359. [Google Scholar] [CrossRef] [PubMed]
- LaConte, L.E.W.; Chavan, V.; Elias, A.F.; Hudson, C.; Schwanke, C.; Styren, K.; Shoof, J.; Kok, F.; Srivastava, S.; Mukherjee, K. Two microcephaly-associated novel missense mutations in CASK specifically disrupt the CASK-neurexin interaction. Hum. Genet. 2018, 137, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.A.; Liang, C.; Arora, A.; Vijayan, S.; Ahuja, S.; Wagley, P.K.; Settlage, R.; LaConte, L.E.W.; Goodkin, H.P.; Lazar, I.; et al. Haploinsufficiency of X-linked intellectual disability gene CASK induces post-transcriptional changes in synaptic and cellular metabolic pathways. Exp. Neurol. 2020, 329, 113319. [Google Scholar] [CrossRef] [PubMed]
- Cerase, A.; Pintacuda, G.; Tattermusch, A.; Avner, P. Xist localization and function: New insights from multiple levels. Genome Biol. 2015, 16, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, T.T.; Weston, M.K.; Wilson, M.J. Selection and evaluation of reference genes for analysis of mouse (Mus musculus) sex-dimorphic brain development. PeerJ 2017, 5, e2909. [Google Scholar] [CrossRef] [Green Version]
- Berletch, J.B.; Ma, W.; Yang, F.; Shendure, J.; Noble, W.S.; Disteche, C.M.; Deng, X. Escape from X inactivation varies in mouse tissues. PLoS Genet. 2015, 11, e1005079. [Google Scholar] [CrossRef]
- Lee, J.; Pinares-Garcia, P.; Loke, H.; Ham, S.; Vilain, E.; Harley, V.R. Sex-specific neuroprotection by inhibition of the Y-chromosome gene, SRY, in experimental Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2019, 116, 16577–16582. [Google Scholar] [CrossRef] [Green Version]
- Mayer, A.; Mosler, G.; Just, W.; Pilgrim, C.; Reisert, I. Developmental profile of Sry transcripts in mouse brain. Neurogenetics 2000, 3, 25–30. [Google Scholar] [CrossRef]
- Rosenfeld, C.S. Brain Sexual Differentiation and Requirement of SRY: Why or Why Not? Front. Neurosci. 2017, 11, 632. [Google Scholar] [CrossRef] [Green Version]
- Dewing, P.; Chiang, C.W.K.; Sinchak, K.; Sim, H.; Fernagut, P.-O.; Kelly, S.; Chesselet, M.-F.; Micevych, P.E.; Albrecht, K.H.; Harley, V.R.; et al. Direct regulation of adult brain function by the male-specific factor SRY. Curr. Biol. 2006, 16, 415–420. [Google Scholar] [CrossRef] [Green Version]
- Warburton, A.L.; Santer, R.M. Localisation of NADPH-diaphorase and acetylcholinesterase activities and of tyrosine hydroxylase and neuropeptide-Y immunoreactivity in neurons of the hypogastric ganglion of young adult and aged rats. J. Auton. Nerv. Syst. 1993, 45, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Reisert, I.; Pilgrim, C. Sexual differentiation of monoaminergic neurons--genetic or epigenetic? Trends Neurosci. 1991, 14, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Earls, L.R.; Westmoreland, J.J.; Zakharenko, S.S. Non-coding RNA regulation of synaptic plasticity and memory: Implications for aging. Ageing Res. Rev. 2014, 17, 34–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Presutti, C.; Rosati, J.; Vincenti, S.; Nasi, S. Non coding RNA and brain. BMC Neurosci. 2006, 7, S5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Li, N.; Li, C.; Zhang, Z.; Teng, H.; Wang, Y.; Zhao, T.; Shi, L.; Zhang, K.; Xia, K.; et al. Genetic evidence of gender difference in autism spectrum disorder supports the female-protective effect. Transl. Psychiatry 2020, 10, 4. [Google Scholar] [CrossRef] [Green Version]
- Davis, E.J.; Lobach, I.; Dubal, D.B. Female XX sex chromosomes increase survival and extend lifespan in aging mice. Aging Cell 2019, 18, e12871. [Google Scholar] [CrossRef] [Green Version]
- Davis, E.J.; Broestl, L.; Abdulai-Saiku, S.; Worden, K.; Bonham, L.W.; Miñones-Moyano, E.; Moreno, A.J.; Wang, D.; Chang, K.; Williams, G.; et al. A second X chromosome contributes to resilience in a mouse model of Alzheimer’s disease. Sci. Transl. Med. 2020, 12, eaaz5677. [Google Scholar] [CrossRef]
- Glaab, E.; Antony, P.; Köglsberger, S.; Forster, J.I.; Cordero-Maldonado, M.L.; Crawford, A.; Garcia, P.; Buttini, M. Transcriptome profiling data reveals ubiquitin-specific peptidase 9 knockdown effects. Data Brief 2019, 25, 104130. [Google Scholar] [CrossRef]
- Xu, J.; Taya, S.; Kaibuchi, K.; Arnold, A.P. Spatially and temporally specific expression in mouse hippocampus of Usp9x, a ubiquitin-specific protease involved in synaptic development. J. Neurosci. Res. 2005, 80, 47–55. [Google Scholar] [CrossRef]
- Kasherman, M.A.; Premarathne, S.; Burne, T.H.J.; Wood, S.A.; Piper, M. The Ubiquitin System: A Regulatory Hub for Intellectual Disability and Autism Spectrum Disorder. Mol. Neurobiol. 2020, 57, 2179–2193. [Google Scholar] [CrossRef]
- Vogt, P.H.; Zimmer, J.; Bender, U.; Strowitzki, T. AZFa candidate gene UTY and its X homologue UTX are expressed in human germ cells. Reprod. Fertil. 2021, 2, 151–160. [Google Scholar] [CrossRef]
- Xu, J.; Deng, X.; Watkins, R.; Disteche, C.M. Sex-specific differences in expression of histone demethylases Utx and Uty in mouse brain and neurons. J. Neurosci. 2008, 28, 4521–4527. [Google Scholar] [CrossRef] [Green Version]
- Nardello, R.; Antona, V.; Mangano, G.D.; Salpietro, V.; Mangano, S.; Fontana, A. A paradigmatic autistic phenotype associated with loss of PCDH11Y and NLGN4Y genes. BMC Med. Genom. 2021, 14, 98. [Google Scholar] [CrossRef]
- Bemben, M.A.; Shipman, S.L.; Nicoll, R.A.; Roche, K.W. The cellular and molecular landscape of neuroligins. Trends Neurosci. 2015, 38, 496–505. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Wu, K.; Pandey, S.; Lehr, A.W.; Li, Y.; Bemben, M.A.; Badger, J.D.; Lauzon, J.L.; Wang, T.; Zaghloul, K.A.; et al. A Cluster of Autism-Associated Variants on X-Linked NLGN4X Functionally Resemble NLGN4Y. Neuron 2020, 106, 759–768.e7. [Google Scholar] [CrossRef]
- Xie, Q.; Li, Z.; Wang, Y.; Zaidi, S.; Baranova, A.; Zhang, F.; Cao, H. Preeclampsia Drives Molecular Networks to Shift Toward Greater Vulnerability to the Development of Autism Spectrum Disorder. Front. Neurol. 2020, 11, 590. [Google Scholar] [CrossRef]
- Venkataramanan, S.; Gadek, M.; Calviello, L.; Wilkins, K.; Floor, S.N. DDX3X and DDX3Y are redundant in protein synthesis. RNA 2021, 27, 1577–1588. [Google Scholar] [CrossRef]
- Sun, A.-G.; Wang, J.; Shan, Y.-Z.; Yu, W.-J.; Li, X.; Cong, C.-H.; Wang, X. Identifying distinct candidate genes for early Parkinson’s disease by analysis of gene expression in whole blood. Neuro Endocrinol. Lett. 2014, 35, 398–404. [Google Scholar]
- Di Stazio, M.; Collesi, C.; Vozzi, D.; Liu, W.; Myers, M.; Morgan, A.; D’Adamo, P.A.; Girotto, G.; Rubinato, E.; Giacca, M.; et al. TBL1Y: A new gene involved in syndromic hearing loss. Eur. J. Hum. Genet. 2019, 27, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Naidoo, M.; Anthony, K. Dystrophin Dp71 and the Neuropathophysiology of Duchenne Muscular Dystrophy. Mol. Neurobiol. 2020, 57, 1748–1767. [Google Scholar] [CrossRef] [Green Version]
- Skare, Ø.; Lie, R.T.; Haaland, Ø.A.; Gjerdevik, M.; Romanowska, J.; Gjessing, H.K.; Jugessur, A. Analysis of Parent-of-Origin Effects on the X Chromosome in Asian and European Orofacial Cleft Triads Identifies Associations with DMD, FGF13, EGFL6, and Additional Loci at Xp22.2. Front. Genet. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Zhang, Y.; Qiao, R.; Kong, X.; Zhong, H.; Wang, X.; Zhu, J.; Li, B. Integrated bioinformatics-based identification of diagnostic markers in Alzheimer disease. Front. Aging Neurosci. 2022, 14, 988143. [Google Scholar] [CrossRef] [PubMed]
- Ozçelik, T.; Lafreniere, R.G.; Archer, B.T.; Johnston, P.A.; Willard, H.F.; Francke, U.; Südhof, T.C. Synaptophysin: Structure of the human gene and assignment to the X chromosome in man and mouse. Am. J. Hum. Genet. 1990, 47, 551–561. [Google Scholar] [PubMed]
- Yang, S.-Y.; Dobkin, C.; He, X.-Y.; Brown, W.T. Transcription start sites and epigenetic analysis of the HSD17B10 proximal promoter. BMC Biochem. 2013, 14, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gubb, S.J.A.; Brcic, L.; Underwood, J.F.G.; Kendall, K.M.; Caseras, X.; Kirov, G.; Davies, W. Medical and neurobehavioural phenotypes in male and female carriers of Xp22.31 duplications in the UK Biobank. Hum. Mol. Genet. 2020, 29, 2872–2881. [Google Scholar] [CrossRef]
- Ross, P.J.; Zhang, W.-B.; Mok, R.S.F.; Zaslavsky, K.; Deneault, E.; D’Abate, L.; Rodrigues, D.C.; Yuen, R.K.C.; Faheem, M.; Mufteev, M.; et al. Synaptic Dysfunction in Human Neurons With Autism-Associated Deletions in PTCHD1-AS. Biol. Psychiatry 2020, 87, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, S.; McMillan, R.; Willis, J.; Clark, H.; Chavan, V.; Liang, C.; Zhang, H.; Hulver, M.; Mukherjee, K. X-linked intellectual disability gene CASK regulates postnatal brain growth in a non-cell autonomous manner. Acta Neuropathol. Commun. 2016, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Patel, P.A.; Hegert, J.V.; Cristian, I.; Kerr, A.; LaConte, L.E.W.; Fox, M.A.; Srivastava, S.; Mukherjee, K. Complete loss of the X-linked gene CASK causes severe cerebellar degeneration. J. Med. Genet. 2022, 59, 1044–1057. [Google Scholar] [CrossRef]
- Catino, G.; Genovese, S.; Di Tommaso, S.; Orlando, V.; Petti, M.T.; de Bernardi, M.L.; Dallapiccola, B.; Novelli, A.; Ulgheri, L.; Piscopo, C.; et al. Reciprocal Xp11.4p11.3 microdeletion/microduplication spanning USP9X, DDX3X, and CASK genes in two patients with syndromic intellectual disability. Am. J. Med. Genet. A 2022, 188, 1836–1847. [Google Scholar] [CrossRef]
- Xu, J.; Taya, S.; Kaibuchi, K.; Arnold, A.P. Sexually dimorphic expression of Usp9x is related to sex chromosome complement in adult mouse brain. Eur. J. Neurosci. 2005, 21, 3017–3022. [Google Scholar] [CrossRef]
- Qureshi, I.A.; Mehler, M.F. Genetic and epigenetic underpinnings of sex differences in the brain and in neurological and psychiatric disease susceptibility. Prog. Brain Res. 2010, 186, 77–95. [Google Scholar] [CrossRef] [Green Version]
- Priddle, T.H.; Crow, T.J. The protocadherin 11X/Y (PCDH11X/Y) gene pair as determinant of cerebral asymmetry in modern Homo sapiens. Ann. N. Y. Acad. Sci. 2013, 1288, 36–47. [Google Scholar] [CrossRef] [Green Version]
- Jamain, S.; Quach, H.; Betancur, C.; Råstam, M.; Colineaux, C.; Gillberg, I.C.; Soderstrom, H.; Giros, B.; Leboyer, M.; Gillberg, C.; et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet. 2003, 34, 27–29. [Google Scholar] [CrossRef] [Green Version]
- Andrés, O.; Kellermann, T.; López-Giráldez, F.; Rozas, J.; Domingo-Roura, X.; Bosch, M. RPS4Y gene family evolution in primates. BMC Evol. Biol. 2008, 8, 142. [Google Scholar] [CrossRef] [Green Version]
- Garand, C.; Guay, D.; Sereduk, C.; Chow, D.; Tsofack, S.P.; Langlois, M.; Perreault, E.; Yin, H.H.; Lebel, M. An integrative approach to identify YB-1-interacting proteins required for cisplatin resistance in MCF7 and MDA-MB-231 breast cancer cells. Cancer Sci. 2011, 102, 1410–1417. [Google Scholar] [CrossRef]
- Austad, S.N.; Fischer, K.E. Sex Differences in Lifespan. Cell Metab. 2016, 23, 1022–1033. [Google Scholar] [CrossRef] [Green Version]
- Castillo-Morales, A.; Monzón-Sandoval, J.; Urrutia, A.O.; Gutiérrez, H. Postmitotic cell longevity-associated genes: A transcriptional signature of postmitotic maintenance in neural tissues. Neurobiol. Aging 2019, 74, 147–160. [Google Scholar] [CrossRef]
- Wruck, W.; Adjaye, J. Meta-analysis of human prefrontal cortex reveals activation of GFAP and decline of synaptic transmission in the aging brain. Acta Neuropathol. Commun. 2020, 8, 26. [Google Scholar] [CrossRef] [Green Version]
- Farias Quipildor, G.E.; Mao, K.; Hu, Z.; Novaj, A.; Cui, M.-H.; Gulinello, M.; Branch, C.A.; Gubbi, S.; Patel, K.; Moellering, D.R.; et al. Central IGF-1 protects against features of cognitive and sensorimotor decline with aging in male mice. Geroscience 2019, 41, 185–208. [Google Scholar] [CrossRef]
- Herrera, M.L.; Basmadjian, O.M.; Falomir Lockhart, E.; Dolcetti, F.J.-C.; Hereñú, C.B.; Bellini, M.J. Novel adenoviral IGF-1 administration modulates the association between depressive symptoms and aging: Does gender matter? Behav. Brain Res. 2019, 372, 112050. [Google Scholar] [CrossRef]
- Nelson, J.F.; Strong, R.; Bokov, A.; Diaz, V.; Ward, W. Probing the relationship between insulin sensitivity and longevity using genetically modified mice. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1332–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.J.; Liu, J.; Chen, E.B.; Wang, J.J.; Cao, L.; Narayan, N.; Fergusson, M.M.; Rovira, I.I.; Allen, M.; Springer, D.A.; et al. Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell Rep. 2013, 4, 913–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, R.A.; Harrison, D.E.; Astle, C.M.; Baur, J.A.; Boyd, A.R.; de Cabo, R.; Fernandez, E.; Flurkey, K.; Javors, M.A.; Nelson, J.F.; et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.E.; Strong, R.; Sharp, Z.D.; Nelson, J.F.; Astle, C.M.; Flurkey, K.; Nadon, N.L.; Wilkinson, J.E.; Frenkel, K.; Carter, C.S.; et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009, 460, 392–395. [Google Scholar] [CrossRef] [Green Version]
- Letra, L.; Santana, I. The Influence of Adipose Tissue on Brain Development, Cognition, and Risk of Neurodegenerative Disorders. Adv. Neurobiol. 2017, 19, 151–161. [Google Scholar] [CrossRef]
- Kacířová, M.; Železná, B.; Blažková, M.; Holubová, M.; Popelová, A.; Kuneš, J.; Šedivá, B.; Maletínská, L. Aging and high-fat diet feeding lead to peripheral insulin resistance and sex-dependent changes in brain of mouse model of tau pathology THY-Tau22. J. Neuroinflamm. 2021, 18, 141. [Google Scholar] [CrossRef]
- Ortiz-Huidobro, R.I.; Larqué, C.; Velasco, M.; Chávez-Maldonado, J.P.; Sabido, J.; Sanchez-Zamora, Y.I.; Hiriart, M. Sexual dimorphism in the molecular mechanisms of insulin resistance during a critical developmental window in Wistar rats. Cell Commun. Signal. 2022, 20, 154. [Google Scholar] [CrossRef]
- Arif, A.; Terenzi, F.; Potdar, A.A.; Jia, J.; Sacks, J.; China, A.; Halawani, D.; Vasu, K.; Li, X.; Brown, J.M.; et al. EPRS is a critical mTORC1-S6K1 effector that influences adiposity in mice. Nature 2017, 542, 357–361. [Google Scholar] [CrossRef] [Green Version]
- Enns, L.C.; Morton, J.F.; Treuting, P.R.; Emond, M.J.; Wolf, N.S.; Dai, D.-F.; McKnight, G.S.; Rabinovitch, P.S.; Ladiges, W.C. Disruption of protein kinase A in mice enhances healthy aging. PLoS ONE 2009, 4, e5963. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Xu, Y.; Yi, C.-X.; Tong, Q.; Cai, D. The hypothalamus for whole-body physiology: From metabolism to aging. Protein Cell 2022, 13, 394–421. [Google Scholar] [CrossRef]
- Garcia-Venzor, A.; Toiber, D. SIRT6 Through the Brain Evolution, Development, and Aging. Front. Aging Neurosci. 2021, 13, 747989. [Google Scholar] [CrossRef]
- Michishita, E.; McCord, R.A.; Berber, E.; Kioi, M.; Padilla-Nash, H.; Damian, M.; Cheung, P.; Kusumoto, R.; Kawahara, T.L.A.; Barrett, J.C.; et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature 2008, 452, 492–496. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.; Gao, Y.; Liu, Q.; Yang, X.; Wu, T.; Huang, C.; Huang, Y.; Zhang, J.; Zhang, Z.; Li, R.; et al. Sirt6 in pro-opiomelanocortin neurons controls energy metabolism by modulating leptin signaling. Mol. Metab. 2020, 37, 100994. [Google Scholar] [CrossRef]
- Niu, J.; Cao, Y.; Ji, Y. Resveratrol, a SIRT1 Activator, Ameliorates MK-801-Induced Cognitive and Motor Impairments in a Neonatal Rat Model of Schizophrenia. Front. Psychiatry 2020, 11, 716. [Google Scholar] [CrossRef]
- Jęśko, H.; Wencel, P.; Strosznajder, R.P.; Strosznajder, J.B. Sirtuins and Their Roles in Brain Aging and Neurodegenerative Disorders. Neurochem. Res. 2017, 42, 876–890. [Google Scholar] [CrossRef] [Green Version]
- Braidy, N.; Poljak, A.; Grant, R.; Jayasena, T.; Mansour, H.; Chan-Ling, T.; Smythe, G.; Sachdev, P.; Guillemin, G.J. Differential expression of sirtuins in the aging rat brain. Front. Cell. Neurosci. 2015, 9, 167. [Google Scholar] [CrossRef] [Green Version]
- Kanfi, Y.; Naiman, S.; Amir, G.; Peshti, V.; Zinman, G.; Nahum, L.; Bar-Joseph, Z.; Cohen, H.Y. The sirtuin SIRT6 regulates lifespan in male mice. Nature 2012, 483, 218–221. [Google Scholar] [CrossRef]
- Van Gastel, J.; Cai, H.; Cong, W.-N.; Chadwick, W.; Daimon, C.; Leysen, H.; Hendrickx, J.O.; de Schepper, R.; Vangenechten, L.; van Turnhout, J.; et al. Multidimensional informatic deconvolution defines gender-specific roles of hypothalamic GIT2 in aging trajectories. Mech. Ageing Dev. 2019, 184, 111150. [Google Scholar] [CrossRef]
- Lu, D.; Cai, H.; Park, S.-S.; Siddiqui, S.; Premont, R.T.; Schmalzigaug, R.; Paramasivam, M.; Seidman, M.; Bodogai, I.; Biragyn, A.; et al. Nuclear GIT2 is an ATM substrate and promotes DNA repair. Mol. Cell. Biol. 2015, 35, 1081–1096. [Google Scholar] [CrossRef] [Green Version]
- Roberts, M.N.; Wallace, M.A.; Tomilov, A.A.; Zhou, Z.; Marcotte, G.R.; Tran, D.; Perez, G.; Gutierrez-Casado, E.; Koike, S.; Knotts, T.A.; et al. A Ketogenic Diet Extends Longevity and Healthspan in Adult Mice. Cell Metab. 2017, 26, 539–546.e5. [Google Scholar] [CrossRef] [Green Version]
- Villa, A.; Della Torre, S.; Maggi, A. Sexual differentiation of microglia. Front. Neuroendocrinol. 2019, 52, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Rahimian, R.; Cordeau, P.; Kriz, J. Brain Response to Injuries: When Microglia Go Sexist. Neuroscience 2019, 405, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.M.; McCarthy, M.M. A Starring Role for Microglia in Brain Sex Differences. Neuroscientist 2015, 21, 306–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouton, P.R.; Long, J.M.; Lei, D.-L.; Howard, V.; Jucker, M.; Calhoun, M.E.; Ingram, D.K. Age and gender effects on microglia and astrocyte numbers in brains of mice. Brain Res. 2002, 956, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Mapplebeck, J.C.S.; Beggs, S.; Salter, M.W. Molecules in pain and sex: A developing story. Mol. Brain 2017, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Kopec, A.M.; Smith, C.J.; Ayre, N.R.; Sweat, S.C.; Bilbo, S.D. Microglial dopamine receptor elimination defines sex-specific nucleus accumbens development and social behavior in adolescent rats. Nat. Commun. 2018, 9, 3769. [Google Scholar] [CrossRef] [Green Version]
- Weinhard, L.; Neniskyte, U.; Vadisiute, A.; Di Bartolomei, G.; Aygün, N.; Riviere, L.; Zonfrillo, F.; Dymecki, S.; Gross, C. Sexual dimorphism of microglia and synapses during mouse postnatal development. Dev. Neurobiol. 2018, 78, 618–626. [Google Scholar] [CrossRef] [Green Version]
- Yanguas-Casás, N.; Crespo-Castrillo, A.; Arevalo, M.-A.; Garcia-Segura, L.M. Aging and sex: Impact on microglia phagocytosis. Aging Cell 2020, 19, e13182. [Google Scholar] [CrossRef]
- Crain, J.M.; Nikodemova, M.; Watters, J.J. Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice. J. Neurosci. Res. 2013, 91, 1143–1151. [Google Scholar] [CrossRef] [Green Version]
- Kerr, N.; Dietrich, D.W.; Bramlett, H.M.; Raval, A.P. Sexually dimorphic microglia and ischemic stroke. CNS Neurosci. Ther. 2019, 25, 1308–1317. [Google Scholar] [CrossRef]
- Dubbelaar, M.L.; Kracht, L.; Eggen, B.J.L.; Boddeke, E.W.G.M. The Kaleidoscope of Microglial Phenotypes. Front. Immunol. 2018, 9, 1753. [Google Scholar] [CrossRef]
- Mangold, C.A.; Wronowski, B.; Du, M.; Masser, D.R.; Hadad, N.; Bixler, G.V.; Brucklacher, R.M.; Ford, M.M.; Sonntag, W.E.; Freeman, W.M. Sexually divergent induction of microglial-associated neuroinflammation with hippocampal aging. J. Neuroinflamm. 2017, 14, 141. [Google Scholar] [CrossRef] [Green Version]
- Yanguas-Casás, N. Physiological sex differences in microglia and their relevance in neurological disorders. Neuroimmunol. Neuroinflamm. 2020, 7, 13–22. [Google Scholar] [CrossRef]
- Villa, A.; Vegeto, E.; Poletti, A.; Maggi, A. Estrogens, Neuroinflammation, and Neurodegeneration. Endocr. Rev. 2016, 37, 372–402. [Google Scholar] [CrossRef] [Green Version]
- Cordeau, P.; Lalancette-Hébert, M.; Weng, Y.C.; Kriz, J. Estrogen receptors alpha mediates postischemic inflammation in chronically estrogen-deprived mice. Neurobiol. Aging 2016, 40, 50–60. [Google Scholar] [CrossRef]
- Brawek, B.; Skok, M.; Garaschuk, O. Changing Functional Signatures of Microglia along the Axis of Brain Aging. Int. J. Mol. Sci. 2021, 22, 1091. [Google Scholar] [CrossRef]
- Olmedillas Del Moral, M.; Fröhlich, N.; Figarella, K.; Mojtahedi, N.; Garaschuk, O. Effect of Caloric Restriction on the in vivo Functional Properties of Aging Microglia. Front. Immunol. 2020, 11, 750. [Google Scholar] [CrossRef]
- Guneykaya, D.; Ivanov, A.; Hernandez, D.P.; Haage, V.; Wojtas, B.; Meyer, N.; Maricos, M.; Jordan, P.; Buonfiglioli, A.; Gielniewski, B.; et al. Transcriptional and Translational Differences of Microglia from Male and Female Brains. Cell Rep. 2018, 24, 2773–2783.e6. [Google Scholar] [CrossRef] [Green Version]
- Sumbria, R.K.; Grigoryan, M.M.; Vasilevko, V.; Paganini-Hill, A.; Kilday, K.; Kim, R.; Cribbs, D.H.; Fisher, M.J. Aging exacerbates development of cerebral microbleeds in a mouse model. J. Neuroinflamm. 2018, 15, 69. [Google Scholar] [CrossRef] [Green Version]
- Hanamsagar, R.; Alter, M.D.; Block, C.S.; Sullivan, H.; Bolton, J.L.; Bilbo, S.D. Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia 2017, 65, 1504–1520. [Google Scholar] [CrossRef]
- Doyle, H.H.; Eidson, L.N.; Sinkiewicz, D.M.; Murphy, A.Z. Sex Differences in Microglia Activity within the Periaqueductal Gray of the Rat: A Potential Mechanism Driving the Dimorphic Effects of Morphine. J. Neurosci. 2017, 37, 3202–3214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Sato, Y.; Falcone-Juengert, J.; Kurisu, K.; Shi, J.; Yenari, M.A. Sexual dimorphism in immune cell responses following stroke. Neurobiol. Dis. 2022, 172, 105836. [Google Scholar] [CrossRef] [PubMed]
- Lauro, C.; Limatola, C. Metabolic Reprograming of Microglia in the Regulation of the Innate Inflammatory Response. Front. Immunol. 2020, 11, 493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mela, V.; Sayd Gaban, A.; O’Neill, E.; Bechet, S.; Walsh, A.; Lynch, M.A. The Modulatory Effects of DMF on Microglia in Aged Mice Are Sex-Specific. Cells 2022, 11, 729. [Google Scholar] [CrossRef] [PubMed]
- Milanova, I.V.; Correa-da-Silva, F.; Kalsbeek, A.; Yi, C.-X. Mapping of Microglial Brain Region, Sex and Age Heterogeneity in Obesity. Int. J. Mol. Sci. 2021, 22, 3141. [Google Scholar] [CrossRef]
- Trautman, M.E.; Richardson, N.E.; Lamming, D.W. Protein restriction and branched-chain amino acid restriction promote geroprotective shifts in metabolism. Aging Cell 2022, 21, e13626. [Google Scholar] [CrossRef]
- Dorfman, M.D.; Krull, J.E.; Douglass, J.D.; Fasnacht, R.; Lara-Lince, F.; Meek, T.H.; Shi, X.; Damian, V.; Nguyen, H.T.; Matsen, M.E.; et al. Sex differences in microglial CX3CR1 signalling determine obesity susceptibility in mice. Nat. Commun. 2017, 8, 14556. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, P.; Shou, Q.; Lu, Y.; Wang, G.; Qiu, J.; Wang, J.; He, L.; Chen, J.; Jiao, J.; Zhang, Y. Arachidonic acid sex-dependently affects obesity through linking gut microbiota-driven inflammation to hypothalamus-adipose-liver axis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2715–2726. [Google Scholar] [CrossRef]
- Sherman, M.; Liu, M.-M.; Birnbaum, S.; Wolf, S.E.; Minei, J.P.; Gatson, J.W. Adult obese mice suffer from chronic secondary brain injury after mild TBI. J. Neuroinflamm. 2016, 13, 171. [Google Scholar] [CrossRef] [Green Version]
- Kodama, L.; Guzman, E.; Etchegaray, J.I.; Li, Y.; Sayed, F.A.; Zhou, L.; Zhou, Y.; Zhan, L.; Le, D.; Udeochu, J.C.; et al. Microglial microRNAs mediate sex-specific responses to tau pathology. Nat. Neurosci. 2020, 23, 167–171. [Google Scholar] [CrossRef]
- Biechele, G.; Franzmeier, N.; Blume, T.; Ewers, M.; Luque, J.M.; Eckenweber, F.; Sacher, C.; Beyer, L.; Ruch-Rubinstein, F.; Lindner, S.; et al. Glial activation is moderated by sex in response to amyloidosis but not to tau pathology in mouse models of neurodegenerative diseases. J. Neuroinflamm. 2020, 17, 374. [Google Scholar] [CrossRef]
- Cao, M.; Li, H.; Zhao, J.; Cui, J.; Hu, G. Identification of age- and gender-associated long noncoding RNAs in the human brain with Alzheimer’s disease. Neurobiol. Aging 2019, 81, 116–126. [Google Scholar] [CrossRef]
- Sala Frigerio, C.; Wolfs, L.; Fattorelli, N.; Thrupp, N.; Voytyuk, I.; Schmidt, I.; Mancuso, R.; Chen, W.-T.; Woodbury, M.E.; Srivastava, G.; et al. The Major Risk Factors for Alzheimer’s Disease: Age, Sex, and Genes Modulate the Microglia Response to Aβ Plaques. Cell Rep. 2019, 27, 1293–1306.e6. [Google Scholar] [CrossRef] [Green Version]
- Laws, K.R.; Irvine, K.; Gale, T.M. Sex differences in Alzheimer’s disease. Curr. Opin. Psychiatry 2018, 31, 133–139. [Google Scholar] [CrossRef]
- Guillot-Sestier, M.-V.; Araiz, A.R.; Mela, V.; Gaban, A.S.; O’Neill, E.; Joshi, L.; Chouchani, E.T.; Mills, E.L.; Lynch, M.A. Microglial metabolism is a pivotal factor in sexual dimorphism in Alzheimer’s disease. Commun. Biol. 2021, 4, 711. [Google Scholar] [CrossRef]
- Coales, I.; Tsartsalis, S.; Fancy, N.; Weinert, M.; Clode, D.; Owen, D.; Matthews, P.M. Alzheimer’s disease-related transcriptional sex differences in myeloid cells. J. Neuroinflamm. 2022, 19, 247. [Google Scholar] [CrossRef]
- Guo, L.; Zhong, M.B.; Zhang, L.; Zhang, B.; Cai, D. Sex Differences in Alzheimer’s Disease: Insights from the Multiomics Landscape. Biol. Psychiatry 2022, 91, 61–71. [Google Scholar] [CrossRef]
- Islam, R.; Rajan, R.; Choudhary, H.; Vrionis, F.; Hanafy, K.A. Gender differences in Alzheimer’s may be associated with TLR4-LYN expression in damage associated microglia and neuronal phagocytosis. J. Cell. Physiol. 2022, 1–11. [Google Scholar] [CrossRef]
- Neman, J. Microglial sex affects Alzheimer’s disease. Sci. Transl. Med. 2020, 12, eaba2905. [Google Scholar] [CrossRef]
- Kang, S.S.; Ebbert, M.T.W.; Baker, K.E.; Cook, C.; Wang, X.; Sens, J.P.; Kocher, J.-P.; Petrucelli, L.; Fryer, J.D. Microglial translational profiling reveals a convergent APOE pathway from aging, amyloid, and tau. J. Exp. Med. 2018, 215, 2235–2245. [Google Scholar] [CrossRef]
- Kiernan, E.A.; Wang, T.; Vanderplow, A.M.; Cherukuri, S.; Cahill, M.E.; Watters, J.J. Neonatal Intermittent Hypoxia Induces Lasting Sex-Specific Augmentation of Rat Microglial Cytokine Expression. Front. Immunol. 2019, 10, 1479. [Google Scholar] [CrossRef]
- Villa, A.; Gelosa, P.; Castiglioni, L.; Cimino, M.; Rizzi, N.; Pepe, G.; Lolli, F.; Marcello, E.; Sironi, L.; Vegeto, E.; et al. Sex-Specific Features of Microglia from Adult Mice. Cell Rep. 2018, 23, 3501–3511. [Google Scholar] [CrossRef] [PubMed]
- Barko, K.; Shelton, M.; Xue, X.; Afriyie-Agyemang, Y.; Puig, S.; Freyberg, Z.; Tseng, G.C.; Logan, R.W.; Seney, M.L. Brain region- and sex-specific transcriptional profiles of microglia. Front. Psychiatry 2022, 13, 945548. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.H.; Fatima, M.; Mondal, A.C. Role of Hypothalamic-Pituitary-Adrenal Axis, Hypothalamic-Pituitary-Gonadal Axis and Insulin Signaling in the Pathophysiology of Alzheimer’s Disease. Neuropsychobiology 2019, 77, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Zárate, S.; Stevnsner, T.; Gredilla, R. Role of Estrogen and Other Sex Hormones in Brain Aging. Neuroprotection and DNA Repair. Front. Aging Neurosci. 2017, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- Giatti, S.; Diviccaro, S.; Serafini, M.M.; Caruso, D.; Garcia-Segura, L.M.; Viviani, B.; Melcangi, R.C. Sex differences in steroid levels and steroidogenesis in the nervous system: Physiopathological role. Front. Neuroendocrinol. 2020, 56, 100804. [Google Scholar] [CrossRef]
- Wise, P.M.; Suzuki, S.; Brown, C.M. Estradiol: A hormone with diverse and contradictory neuroprotective actions. Dialogues Clin. Neurosci. 2009, 11, 297–303. [Google Scholar] [CrossRef]
- Blair, J.A.; McGee, H.; Bhatta, S.; Palm, R.; Casadesus, G. Hypothalamic-pituitary-gonadal axis involvement in learning and memory and Alzheimer’s disease: More than “just” estrogen. Front. Endocrinol. 2015, 6, 45. [Google Scholar] [CrossRef] [Green Version]
- Doran, S.J.; Ritzel, R.M.; Glaser, E.P.; Henry, R.J.; Faden, A.I.; Loane, D.J. Sex Differences in Acute Neuroinflammation after Experimental Traumatic Brain Injury Are Mediated by Infiltrating Myeloid Cells. J. Neurotrauma 2019, 36, 1040–1053. [Google Scholar] [CrossRef]
- Russell, J.K.; Jones, C.K.; Newhouse, P.A. The Role of Estrogen in Brain and Cognitive Aging. Neurotherapeutics 2019, 16, 649–665. [Google Scholar] [CrossRef]
- ThyagaRajan, S.; Hima, L.; Pratap, U.P.; Priyanka, H.P.; Vasantharekha, R. Estrogen-induced neuroimmunomodulation as facilitator of and barrier to reproductive aging in brain and lymphoid organs. J. Chem. Neuroanat. 2019, 95, 6–12. [Google Scholar] [CrossRef]
- Pike, C.J. Sex and the development of Alzheimer’s disease. J. Neurosci. Res. 2017, 95, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Shen, Y.; Li, R. Estrogen synthesis and signaling pathways during aging: From periphery to brain. Trends Mol. Med. 2013, 19, 197–209. [Google Scholar] [CrossRef] [Green Version]
- Gillies, G.E.; Virdee, K.; McArthur, S.; Dalley, J.W. Sex-dependent diversity in ventral tegmental dopaminergic neurons and developmental programing: A molecular, cellular and behavioral analysis. Neuroscience 2014, 282, 69–85. [Google Scholar] [CrossRef] [Green Version]
- Toro, C.A.; Zhang, L.; Cao, J.; Cai, D. Sex differences in Alzheimer’s disease: Understanding the molecular impact. Brain Res. 2019, 1719, 194–207. [Google Scholar] [CrossRef]
- Duong, P.; Tenkorang, M.A.A.; Trieu, J.; McCuiston, C.; Rybalchenko, N.; Cunningham, R.L. Neuroprotective and neurotoxic outcomes of androgens and estrogens in an oxidative stress environment. Biol. Sex Differ. 2020, 11, 12. [Google Scholar] [CrossRef]
- Perez-Laso, C.; Cerdan, S.; Junque, C.; Gómez, Á.; Ortega, E.; Mora, M.; Avendaño, C.; Gómez-Gil, E.; Del Cerro, M.C.R.; Guillamon, A. Effects of Adult Female Rat Androgenization on Brain Morphology and Metabolomic Profile. Cereb. Cortex 2018, 28, 2846–2853. [Google Scholar] [CrossRef] [Green Version]
- Jaeger, E.C.B.; Miller, L.E.; Goins, E.C.; Super, C.E.; Chyr, C.U.; Lower, J.W.; Honican, L.S.; Morrison, D.E.; Ramdev, R.A.; Spritzer, M.D. Testosterone replacement causes dose-dependent improvements in spatial memory among aged male rats. Psychoneuroendocrinology 2020, 113, 104550. [Google Scholar] [CrossRef]
- Fattoretti, P.; Malatesta, M.; Mariotti, R.; Zancanaro, C. Testosterone administration increases synaptic density in the gyrus dentatus of old mice independently of physical exercise. Exp. Gerontol. 2019, 125, 110664. [Google Scholar] [CrossRef]
- Bianchi, V.E.; Locatelli, V.; Rizzi, L. Neurotrophic and Neuroregenerative Effects of GH/IGF1. Int. J. Mol. Sci. 2017, 18, 2441. [Google Scholar] [CrossRef] [Green Version]
- Vitale, G.; Pellegrino, G.; Vollery, M.; Hofland, L.J. ROLE of IGF-1 System in the Modulation of Longevity: Controversies and New Insights From a Centenarians’ Perspective. Front. Endocrinol. 2019, 10, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veldhuis, J.D. Aging and hormones of the hypothalamo-pituitary axis: Gonadotropic axis in men and somatotropic axes in men and women. Ageing Res. Rev. 2008, 7, 189–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bleach, R.; Sherlock, M.; O’Reilly, M.W.; McIlroy, M. Growth Hormone/Insulin Growth Factor Axis in Sex Steroid Associated Disorders and Related Cancers. Front. Cell Dev. Biol. 2021, 9, 630503. [Google Scholar] [CrossRef] [PubMed]
- Gesing, A.; Wiesenborn, D.; Do, A.; Menon, V.; Schneider, A.; Victoria, B.; Stout, M.B.; Kopchick, J.J.; Bartke, A.; Masternak, M.M. A Long-lived Mouse Lacking Both Growth Hormone and Growth Hormone Receptor: A New Animal Model for Aging Studies. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1054–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candeias, E.; Duarte, A.I.; Sebastião, I.; Fernandes, M.A.; Plácido, A.I.; Carvalho, C.; Correia, S.; Santos, R.X.; Seiça, R.; Santos, M.S.; et al. Middle-Aged Diabetic Females and Males Present Distinct Susceptibility to Alzheimer Disease-like Pathology. Mol. Neurobiol. 2017, 54, 6471–6489. [Google Scholar] [CrossRef]
- Genua, M.; Pandini, G.; Sisci, D.; Castoria, G.; Maggiolini, M.; Vigneri, R.; Belfiore, A. Role of cyclic AMP response element-binding protein in insulin-like growth factor-i receptor up-regulation by sex steroids in prostate cancer cells. Cancer Res. 2009, 69, 7270–7277. [Google Scholar] [CrossRef] [Green Version]
- Van Nieuwpoort, I.C.; Vlot, M.C.; Schaap, L.A.; Lips, P.; Drent, M.L. The relationship between serum IGF-1, handgrip strength, physical performance and falls in elderly men and women. Eur. J. Endocrinol. 2018, 179, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Gegenhuber, B.; Wu, M.V.; Bronstein, R.; Tollkuhn, J. Gene regulation by gonadal hormone receptors underlies brain sex differences. Nature 2022, 606, 153–159. [Google Scholar] [CrossRef]
- De Winther, M.P.J.; Palaga, T. Editorial: Epigenetic Regulation of Innate Immunity. Front. Immunol. 2021, 12, 713758. [Google Scholar] [CrossRef]
- Shepherd, R.; Cheung, A.S.; Pang, K.; Saffery, R.; Novakovic, B. Sexual Dimorphism in Innate Immunity: The Role of Sex Hormones and Epigenetics. Front. Immunol. 2020, 11, 604000. [Google Scholar] [CrossRef]
- Migliore, L.; Nicolì, V.; Stoccoro, A. Gender Specific Differences in Disease Susceptibility: The Role of Epigenetics. Biomedicines 2021, 9, 652. [Google Scholar] [CrossRef]
- Taneja, V. Sexual dimorphism, aging and immunity. Vitam. Horm. 2021, 115, 367–399. [Google Scholar] [CrossRef]
- Frick, K.M.; Tuscher, J.J.; Koss, W.A.; Kim, J.; Taxier, L.R. Estrogenic regulation of memory consolidation: A look beyond the hippocampus, ovaries, and females. Physiol. Behav. 2018, 187, 57–66. [Google Scholar] [CrossRef]
- Singh, G.; Stefanelli, G.; Narkaj, K.; Brimble, M.A.; Creighton, S.D.; McLean, T.A.B.; Hall, M.; Mitchnick, K.A.; Zakaria, J.; Phung, T.; et al. Histone macroH2A1 is a stronger regulator of hippocampal transcription and memory than macroH2A2 in mice. Commun. Biol. 2022, 5, 482. [Google Scholar] [CrossRef]
- Ramzan, F.; Baumbach, J.; Monks, A.D.; Zovkic, I.B. Histone H2A.Z is required for androgen receptor-mediated effects on fear memory. Neurobiol. Learn. Mem. 2020, 175, 107311. [Google Scholar] [CrossRef]
- McCarthy, M.; Raval, A.P. The peri-menopause in a woman’s life: A systemic inflammatory phase that enables later neurodegenerative disease. J. Neuroinflamm. 2020, 17, 317. [Google Scholar] [CrossRef]
- Crespo-Castrillo, A.; Arevalo, M.-A. Microglial and Astrocytic Function in Physiological and Pathological Conditions: Estrogenic Modulation. Int. J. Mol. Sci. 2020, 21, 3219. [Google Scholar] [CrossRef]
- Petralla, S.; de Chirico, F.; Miti, A.; Tartagni, O.; Massenzio, F.; Poeta, E.; Virgili, M.; Zuccheri, G.; Monti, B. Epigenetics and Communication Mechanisms in Microglia Activation with a View on Technological Approaches. Biomolecules 2021, 11, 306. [Google Scholar] [CrossRef]
- Sheppard, P.A.S.; Choleris, E.; Galea, L.A.M. Structural plasticity of the hippocampus in response to estrogens in female rodents. Mol. Brain 2019, 12, 22. [Google Scholar] [CrossRef] [Green Version]
- Marrocco, J.; McEwen, B.S. Sex in the brain: Hormones and sex differences. Dialogues Clin. Neurosci. 2016, 18, 373–383. [Google Scholar] [CrossRef]
- Beekman, M.; Dowling, D.K.; Aanen, D.K. The costs of being male: Are there sex-specific effects of uniparental mitochondrial inheritance? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Innocenti, P.; Morrow, E.H.; Dowling, D.K. Experimental evidence supports a sex-specific selective sieve in mitochondrial genome evolution. Science 2011, 332, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Camus, M.F.; Clancy, D.J.; Dowling, D.K. Mitochondria, maternal inheritance, and male aging. Curr. Biol. 2012, 22, 1717–1721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristensen, T.N.; Loeschcke, V.; Tan, Q.; Pertoldi, C.; Mengel-From, J. Sex and age specific reduction in stress resistance and mitochondrial DNA copy number in Drosophila melanogaster. Sci. Rep. 2019, 9, 12305. [Google Scholar] [CrossRef] [Green Version]
- Ventura-Clapier, R.; Moulin, M.; Piquereau, J.; Lemaire, C.; Mericskay, M.; Veksler, V.; Garnier, A. Mitochondria: A central target for sex differences in pathologies. Clin. Sci. 2017, 131, 803–822. [Google Scholar] [CrossRef]
- Hägg, S.; Jylhävä, J. Sex differences in biological aging with a focus on human studies. Elife 2021, 10, e63425. [Google Scholar] [CrossRef]
- Bellissimo, M.P.; Fleischer, C.C.; Reiter, D.A.; Goss, A.M.; Zhou, L.; Smith, M.R.; Kohlmeier, J.; Tirouvanziam, R.; Tran, P.H.; Hao, L.; et al. Sex differences in the relationships between body composition, fat distribution, and mitochondrial energy metabolism: A pilot study. Nutr. Metab. 2022, 19, 37. [Google Scholar] [CrossRef]
- Ratner, M.H.; Kumaresan, V.; Farb, D.H. Neurosteroid Actions in Memory and Neurologic/Neuropsychiatric Disorders. Front. Endocrinol. 2019, 10, 169. [Google Scholar] [CrossRef]
- Porcu, P.; Barron, A.M.; Frye, C.A.; Walf, A.A.; Yang, S.-Y.; He, X.-Y.; Morrow, A.L.; Panzica, G.C.; Melcangi, R.C. Neurosteroidogenesis Today: Novel Targets for Neuroactive Steroid Synthesis and Action and Their Relevance for Translational Research. J. Neuroendocrinol. 2016, 28, 12351. [Google Scholar] [CrossRef] [Green Version]
- Lejri, I.; Grimm, A.; Eckert, A. Mitochondria, Estrogen and Female Brain Aging. Front. Aging Neurosci. 2018, 10, 124. [Google Scholar] [CrossRef] [Green Version]
- Picard, M.; McEwen, B.S. Mitochondria impact brain function and cognition. Proc. Natl. Acad. Sci. USA 2014, 111, 7–8. [Google Scholar] [CrossRef] [Green Version]
- Niveditha, S.; Deepashree, S.; Ramesh, S.R.; Shivanandappa, T. Sex differences in oxidative stress resistance in relation to longevity in Drosophila melanogaster. J. Comp. Physiol. B 2017, 187, 899–909. [Google Scholar] [CrossRef]
- Fang, E.F.; Scheibye-Knudsen, M.; Chua, K.F.; Mattson, M.P.; Croteau, D.L.; Bohr, V.A. Nuclear DNA damage signalling to mitochondria in ageing. Nat. Rev. Mol. Cell Biol. 2016, 17, 308–321. [Google Scholar] [CrossRef] [Green Version]
- Razmara, A.; Sunday, L.; Stirone, C.; Wang, X.B.; Krause, D.N.; Duckles, S.P.; Procaccio, V. Mitochondrial effects of estrogen are mediated by estrogen receptor alpha in brain endothelial cells. J. Pharmacol. Exp. Ther. 2008, 325, 782–790. [Google Scholar] [CrossRef] [Green Version]
- Lejri, I.; Grimm, A.; Miesch, M.; Geoffroy, P.; Eckert, A.; Mensah-Nyagan, A.-G. Allopregnanolone and its analog BR 297 rescue neuronal cells from oxidative stress-induced death through bioenergetic improvement. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 631–642. [Google Scholar] [CrossRef]
- Gaignard, P.; Liere, P.; Thérond, P.; Schumacher, M.; Slama, A.; Guennoun, R. Role of Sex Hormones on Brain Mitochondrial Function, with Special Reference to Aging and Neurodegenerative Diseases. Front. Aging Neurosci. 2017, 9, 406. [Google Scholar] [CrossRef]
- Grimm, A.; Eckert, A. Brain aging and neurodegeneration: From a mitochondrial point of view. J. Neurochem. 2017, 143, 418–431. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Zhan, M.; Duan, W.; Prabhu, V.; Brenneman, R.; Wood, W.; Firman, J.; Li, H.; Zhang, P.; Ibe, C.; et al. Gene expression atlas of the mouse central nervous system: Impact and interactions of age, energy intake and gender. Genome Biol. 2007, 8, R234. [Google Scholar] [CrossRef] [Green Version]
- Berchtold, N.C.; Cribbs, D.H.; Coleman, P.D.; Rogers, J.; Head, E.; Kim, R.; Beach, T.; Miller, C.; Troncoso, J.; Trojanowski, J.Q.; et al. Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc. Natl. Acad. Sci. USA 2008, 105, 15605–15610. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Brinton, R.D. Triad of Risk for Late Onset Alzheimer’s: Mitochondrial Haplotype, APOE Genotype and Chromosomal Sex. Front. Aging Neurosci. 2016, 8, 232. [Google Scholar] [CrossRef] [Green Version]
- Chinnery, P.F.; Gomez-Duran, A. Oldies but Goldies mtDNA Population Variants and Neurodegenerative Diseases. Front. Neurosci. 2018, 12, 682. [Google Scholar] [CrossRef] [Green Version]
- Gallart-Palau, X.; Lee, B.S.T.; Adav, S.S.; Qian, J.; Serra, A.; Park, J.E.; Lai, M.K.P.; Chen, C.P.; Kalaria, R.N.; Sze, S.K. Gender differences in white matter pathology and mitochondrial dysfunction in Alzheimer’s disease with cerebrovascular disease. Mol. Brain 2016, 9, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotz, C.; Lin, A.J.; Black, C.M.; Zhang, J.; Lau, E.; Deng, N.; Wang, Y.; Zong, N.C.; Choi, J.H.; Xu, T.; et al. Characterization, design, and function of the mitochondrial proteome: From organs to organisms. J. Proteome Res. 2014, 13, 433–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, J.N.; Pichaud, N.; Camus, M.F.; Côté, G.; Blier, P.U.; Dowling, D.K. Evolutionary implications of mitochondrial genetic variation: Mitochondrial genetic effects on OXPHOS respiration and mitochondrial quantity change with age and sex in fruit flies. J. Evol. Biol. 2016, 29, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, H.; Hu, Q.; Wang, L.; Liu, J.; Zheng, Z.; Zhang, W.; Ren, J.; Zhu, F.; Liu, G.-H. Epigenetic regulation of aging: Implications for interventions of aging and diseases. Signal Transduct. Target. Ther. 2022, 7, 374. [Google Scholar] [CrossRef]
- Molina-Serrano, D.; Kyriakou, D.; Kirmizis, A. Histone Modifications as an Intersection Between Diet and Longevity. Front. Genet. 2019, 10, 192. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, Q.; Xie, L. Histone Modifications in Aging: The Underlying Mechanisms and Implications. Curr. Stem Cell Res. Ther. 2018, 13, 125–135. [Google Scholar] [CrossRef]
- Eckschlager, T.; Plch, J.; Stiborova, M.; Hrabeta, J. Histone Deacetylase Inhibitors as Anticancer Drugs. Int. J. Mol. Sci. 2017, 18, 1414. [Google Scholar] [CrossRef] [Green Version]
- Nativio, R.; Donahue, G.; Berson, A.; Lan, Y.; Amlie-Wolf, A.; Tuzer, F.; Toledo, J.B.; Gosai, S.J.; Gregory, B.D.; Torres, C.; et al. Dysregulation of the epigenetic landscape of normal aging in Alzheimer’s disease. Nat. Neurosci. 2018, 21, 497–505. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, T.M.; Zürcher, N.R.; Catanese, M.C.; Tseng, C.-E.J.; Di Biase, M.A.; Lyall, A.E.; Hightower, B.G.; Parmar, A.J.; Bhanot, A.; Wu, C.J.; et al. Neuroepigenetic signatures of age and sex in the living human brain. Nat. Commun. 2019, 10, 2945. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.; Tyler, J.K. Epigenetics and aging. Sci. Adv. 2016, 2, e1600584. [Google Scholar] [CrossRef] [Green Version]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Selvamani, A.; Williams, M.H.; Miranda, R.C.; Sohrabji, F. Circulating miRNA profiles provide a biomarker for severity of stroke outcomes associated with age and sex in a rat model. Clin. Sci. 2014, 127, 77–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chisholm, N.C.; Henderson, M.L.; Selvamani, A.; Park, M.J.; Dindot, S.; Miranda, R.C.; Sohrabji, F. Histone methylation patterns in astrocytes are influenced by age following ischemia. Epigenetics 2015, 10, 142–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrini, C.; Pirazzini, C.; Sala, C.; Sambati, L.; Yusipov, I.; Kalyakulina, A.; Ravaioli, F.; Kwiatkowska, K.M.; Durso, D.F.; Ivanchenko, M.; et al. A Meta-Analysis of Brain DNA Methylation Across Sex, Age, and Alzheimer’s Disease Points for Accelerated Epigenetic Aging in Neurodegeneration. Front. Aging Neurosci. 2021, 13, 639428. [Google Scholar] [CrossRef] [PubMed]
- Inkster, A.M.; Duarte-Guterman, P.; Albert, A.Y.; Barha, C.K.; Galea, L.A.M.; Robinson, W.P. Are sex differences in cognitive impairment reflected in epigenetic age acceleration metrics? Neurobiol. Aging 2022, 109, 192–194. [Google Scholar] [CrossRef]
- Barter, J.D.; Foster, T.C. Aging in the Brain: New Roles of Epigenetics in Cognitive Decline. Neuroscientist 2018, 24, 516–525. [Google Scholar] [CrossRef]
- Rani, A.; Barter, J.; Kumar, A.; Stortz, J.A.; Hollen, M.; Nacionales, D.; Moldawer, L.L.; Efron, P.A.; Foster, T.C. Influence of age and sex on microRNA response and recovery in the hippocampus following sepsis. Aging 2022, 14, 728–746. [Google Scholar] [CrossRef]
- Becker, J.; Sun, B.; Alammari, F.; Haerty, W.; Vance, K.W.; Szele, F.G. What has single-cell transcriptomics taught us about long non-coding RNAs in the ventricular-subventricular zone? Stem Cell Rep. 2023, 18, 354–376. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Chen, D.; Zhang, B.; Tian, R.-R.; Wu, J.; Zhang, Y.; Xu, K.; Yang, L.-M.; Cheng, C.; et al. Annotation and cluster analysis of spatiotemporal- and sex-related lncRNA expression in rhesus macaque brain. Genome Res. 2017, 27, 1608–1620. [Google Scholar] [CrossRef] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirokova, O.; Zaborskaya, O.; Pchelin, P.; Kozliaeva, E.; Pershin, V.; Mukhina, I. Genetic and Epigenetic Sexual Dimorphism of Brain Cells during Aging. Brain Sci. 2023, 13, 195. https://doi.org/10.3390/brainsci13020195
Shirokova O, Zaborskaya O, Pchelin P, Kozliaeva E, Pershin V, Mukhina I. Genetic and Epigenetic Sexual Dimorphism of Brain Cells during Aging. Brain Sciences. 2023; 13(2):195. https://doi.org/10.3390/brainsci13020195
Chicago/Turabian StyleShirokova, Olesya, Olga Zaborskaya, Pavel Pchelin, Elizaveta Kozliaeva, Vladimir Pershin, and Irina Mukhina. 2023. "Genetic and Epigenetic Sexual Dimorphism of Brain Cells during Aging" Brain Sciences 13, no. 2: 195. https://doi.org/10.3390/brainsci13020195
APA StyleShirokova, O., Zaborskaya, O., Pchelin, P., Kozliaeva, E., Pershin, V., & Mukhina, I. (2023). Genetic and Epigenetic Sexual Dimorphism of Brain Cells during Aging. Brain Sciences, 13(2), 195. https://doi.org/10.3390/brainsci13020195






