The Effects of a New Integrated and Multidisciplinary Cognitive Rehabilitation Program Based on Mindfulness and Reminiscence Therapy in Patients with Parkinson’s Disease and Mild Cognitive Impairment: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Rehabilitation Protocols
2.3. Study Design
2.4. Cognitive and Psychological Assessment
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giovanni, A.D.; Paolo, E.D.; Cuzzoni, G. Depressione in pazienti con Mild Cognitive Impairment, trattamento con SSRI ed esiti. Boll. Della Soc. Med. Chir. Di Pavia 2013, 126, 643–648. [Google Scholar] [CrossRef]
- Petersen, R.C.; Roberts, R.O.; Knopman, D.S.; Geda, Y.E.; Cha, R.H.; Pankratz, V.S.; Boeve, B.F.; Tangalos, E.G.; Ivnik, R.J.; Rocca, W.A. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology 2010, 75, 889–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanford, A.M. Mild Cognitive Impairment. Clin. Geriatr. Med. 2017, 33, 325–337. [Google Scholar] [CrossRef]
- Pandya, S.Y.; Clem, M.A.; Silva, L.M.; Woon, F.L. Does mild cognitive impairment always lead to dementia? A review. J. Neurol. Sci. 2016, 369, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, M.; Dodge, H.H.; Shen, C.; DeKosky, S.T. Mild cognitive impairment, amnestic type: An epidemiologic study. Neurology 2004, 63, 115–121. [Google Scholar] [CrossRef]
- Hänninen, T.; Hallikainen, M.; Tuomainen, S.; Vanhanen, M.; Soininen, H. Prevalence of mild cognitive impairment: A population-based study in elderly subjects. Acta Neurol. Scand. 2002, 106, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C. Mild cognitive impairment is early-stage Alzheimer disease: Time to revise diagnostic criteria. Arch. Neurol. 2006, 63, 15–16. [Google Scholar] [CrossRef]
- Sachdev, P.S.; Blacker, D.; Blazer, D.G.; Ganguli, M.; Jeste, D.V.; Paulsen, J.S.; Petersen, R.C. Classifying neurocognitive disorders: The DSM-5 approach. Nat. Rev. Neurol. 2014, 10, 634–642. [Google Scholar] [CrossRef]
- Beretta, V.S.; Conceição, N.R.; Nóbrega-Sousa, P.; Orcioli-Silva, D.; Dantas, L.K.B.F.; Gobbi, L.T.B.; Vitório, R. Transcranial direct current stimulation combined with physical or cognitive training in people with Parkinson’s disease: A systematic review. J. Neuroeng. Rehabil. 2020, 17, 74. [Google Scholar] [CrossRef]
- Berg, D.; Postuma, R.B.; Bloem, B.; Chan, P.; Dubois, B.; Gasser, T.; Goetz, C.G.; Halliday, G.M.; Hardy, J.; Lang, A.E.; et al. Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson’s disease. Mov. Disord. 2014, 29, 454–462. [Google Scholar] [CrossRef]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aarsland, D.; Marsh, L.; Schrag, A. Neuropsychiatric symptoms in Parkinson’s disease. Mov. Disord. 2009, 24, 2175–2186. [Google Scholar] [CrossRef] [PubMed]
- Ransmayr, G. Cognitive impairment in Parkinson’s disease. Psychiatr. Danub. 2015, 27, 458–461. [Google Scholar] [PubMed]
- Reijnders, J.S.A.M.; Ehrt, U.; Weber, W.E.J.; Aarsland, D.; Leentjens, A.F.G. A systematic review of prevalence studies of depression in Parkinson’s disease. Mov. Disord. 2008, 23, 183–189, quiz 313. [Google Scholar] [CrossRef] [Green Version]
- Aarsland, D.; Brønnick, K.; Fladby, T. Mild cognitive impairment in Parkinson’s disease. Curr. Neurol. Neurosci. Rep. 2011, 11, 371–378. [Google Scholar] [CrossRef]
- Monastero, R.; Cicero, C.E.; Baschi, R.; Davì, M.; Luca, A.; Restivo, V.; Zangara, C.; Fierro, B.; Zappia, M.; Nicoletti, A. Mild cognitive impairment in Parkinson’s disease: The Parkinson’s disease cognitive study (PACOS). J. Neurol. 2018, 265, 1050–1058. [Google Scholar] [CrossRef]
- Balestrino, R.; Schapira, A.H.V. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef]
- Guidetti, M.; Bertini, A.; Pirone, F.; Sala, G.; Signorelli, P.; Ferrarese, C.; Priori, A.; Bocci, T. Neuroprotection and Non-Invasive Brain Stimulation: Facts or Fiction? Int. J. Mol. Sci. 2022, 23, 13775. [Google Scholar] [CrossRef]
- Díez-Cirarda, M.; Ibarretxe-Bilbao, N.; Peña, J.; Ojeda, N. Efficacy of cognitive rehabilitation in Parkinson’s disease. Neural. Regen. Res. 2018, 13, 226–227. [Google Scholar] [CrossRef]
- Huckans, M.; Hutson, L.; Twamley, E.; Jak, A.; Kaye, J.; Storzbach, D. Efficacy of cognitive rehabilitation therapies for mild cognitive impairment (MCI) in older adults: Working toward a theoretical model and evidence-based interventions. Neuropsychol. Rev. 2013, 23, 63–80. [Google Scholar] [CrossRef]
- Cash, T.V.; Ekouevi, V.S.; Kilbourn, C.; Lageman, S.K. Pilot study of a mindfulness-based group intervention for individuals with Parkinson’s disease and their caregivers. Mindfulness 2016, 7, 361–371. [Google Scholar] [CrossRef]
- Larouche, E.; Hudon, C.; Goulet, S. Potential benefits of mindfulness-based interventions in mild cognitive impairment and Alzheimer’s disease: An interdisciplinary perspective. Behav. Brain Res. 2015, 276, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Pickut, B.A.; Van Hecke, W.; Kerckhofs, E.; Mariën, P.; Vanneste, S.; Cras, P.; Parizel, P.M. Mindfulness based intervention in Parkinson’s disease leads to structural brain changes on MRI: A randomized controlled longitudinal trial. Clin. Neurol. Neurosurg. 2013, 115, 2419–2425. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Rawtaer, I.; Feng, L.; Fam, J.; Kumar, A.P.; Kee-Mun Cheah, I.; Honer, W.G.; Su, W.; Lee, Y.K.; Tan, E.C.; et al. Mindfulness intervention for mild cognitive impairment led to attention-related improvements and neuroplastic changes: Results from a 9-month randomized control trial. J. Psychiatr. Res. 2021, 135, 203–211. [Google Scholar] [CrossRef]
- Gotink, R.A.; Meijboom, R.; Vernooij, M.W.; Smits, M.; Hunink, M.G.M. 8-week Mindfulness Based Stress Reduction induces brain changes similar to traditional long-term meditation practice—A systematic review. Brain Cogn. 2016, 108, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Bishop, S.R.; Lau, M.; Shapiro, S.; Carlson, L.; Anderson, N.D.; Carmody, J.; Segal, Z.V.; Abbey, S.; Speca, M.; Velting, D.; et al. Mindfulness: A proposed operational definition. Clin. Psychol. Sci. Pract. 2004, 11, 230–241. [Google Scholar] [CrossRef]
- Brown, K.W.; Ryan, R.M. The benefits of being present: Mindfulness and its role in psychological well-being. J. Personal. Soc. Psychol. 2003, 84, 822–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabat-Zinn, J. Mindfulness-based interventions in context: Past, present, and future. Clin. Psychol. Sci. Pract. 2003, 10, 144–156. [Google Scholar] [CrossRef]
- Kabat-Zinn, J.; Lipworth, L.; Burney, R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. J. Behav. Med. 1985, 8, 163–190. [Google Scholar] [CrossRef]
- Montero-Marin, J.; Garcia-Campayo, J.; Pérez-Yus, M.C.; Zabaleta-Del-Olmo, E.; Cuijpers, P. Meditation techniques v. relaxation therapies when treating anxiety: A meta-analytic review. Psychol. Med. 2019, 49, 2118–2133. [Google Scholar] [CrossRef]
- Khusid, M.A.; Vythilingam, M. The Emerging Role of Mindfulness Meditation as Effective Self-Management Strategy, Part 1: Clinical Implications for Depression, Post-Traumatic Stress Disorder, and Anxiety. Mil. Med. 2016, 181, 961–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juul, L.; Brorsen, E.; Gøtzsche, K.; Nielsen, B.L.; Fjorback, L.O. The Effects of a Mindfulness Program on Mental Health in Students at an Undergraduate Program for Teacher Education: A Randomized Controlled Trial in Real-Life. Front. Psychol. 2021, 12, 722771. [Google Scholar] [CrossRef] [PubMed]
- Krasner, M.S.; Epstein, R.M.; Beckman, H.; Suchman, A.L.; Chapman, B.; Mooney, C.J.; Quill, T.E. Association of an educational program in mindful communication with burnout, empathy, and attitudes among primary care physicians. JAMA 2009, 302, 1284–1293. [Google Scholar] [CrossRef] [Green Version]
- Hölzel, B.K.; Carmody, J.; Vangel, M.; Congleton, C.; Yerramsetti, S.M.; Gard, T.; Lazar, S.W. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res. Neuroimaging 2011, 191, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Depping, M.S.; Schmitgen, M.M.; Kubera, K.M.; Wolf, R.C. Cerebellar Contributions to Major Depression. Front. Psychiatry 2018, 9, 634. [Google Scholar] [CrossRef] [Green Version]
- D’Urso, G.; Dini, M.; Bonato, M.; Gallucci, S.; Parazzini, M.; Maiorana, N.; Bortolomasi, M.; Priori, A.; Ferrucci, R. Simultaneous Bilateral Frontal and Bilateral Cerebellar Transcranial Direct Current Stimulation in Treatment-Resistant Depression—Clinical Effects and Electrical Field Modelling of a Novel Electrodes Montage. Biomedicines 2022, 10, 1681. [Google Scholar] [CrossRef]
- Manto, M.; Argyropoulos, G.P.D.; Bocci, T.; Celnik, P.A.; Corben, L.A.; Guidetti, M.; Koch, G.; Priori, A.; Rothwell, J.C.; Sadnicka, A.; et al. Consensus Paper: Novel Directions and Next Steps of Non-invasive Brain Stimulation of the Cerebellum in Health and Disease. Cerebellum 2021, 21, 1092–1122. [Google Scholar] [CrossRef] [PubMed]
- Luders, E.; Toga, A.W.; Lepore, N.; Gaser, C. The underlying anatomical correlates of long-term meditation: Larger hippocampal and frontal volumes of gray matter. NeuroImage 2009, 45, 672–678. [Google Scholar] [CrossRef] [Green Version]
- Opialla, S.; Lutz, J.; Scherpiet, S.; Hittmeyer, A.; Jäncke, L.; Rufer, M.; Grosse Holtforth, M.; Herwig, U.; Brühl, A.B. Neural circuits of emotion regulation: A comparison of mindfulness-based and cognitive reappraisal strategies. Eur. Arch. Psychiatry Clin. Neurosci. 2015, 265, 45–55. [Google Scholar] [CrossRef]
- Gard, T.; Taquet, M.; Dixit, R.; Hölzel, B.K.; de Montjoye, Y.-A.; Brach, N.; Salat, D.H.; Dickerson, B.C.; Gray, J.R.; Lazar, S.W. Fluid intelligence and brain functional organization in aging yoga and meditation practitioners. Front. Aging Neurosci. 2014, 6, 76. [Google Scholar] [CrossRef]
- Kong, F.; Wang, X.; Song, Y.; Liu, J. Brain regions involved in dispositional mindfulness during resting state and their relation with well-being. Soc. Neurosci. 2016, 11, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Taren, A.A.; Creswell, J.D.; Gianaros, P.J. Dispositional mindfulness co-varies with smaller amygdala and caudate volumes in community adults. PLoS ONE 2013, 8, e64574. [Google Scholar] [CrossRef] [PubMed]
- Smart, C.M.; Segalowitz, S.J.; Mulligan, B.P.; Koudys, J.; Gawryluk, J.R. Mindfulness Training for Older Adults with Subjective Cognitive Decline: Results from a Pilot Randomized Controlled Trial. J. Alzheimers Dis. 2016, 52, 757–774. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.E.; Yeh, G.Y.; Kerr, C.E.; Wolkin, J.; Davis, R.B.; Tan, Y.; Spaeth, R.; Wall, R.B.; Walsh, J.; Kaptchuk, T.J.; et al. Meditation’s impact on default mode network and hippocampus in mild cognitive impairment: A pilot study. Neurosci. Lett. 2013, 556, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Subramaniam, P.; Woods, B.; Whitaker, C. Life review and life story books for people with mild to moderate dementia: A randomised controlled trial. Aging Ment. Health 2014, 18, 363–375. [Google Scholar] [CrossRef]
- Borrini, G.; Dall’Ora, P.; Della Sala, S.; Marinelli, L.; Spinnler, H. Autobiographical memory: Sensitivity to age and education of a standardized enquiry. Psychol. Med. 1989, 19, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Ghidoni, E.; Poletti, M.; Bondavalli, M. An Italian standardized test for autobiographical memory. Arch. Di Psicol. Neurol. E Psichiatr. 1995, 56, 428–443. [Google Scholar]
- Butler, R.N. The life review: An interpretation of reminiscence in the aged. Psychiatry 1963, 26, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.G. Reminiscence as Continuity: Comparison of Young and Older Adults. J. Clin. Geropsychology 1999, 5, 147–157. [Google Scholar] [CrossRef]
- Woods, B.; O’Philbin, L.; Farrell, E.M.; Spector, A.E.; Orrell, M. Reminiscence therapy for dementia. Cochrane Database Syst. Rev. 2018, 3. [Google Scholar] [CrossRef] [Green Version]
- Cammisuli, D.M.; Cipriani, G.; Giusti, E.M.; Castelnuovo, G. Effects of Reminiscence Therapy on Cognition, Depression and Quality of Life in Elderly People with Alzheimer’s Disease: A Systematic Review of Randomized Controlled Trials. J. Clin. Med. 2022, 11, 5752. [Google Scholar] [CrossRef]
- Macleod, F.; Storey, L.; Rushe, T.; McLaughlin, K. Towards an increased understanding of reminiscence therapy for people with dementia: A narrative analysis. Dementia 2021, 20, 1375–1407. [Google Scholar] [CrossRef]
- Pirani, A.; Nasreddine, Z.; Neviani, F.; Fabbo, A.; Rocchi, M.B.; Bertolotti, M.; Tulipani, C.; Galassi, M.; Belvederi Murri, M.; Neri, M. MoCA 7.1: Multicenter Validation of the First Italian Version of Montreal Cognitive Assessment. J. Alzheimers Dis. Rep. 2022, 6, 509–520. [Google Scholar] [CrossRef]
- Santangelo, G.; Siciliano, M.; Pedone, R.; Vitale, C.; Falco, F.; Bisogno, R.; Siano, P.; Barone, P.; Grossi, D.; Santangelo, F.; et al. Normative data for the Montreal Cognitive Assessment in an Italian population sample. Neurol. Sci. 2015, 36, 585–591. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.T.M.; Szewczyk-Królikowski, K.; Tomlinson, P.; Nithi, K.; Rolinski, M.; Murray, C.; Talbot, K.; Ebmeier, K.P.; Mackay, C.E.; Ben-Shlomo, Y. Predictors of cognitive impairment in an early stage Parkinson’s disease cohort. Mov. Disord. 2014, 29, 351–359. [Google Scholar] [CrossRef] [Green Version]
- Scally, E. The Relationship between Performance on Cognitive Screening Tests and Everyday Functioning in Older Adults with Mild Cognitive Impairment. Master’s Thesis, Royal College of Surgeons in Ireland, Dublin, Ireland, 2016. [Google Scholar]
- Sica, C.; Ghisi, M. The Italian versions of the Beck Anxiety Inventory and the Beck Depression Inventory-II: Psychometric properties and discriminant power. In Leading-Edge Psychological Tests and Testing Research; Nova Science Publishers: Hauppauge, NY, USA, 2007; pp. 27–50. ISBN 978-1-60021-571-1. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Ball, R.; Ranieri, W.F. Comparison of Beck Depression Inventories-IA and-II in Psychiatric Outpatients. J. Personal. Assess. 1996, 67, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Montano, A.; Flebus, G.B. Presentation of the Beck Depression Inventory—Second edition (BDI-II): Confirmation of bifactorial structure in a sample of the Italian population. Psicoter. Cogn. Comport. 2006, 12, 67–82. [Google Scholar]
- Carvalho, J.O.; Tan, J.E.; Springate, B.A.; Davis, J.D. Self-reported depressive syndromes in mild cognitive impairment and mild Alzheimer’s disease. Int. Psychogeriatr. 2013, 25, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Stohlman, S.L.; Barrett, M.J.; Sperling, S.A. Factor structure of the BDI-II in Parkinson’s disease. Neuropsychology 2021, 35, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.J.; Kabat-Zinn, J.; Schumacher, J.; Rosenkranz, M.; Muller, D.; Santorelli, S.F.; Urbanowski, F.; Harrington, A.; Bonus, K.; Sheridan, J.F. Alterations in brain and immune function produced by mindfulness meditation. Psychosom. Med. 2003, 65, 564–570. [Google Scholar] [CrossRef]
- Luders, E.; Kurth, F.; Mayer, E.A.; Toga, A.W.; Narr, K.L.; Gaser, C. The unique brain anatomy of meditation practitioners: Alterations in cortical gyrification. Front. Hum. Neurosci. 2012, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Fam, J.; Sun, Y.; Qi, P.; Lau, R.C.; Feng, L.; Kua, E.H.; Mahendran, R. Mindfulness practice alters brain connectivity in community-living elders with mild cognitive impairment. Psychiatry Clin. Neurosci. 2020, 74, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Soler, J.; Elices, M.; Pascual, J.C.; Martín-Blanco, A.; Feliu-Soler, A.; Carmona, C.; Portella, M.J. Effects of mindfulness training on different components of impulsivity in borderline personality disorder: Results from a pilot randomized study. Bord. Pers. Disord. Emot. Dysregul. 2016, 3, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, B.; Bjuhr, H.; Rönnbäck, L. Mindfulness-based stress reduction (MBSR) improves long-term mental fatigue after stroke or traumatic brain injury. Brain Inj. 2012, 26, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Chambers, R.; Lo, B.C.Y.; Allen, N.B. The Impact of Intensive Mindfulness Training on Attentional Control, Cognitive Style, and Affect. Cogn. Res. 2008, 32, 303–322. [Google Scholar] [CrossRef]
- Mrazek, M.D.; Franklin, M.S.; Phillips, D.T.; Baird, B.; Schooler, J.W. Mindfulness training improves working memory capacity and GRE performance while reducing mind wandering. Psychol. Sci. 2013, 24, 776–781. [Google Scholar] [CrossRef] [Green Version]
- Ruocco, A.C.; Wonders, E. Delineating the contributions of sustained attention and working memory to individual differences in mindfulness. Personal. Individ. Differ. 2013, 54, 226–230. [Google Scholar] [CrossRef]
- Quan, P.; Wang, W.; Chu, C.; Hou, L. Seven days of mindfulness-based cognitive therapy improves attention and coping style. Soc. Behav. Personal. 2018, 46, 421–430. [Google Scholar] [CrossRef]
- Becerra, R.; Dandrade, C.; Harms, C. Can Specific Attentional Skills be Modified with Mindfulness Training for Novice Practitioners? Curr. Psychol. 2017, 36, 657–664. [Google Scholar] [CrossRef]
- Isbel, B.D.; Lagopoulos, J.; Hermens, D.F.; Summers, M.J. Mental training affects electrophysiological markers of attention resource allocation in healthy older adults. Neurosci. Lett. 2019, 698, 186–191. [Google Scholar] [CrossRef]
- Allen, M.; Dietz, M.; Blair, K.S.; van Beek, M.; Rees, G.; Vestergaard-Poulsen, P.; Lutz, A.; Roepstorff, A. Cognitive-affective neural plasticity following active-controlled mindfulness intervention. J. Neurosci. 2012, 32, 15601–15610. [Google Scholar] [CrossRef] [Green Version]
- Janssen, S.M.J.; Kristo, G.; Rouw, R.; Murre, J.M.J. The relation between verbal and visuospatial memory and autobiographical memory. Conscious Cogn. 2015, 31, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.C. A Basic-Systems Approach to Autobiographical Memory. Curr. Dir. Psychol. Sci. 2005, 14, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Rubin, D.C. The Basic-Systems Model of Episodic Memory. Perspect. Psychol. Sci. 2006, 1, 277–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boccia, M.; Piccardi, L.; Guariglia, P. The Meditative Mind: A Comprehensive Meta-Analysis of MRI Studies. BioMed. Res. Int. 2015, 2015, e419808. [Google Scholar] [CrossRef] [Green Version]
- Fox, K.C.R.; Nijeboer, S.; Dixon, M.L.; Floman, J.L.; Ellamil, M.; Rumak, S.P.; Sedlmeier, P.; Christoff, K. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci. Biobehav. Rev. 2014, 43, 48–73. [Google Scholar] [CrossRef]
- Tomasino, B.; Fregona, S.; Skrap, M.; Fabbro, F. Meditation-related activations are modulated by the practices needed to obtain it and by the expertise: An ALE meta-analysis study. Front. Hum. Neurosci. 2013, 6, 346. [Google Scholar] [CrossRef] [Green Version]
- BÉdard, M.; Felteau, M.; Mazmanian, D.; Fedyk, K.; Klein, R.; Richardson, J.; Parkinson, W.; Minthorn-Biggs, M.-B. Pilot evaluation of a mindfulness-based intervention to improve quality of life among individuals who sustained traumatic brain injuries. Disabil. Rehabil. 2003, 25, 722–731. [Google Scholar] [CrossRef]
- Pickut, B.; Vanneste, S.; Hirsch, M.A.; Van Hecke, W.; Kerckhofs, E.; Mariën, P.; Parizel, P.M.; Crosiers, D.; Cras, P. Mindfulness Training among Individuals with Parkinson’s Disease: Neurobehavioral Effects. Park. Dis. 2015, 2015, e816404. [Google Scholar] [CrossRef]
- Raes, F.; Williams, J.M.G. The Relationship between Mindfulness and Uncontrollability of Ruminative Thinking. Mindfulness 2010, 1, 199–203. [Google Scholar] [CrossRef]
- Barnes, S.M.; Lynn, S.J. Mindfulness Skills and Depressive Symptoms: A Longitudinal Study. Imagin. Cogn. Personal. 2010, 30, 77–91. [Google Scholar] [CrossRef]
- Martín-Asuero, A.; García-Banda, G. The Mindfulness-Based Stress Reduction Program (MBSR) Reduces Stress-Related Psychological Distress in Healthcare Professionals. Span. J. Psychol. 2010, 13, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Eisendrath, S.J.; Delucchi, K.; Bitner, R.; Fenimore, P.; Smit, M.; McLane, M. Mindfulness-Based Cognitive Therapy for Treatment-Resistant Depression: A Pilot Study. PPS 2008, 77, 319–320. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, A.; Serretti, A. Mindfulness-Based Interventions for Chronic Pain: A Systematic Review of the Evidence. Available online: https://www.liebertpub.com/doi/10.1089/acm.2009.0546 (accessed on 8 December 2022).
- Zhang, M.-F.; Wen, Y.-S.; Liu, W.-Y.; Peng, L.-F.; Wu, X.-D.; Liu, Q.-W. Effectiveness of Mindfulness-based Therapy for Reducing Anxiety and Depression in Patients With Cancer: A Meta-analysis. Medicine 2015, 94, e0897-0. [Google Scholar] [CrossRef]
- Hearn, J.H.; Finlay, K.A. Internet-delivered mindfulness for people with depression and chronic pain following spinal cord injury: A randomized, controlled feasibility trial. Spinal Cord 2018, 56, 750–761. [Google Scholar] [CrossRef] [PubMed]
- van der Heide, A.; Meinders, M.J.; Speckens, A.E.M.; Peerbolte, T.F.; Bloem, B.R.; Helmich, R.C. Stress and Mindfulness in Parkinson’s Disease: Clinical Effects and Potential Underlying Mechanisms. Mov. Disord. 2021, 36, 64–70. [Google Scholar] [CrossRef]
- Kwok, J.Y.Y.; Kwan, J.C.Y.; Auyeung, M.; Mok, V.C.T.; Lau, C.K.Y.; Choi, K.C.; Chan, H.Y.L. Effects of Mindfulness Yoga vs Stretching and Resistance Training Exercises on Anxiety and Depression for People With Parkinson Disease: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 755–763. [Google Scholar] [CrossRef]
- Cai, Z.-Z.; Lin, R.; Wang, X.-X.; Yan, Y.-J.; Li, H. Effects of mindfulness in patients with mild cognitive impairment with insomnia: A double-blind randomized controlled trial. Geriatr. Nurs. 2022, 47, 239–246. [Google Scholar] [CrossRef]
- Marciniak, R.; Šumec, R.; Vyhnálek, M.; Bendíčková, K.; Lázničková, P.; Forte, G.; Jeleník, A.; Římalová, V.; Frič, J.; Hort, J.; et al. The Effect of Mindfulness-Based Stress Reduction (MBSR) on Depression, Cognition, and Immunity in Mild Cognitive Impairment: A Pilot Feasibility Study. Clin. Interv. Aging 2020, 15, 1365–1381. [Google Scholar] [CrossRef]

| Total (n = 33) | Control Group (n = 14) | Experimental Group (n = 19) | |
|---|---|---|---|
| Females—n (%) | 15 (45.4) | 6 (42.9) | 9 (47.4) |
| Age—years (mean ± SD) | 70.5 ± 11 | 72.7 ± 11.5 | 68.8 ± 10.6 |
| Education—years (mean ± SD) | 11.58 ± 3.39 | 10.6 ± 3.2 | 12.31 ± 3.4 |
| Diagnosis MCI—n (%) PD—n (%) | 22 (66.6) 11 (33.3) | 8 (57.1) 6 (42.9) | 14 (73.6) 5 (26.4) |
| Experimental Group | Control Group | |||||||
|---|---|---|---|---|---|---|---|---|
| T0 | T1 | χ2 | p | T0 | T1 | χ2 | p | |
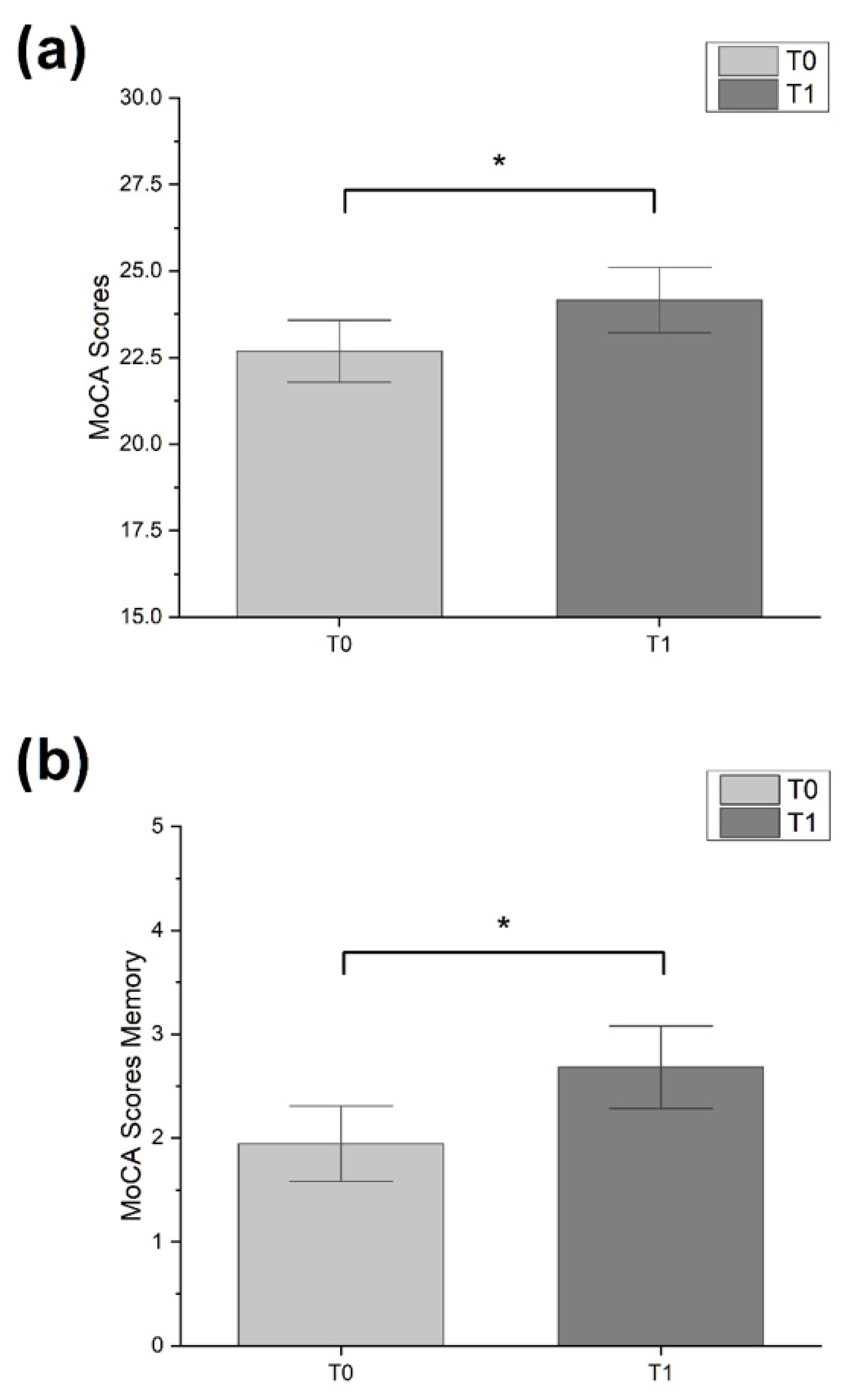
| MoCA | 23.00 ± 6.00 | 26.00 ± 7.00 | 4.000 | 0.046 * | 20.00 ± 5.75 | 18.50 ± 7.00 | 0.091 | >0.05 |
| MoCA—memory | 2.00 ± 3.00 | 2.00 ± 2.00 | 4.571 | 0.033 * | 0.00 ± 1.75 | 0.00 ± 1.75 | 0.333 | >0.05 |
| MoCA—visuospatial ability | 3.00 ± 2.00 | 3.00 ± 2.00 | 0.667 | >0.05 | 2.00 ± 1.00 | 2.50 ± 2.00 | 0.111 | >0.05 |
| MoCA—executive functions | 3.00 ± 2.00 | 3.00 ± 1.00 | 3.600 | >0.05 | 2.50 ± 1.00 | 2.00 ± 1.00 | 0.400 | >0.05 |
| MoCA—attention | 5.00 ± 1.50 | 6.00 ± 1.00 | 0.400 | >0.05 | 5.00 ± 0.75 | 4.00 ± 1.00 | 2.667 | >0.05 |
| MoCA—language | 5.00 ± 2.00 | 5.00 ± 1.00 | 1.286 | >0.05 | 4.50 ± 1.75 | 4.00 ± 1.00 | 0.143 | >0.05 |
| MoCA—orientation | 6.00 ± 1.00 | 6.00 ± 1.00 | 0.000 | >0.05 | 6.00 ± 1.00 | 6.00 ± 1.00 | 0.000 | >0.05 |
| BDI-II | 17.00 ± 16.50 | 13.00 ± 13.50 | 0.000 | >0.05 | 15.00 ± 8.00 | 15.00 ± 10.75 | 0.000 | >0.05 |
| BDI-II, cognitive and affective factors | 9.00 ± 11.50 | 6.00 ± 9.00 | 4.000 | 0.046* | 9.00 ± 4.50 | 8.50 ± 10.75 | 2.273 | >0.05 |
| BDI-II, somato-affective factors | 6.00 ± 7.50 | 6.00 ± 5.00 | 0.067 | >0.05 | 6.00 ± 2.50 | 6.00 ± 2.75 | 0.091 | >0.05 |
| Experimental Group | Control Group | U | p | |
|---|---|---|---|---|
| Δ MoCA | 2.00 ± 3.00 | 0.00 ± 4.50 | 178.50 | >0.05 |
| Δ MoCA—memory | 1.00 ± 1.50 | 0.00 ± 0.00 | 190.50 | 0.035 * |
| Δ MoCA—visuospatial ability | 0.00 ± 0.50 | 0.00 ± 0.75 | 121.50 | >0.05 |
| Δ MoCA—executive functions | 0.00 ± 1.00 | 0.00 ± 1.75 | 177.00 | >0.05 |
| Δ MoCA—attention | 0.00 ± 1.00 | 0.00 ± 1.00 | 171.50 | >0.05 |
| Δ MoCA—language | 0.00 ± 0.50 | 0.00 ± 0.75 | 141.00 | >0.05 |
| Δ MoCA—orientation | 0.00 ± 0.00 | 0.00 ± 0.00 | 129.00 | >0.05 |
| Δ BDI-II | 0.00 ± 6.00 | 0.00 ± 6.50 | 131.50 | >0.05 |
| Δ BDI-II, cognitive and affective factors | −1.00 ± 3.50 | −1.00 ± 3.00 | 124.00 | >0.05 |
| Δ BDI-II, somato-affective factors | 0.00 ± 4.50 | 0.00 ± 2.75 | 137.00 | >0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reitano, M.R.; Guidetti, M.; Maiorana, N.V.; De Sandi, A.; Carusi, F.; Rosci, C.; Ruggiero, F.; Poletti, B.; Ticozzi, N.; Mameli, F.; et al. The Effects of a New Integrated and Multidisciplinary Cognitive Rehabilitation Program Based on Mindfulness and Reminiscence Therapy in Patients with Parkinson’s Disease and Mild Cognitive Impairment: A Pilot Study. Brain Sci. 2023, 13, 201. https://doi.org/10.3390/brainsci13020201
Reitano MR, Guidetti M, Maiorana NV, De Sandi A, Carusi F, Rosci C, Ruggiero F, Poletti B, Ticozzi N, Mameli F, et al. The Effects of a New Integrated and Multidisciplinary Cognitive Rehabilitation Program Based on Mindfulness and Reminiscence Therapy in Patients with Parkinson’s Disease and Mild Cognitive Impairment: A Pilot Study. Brain Sciences. 2023; 13(2):201. https://doi.org/10.3390/brainsci13020201
Chicago/Turabian StyleReitano, Maria Rita, Matteo Guidetti, Natale Vincenzo Maiorana, Angelica De Sandi, Fabrizio Carusi, Chiara Rosci, Fabiana Ruggiero, Barbara Poletti, Nicola Ticozzi, Francesca Mameli, and et al. 2023. "The Effects of a New Integrated and Multidisciplinary Cognitive Rehabilitation Program Based on Mindfulness and Reminiscence Therapy in Patients with Parkinson’s Disease and Mild Cognitive Impairment: A Pilot Study" Brain Sciences 13, no. 2: 201. https://doi.org/10.3390/brainsci13020201
APA StyleReitano, M. R., Guidetti, M., Maiorana, N. V., De Sandi, A., Carusi, F., Rosci, C., Ruggiero, F., Poletti, B., Ticozzi, N., Mameli, F., Barbieri, S., Silani, V., Priori, A., & Ferrucci, R. (2023). The Effects of a New Integrated and Multidisciplinary Cognitive Rehabilitation Program Based on Mindfulness and Reminiscence Therapy in Patients with Parkinson’s Disease and Mild Cognitive Impairment: A Pilot Study. Brain Sciences, 13(2), 201. https://doi.org/10.3390/brainsci13020201








