Wider and Stronger Inhibitory Ring of the Attentional Focus in Schizophrenia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Apparatus and Stimuli
2.3. Procedure
2.4. Data Analysis
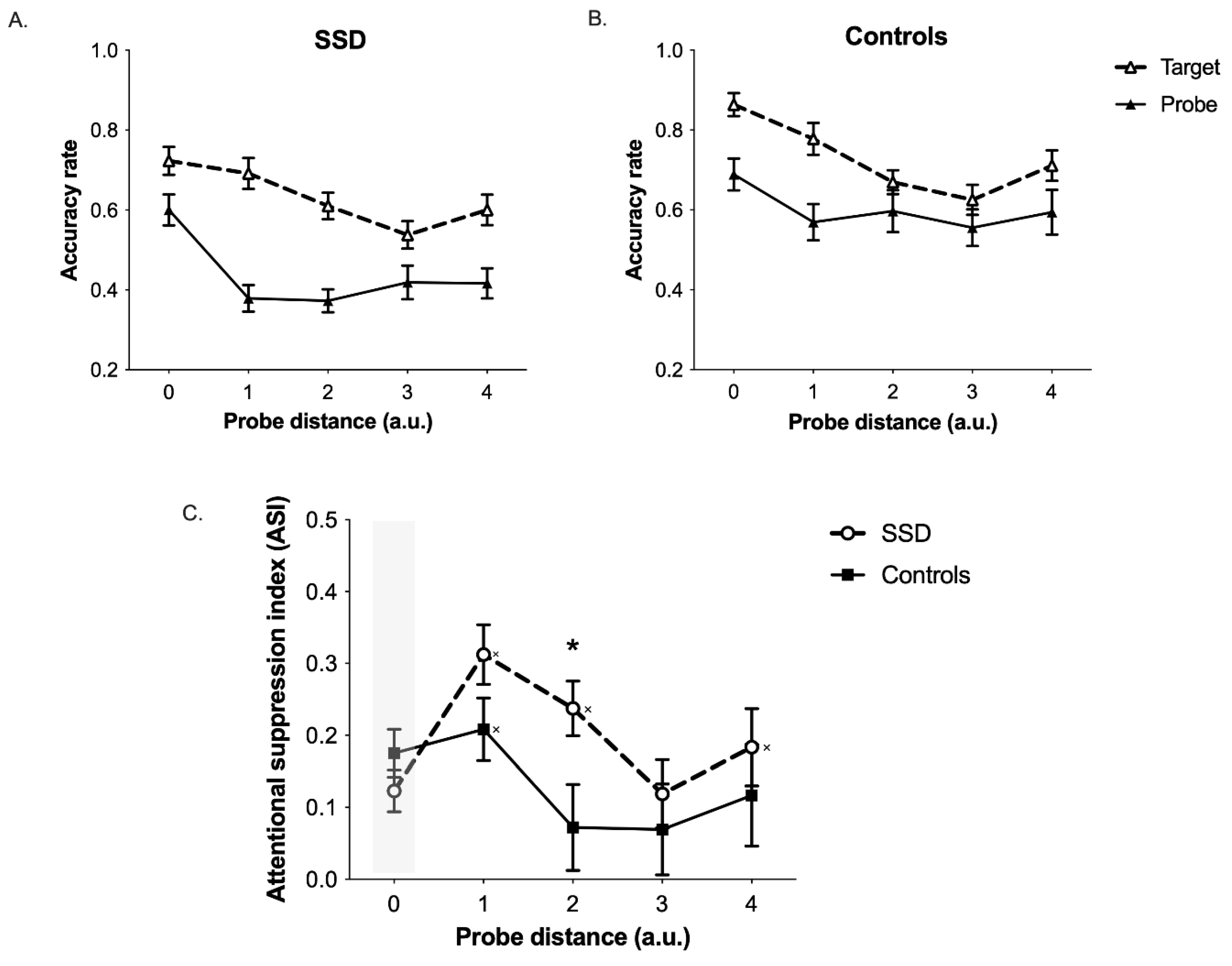
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilmour, G.; Porcelli, S.; Bertaina-Anglade, V.; Arce, E.; Dukart, J.; Hayen, A.; Lobo, A.; Lopez-Anton, R.; Merlo Pich, E.; Pemberton, D.J.; et al. Relating Constructs of Attention and Working Memory to Social Withdrawal in Alzheimer’s Disease and Schizophrenia: Issues Regarding Paradigm Selection. Neurosci. Biobehav. Rev. 2019, 97, 47–69. [Google Scholar] [CrossRef] [PubMed]
- Luck, S.J.; Gold, J.M. The Construct of Attention in Schizophrenia. Biol. Psychiatry 2008, 64, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Carrasco, M. Visual Attention: The Past 25 Years. Vision Res. 2011, 51, 1484–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbetta, M.; Shulman, G.L. Control of Goal-Directed and Stimulus-Driven Attention in the Brain. Nat. Rev. Neurosci. 2002, 3, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.E.; Posner, M.I. The Attention System of the Human Brain: 20 Years After. Annu. Rev. Neurosci. 2012, 35, 73–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castiello, U.; Umiltà, C. Size of the Attentional Focus and Efficiency of Processing. Acta Psychol. 1990, 73, 195–209. [Google Scholar] [CrossRef]
- Eriksen, C.W.; St James, J.D. Visual Attention within and around the Field of Focal Attention: A Zoom Lens Model. Percept. Psychophys. 1986, 40, 225–240. [Google Scholar] [CrossRef] [Green Version]
- Müller, N.G.; Bartelt, O.A.; Donner, T.H.; Villringer, A.; Brandt, S.A. A Physiological Correlate of the “Zoom Lens” of Visual Attention. J. Neurosci. 2003, 23, 3561–3565. [Google Scholar] [CrossRef] [Green Version]
- Ronconi, L.; Basso, D.; Gori, S.; Facoetti, A. TMS on Right Frontal Eye Fields Induces an Inflexible Focus of Attention. Cereb. Cortex 2014, 24, 396–402. [Google Scholar] [CrossRef] [Green Version]
- Spencer, K.M.; Nestor, P.G.; Valdman, O.; Niznikiewicz, M.A.; Shenton, M.E.; McCarley, R.W. Enhanced Facilitation of Spatial Attention in Schizophrenia. Neuropsychology 2011, 25, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Dalmaso, M.; Galfano, G.; Tarqui, L.; Forti, B.; Castelli, L. Is Social Attention Impaired in Schizophrenia? Gaze, but Not Pointing Gestures, Is Associated with Spatial Attention Deficits. Neuropsychology 2013, 27, 608–613. [Google Scholar] [CrossRef]
- Akiyama, T.; Kato, M.; Muramatsu, T.; Maeda, T.; Hara, T.; Kashima, H. Gaze-Triggered Orienting Is Reduced in Chronic Schizophrenia. Psychiatry Res. 2008, 158, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Langdon, R.; Corner, T.; McLaren, J.; Coltheart, M.; Ward, P.B. Attentional Orienting Triggered by Gaze in Schizophrenia. Neuropsychologia 2006, 44, 417–429. [Google Scholar] [CrossRef]
- Langdon, R.; Seymour, K.; Williams, T.; Ward, P.B. Automatic Attentional Orienting to Other People’s Gaze in Schizophrenia. Q. J. Exp. Psychol. 2017, 70, 1549–1558. [Google Scholar] [CrossRef]
- Kreither, J.; Lopez-Calderon, J.; Leonard, C.J.; Robinson, B.M.; Ruffle, A.; Hahn, B.; Gold, J.M.; Luck, S.J. Electrophysiological Evidence for Hyperfocusing of Spatial Attention in Schizophrenia. J. Neurosci. 2017, 37, 3813–3823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonard, C.J.; Robinson, B.M.; Hahn, B.; Luck, S.J.; Gold, J.M. Altered Spatial Profile of Distraction in People with Schizophrenia. J. Abnorm. Psychol. 2017, 126, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Umiltà, C.; Sartori, G. Deficits in The Control of The Attentional Focus in Chronic Schizophrenics. Cortex 1998, 34, 263–270. [Google Scholar] [CrossRef]
- Bellgrove, M.A.; Vance, A.; Bradshaw, J.L. Local–Global Processing in Early-Onset Schizophrenia: Evidence for an Impairment in Shifting the Spatial Scale of Attention. Brain Cogn. 2003, 51, 48–65. [Google Scholar] [CrossRef]
- Müller, N.G.; Mollenhauer, M.; Rösler, A.; Kleinschmidt, A. The Attentional Field Has a Mexican Hat Distribution. Vision Res. 2005, 45, 1129–1137. [Google Scholar] [CrossRef] [Green Version]
- Hopf, J.-M.; Boehler, C.N.; Luck, S.J.; Tsotsos, J.K.; Heinze, H.-J.; Schoenfeld, M.A. Direct Neurophysiological Evidence for Spatial Suppression Surrounding the Focus of Attention in Vision. Proc. Natl. Acad. Sci. USA 2006, 103, 1053–1058. [Google Scholar] [CrossRef] [Green Version]
- Slotnick, S.D.; Hopfinger, J.B.; Klein, S.A.; Sutter, E.E. Darkness beyond the Light: Attentional Inhibition Surrounding the Classic Spotlight. Neuroreport 2002, 13, 773–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronconi, L.; Gori, S.; Federici, A.; Devita, M.; Carna, S.; Sali, M.E.; Molteni, M.; Casartelli, L.; Facoetti, A. Weak Surround Suppression of the Attentional Focus Characterizes Visual Selection in the Ventral Stream in Autism. NeuroImage Clin. 2018, 18, 912–922. [Google Scholar] [CrossRef]
- Hopf, J.M.; Boehler, C.N.; Schoenfeld, M.A.; Heinze, H.J.; Tsotsos, J.K. The Spatial Profile of the Focus of Attention in Visual Search: Insights from MEG Recordings. Vision Res. 2010, 50, 1312–1320. [Google Scholar] [CrossRef] [Green Version]
- Tsotsos, J.K.; Culhane, S.M.; Winky, Y.K.W.; Yuzhong, L.; Davis, N.; Nuflo, F. Modeling Visual Attention via Selective Tuning. Artif. Intell. 1995, 78, 507–545. [Google Scholar] [CrossRef] [Green Version]
- Tsotsos, J.K. Analyzing Vision at the Complexity Level. Behav. Brain Sci. 1990, 13, 423–469. [Google Scholar] [CrossRef]
- Tsotsos, J.K.; Rodríguez-Sánchez, A.J.; Rothenstein, A.L.; Simine, E. The Different Stages of Visual Recognition Need Different Attentional Binding Strategies. Brain Res. 2008, 1225, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Cave, K.R.; Zimmerman, J.M. Flexibility in Spatial Attention Before and After Practice. Psychol. Sci. 1997, 8, 399–403. [Google Scholar] [CrossRef]
- Bahcall, D.O.; Kowler, E. Attentional Interference at Small Spatial Separations. Vision Res. 1999, 39, 71–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Uhlhaas, P.J.; Linden, D.E.J.; Singer, W.; Haenschel, C.; Lindner, M.; Maurer, K.; Rodriguez, E. Dysfunctional Long-Range Coordination of Neural Activity during Gestalt Perception in Schizophrenia. J. Neurosci. 2006, 26, 8168–8175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivolta, D.; Castellanos, N.P.; Stawowsky, C.; Helbling, S.; Wibral, M.; Grutzner, C.; Koethe, D.; Birkner, K.; Kranaster, L.; Enning, F.; et al. Source-Reconstruction of Event-Related Fields Reveals Hyperfunction and Hypofunction of Cortical Circuits in Antipsychotic-Naive, First-Episode Schizophrenia Patients during Mooney Face Processing. J. Neurosci. 2014, 34, 5909–5917. [Google Scholar] [CrossRef] [Green Version]
- Okruszek, Ł.; Pilecka, I. Biological Motion Processing in Schizophrenia—Systematic Review and Meta-Analysis. Schizophr. Res. 2017, 190, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, D.J.; Hodgekins, J.; Chouinard, P.A.; Chouinard, V.-A.; Sperandio, I. A Review of Abnormalities in the Perception of Visual Illusions in Schizophrenia. Psychon. Bull. Rev. 2017, 24, 734–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzolatti, G.; Riggio, L.; Dascola, I.; Umiltá, C. Reorienting Attention across the Horizontal and Vertical Meridians: Evidence in Favor of a Premotor Theory of Attention. Neuropsychologia 1987, 25, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.E.; Subramaniam, B. The Role of Visual Attention in Saccadic Eye Movements. Percept. Psychophys. 1995, 57, 787. [Google Scholar] [CrossRef] [Green Version]
- Findlay, J.M. Covert Attention and Saccadic Eye Movements. In Neurobiology of Attention; Elsevier: Amsterdam, The Netherlands, 2005; pp. 114–116. ISBN 978-0-12-375731-9. [Google Scholar]
- Beedie, S.A.; Benson, P.J.; Giegling, I.; Rujescu, D.; St. Clair, D.M. Smooth Pursuit and Visual Scanpaths: Independence of Two Candidate Oculomotor Risk Markers for Schizophrenia. World J. Biol. Psychiatry 2012, 13, 200–210. [Google Scholar] [CrossRef]
- Sprenger, A.; Friedrich, M.; Nagel, M.; Schmidt, C.S.; Moritz, S.; Lencer, R. Advanced Analysis of Free Visual Exploration Patterns in Schizophrenia. Front. Psychol. 2013, 4, 737. [Google Scholar] [CrossRef] [Green Version]
- Morita, K.; Miura, K.; Fujimoto, M.; Yamamori, H.; Yasuda, Y.; Kudo, N.; Azechi, H.; Okada, N.; Koshiyama, D.; Ikeda, M.; et al. Eye Movement Abnormalities and Their Association with Cognitive Impairments in Schizophrenia. Schizophr. Res. 2019, 209, 255–262. [Google Scholar] [CrossRef]
- Nobre, A.C.; Coull, J.T.; Maquet, P.; Frith, C.D.; Vandenberghe, R.; Mesulam, M.M. Orienting Attention to Locations in Perceptual Versus Mental Representations. J. Cogn. Neurosci. 2004, 16, 363–373. [Google Scholar] [CrossRef]
- Kiyonaga, A.; Egner, T. Center-Surround Inhibition in Working Memory. Curr. Biol. 2016, 26, 64–68. [Google Scholar] [CrossRef] [Green Version]
- Corbetta, M.; Shulman, G.L. Spatial Neglect and Attention Networks. Annu. Rev. Neurosci. 2011, 34, 569–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buschman, T.J.; Miller, E.K. Top-down versus Bottom-up Control of Attention in the Prefrontal and Posterior Parietal Cortices. Science 2007, 315, 1860–1862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boehler, C.N.; Tsotsos, J.K.; Schoenfeld, M.A.; Heinze, H.J.; Hopf, J.M. The center- surround profile of the focus of attention arises from recurrent processing in visual cortex. Cereb. Cortex. 2009, 19, 982–991. [Google Scholar] [CrossRef]
- Michalareas, G.; Vezoli, J.; van Pelt, S.; Schoffelen, J.-M.; Kennedy, H.; Fries, P. Alpha-Beta and Gamma Rhythms Subserve Feedback and Feedforward Influences among Human Visual Cortical Areas. Neuron 2016, 89, 384–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastos, A.M.; Vezoli, J.; Bosman, C.A.; Schoffelen, J.-M.; Oostenveld, R.; Dowdall, J.R.; De Weerd, P.; Kennedy, H.; Fries, P. Visual Areas Exert Feedforward and Feedback Influences through Distinct Frequency Channels. Neuron 2015, 85, 390–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastos, A.M.; Lundqvist, M.; Waite, A.S.; Kopell, N.; Miller, E.K. Layer and Rhythm Specificity for Predictive Routing. Proc. Natl. Acad. Sci. USA 2020, 117, 31459–31469. [Google Scholar] [CrossRef]
- Ronconi, L.; Bertoni, S.; Marotti, R.B. The Neural Origins of Visual Crowding as Revealed by Event-Related Potentials and Oscillatory Dynamics. Cortex 2016, 79, 87–98. [Google Scholar] [CrossRef]
- Battaglini, L.; Ghiani, A.; Casco, C.; Ronconi, L. Parietal TACS at Beta Frequency Improves Vision in a Crowding Regime. NeuroImage 2020, 208, 116451. [Google Scholar] [CrossRef]
- Kloosterman, N.A.; de Gee, J.W.; Werkle-Bergner, M.; Lindenberger, U.; Garrett, D.D.; Fahrenfort, J.J. Humans Strategically Shift Decision Bias by Flexibly Adjusting Sensory Evidence Accumulation. eLife 2019, 8, e37321. [Google Scholar] [CrossRef]
- Battaglini, L.; Mena, F.; Ghiani, A.; Casco, C.; Melcher, D.; Ronconi, L. The Effect of Alpha TACS on the Temporal Resolution of Visual Perception. Front. Psychol. 2020, 11, 1765. [Google Scholar] [CrossRef]
- Ronconi, L.; Vitale, A.; Federici, A.; Pini, E.; Molteni, M.; Casartelli, L. Altered Neural Oscillations and Connectivity in the Beta Band Underlie Detail-Oriented Visual Processing in Autism. NeuroImage Clin. 2020, 28, 102484. [Google Scholar] [CrossRef]
- Tarasi, L.; Trajkovic, J.; Diciotti, S.; di Pellegrino, G.; Ferri, F.; Ursino, M.; Romei, V. Predictive Waves in the Autism-Schizophrenia Continuum: A Novel Biobehavioral Model. Neurosci. Biobehav. Rev. 2022, 132, 1–22. [Google Scholar] [CrossRef]
- Baskin-Sommers, A.R.; Curtin, J.J.; Newman, J.P. Specifying the attentional selection that moderates the fearlessness of psychopathic offenders. Psychol. Sci. 2011, 22, 226–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baskin-Sommers, A.; Brazil, I.A. The importance of an exaggerated attention bottleneck for understanding psychopathy. Trends Cogn. Sci. 2022, 26, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.C.; Carpenter, R.W.; Warren, C.M.; Zeier, J.D.; Baskin-Sommers, A.R.; Newman, J.P. Reduced susceptibility to the attentional blink in psychopathic offenders: Implications for the attention bottleneck hypothesis. Neuropsychology 2012, 26, 102. [Google Scholar] [CrossRef] [Green Version]
- Tillem, S.; Weinstein, H.; Baskin-Sommers, A. Psychopathy is associated with an exaggerated attention bottleneck: EEG and behavioral evidence from a dual-task paradigm. Cogn. Affect. Behav. Neurosci. 2021, 21, 881–893. [Google Scholar] [CrossRef]
- Fatouros-Bergman, H.; Cervenka, S.; Flyckt, L.; Edman, G.; Farde, L. Meta-analysis of cognitive performance in drug-naïve patients with schizophrenia. Schizophr. Res. 2014, 158, 156–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesholam-Gately, R.I.; Giuliano, A.J.; Goff, K.P.; Faraone, S.V.; Seidman, L.J. Neurocognition in first-episode schizophrenia: A meta-analytic review. Neuropsychology 2009, 23, 315. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Drug | Number of Patients | Average Daily Dosage |
|---|---|---|
| Clozapine | 7 | 270 mg |
| Olanzapine | 6 | 10 mg |
| Aripiprazole | 6 | 10 mg |
| Paliperidone | 3 | 5 mg |
| Fluphenazine | 1 | 1 mg |
| Quetiapine | 1 | 100 mg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ronconi, L.; Florio, V.; Bronzoni, S.; Salvetti, B.; Raponi, A.; Giupponi, G.; Conca, A.; Basso, D. Wider and Stronger Inhibitory Ring of the Attentional Focus in Schizophrenia. Brain Sci. 2023, 13, 211. https://doi.org/10.3390/brainsci13020211
Ronconi L, Florio V, Bronzoni S, Salvetti B, Raponi A, Giupponi G, Conca A, Basso D. Wider and Stronger Inhibitory Ring of the Attentional Focus in Schizophrenia. Brain Sciences. 2023; 13(2):211. https://doi.org/10.3390/brainsci13020211
Chicago/Turabian StyleRonconi, Luca, Vincenzo Florio, Silvia Bronzoni, Beatrice Salvetti, Agnese Raponi, Giancarlo Giupponi, Andreas Conca, and Demis Basso. 2023. "Wider and Stronger Inhibitory Ring of the Attentional Focus in Schizophrenia" Brain Sciences 13, no. 2: 211. https://doi.org/10.3390/brainsci13020211





