Current and Future Nano-Carrier-Based Approaches in the Treatment of Alzheimer’s Disease
Abstract
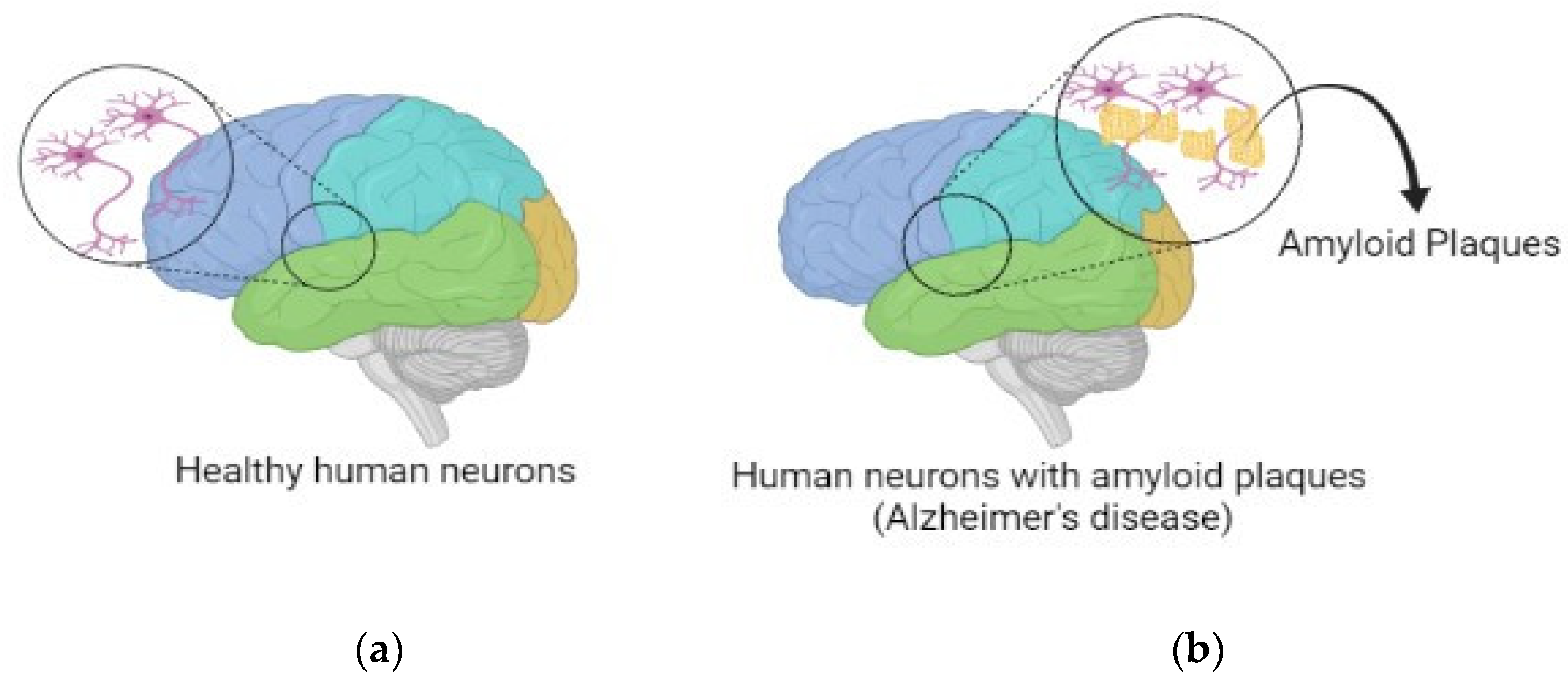
:1. Introduction
2. Pharmacotherapy Approaches in AD Treatment
2.1. Drug Repurposing
2.1.1. Thalidomide
2.1.2. Bexarotene
2.1.3. PD 1 Blocker
2.1.4. Anti-Microbial
2.1.5. Clioquinol
2.1.6. Anti-Diabetic
2.2. Oral Administration
2.2.1. Conventional Oral-Based Delivery
Donepezil
Rivastigmine
Galantamine
Memantine
2.3. Oral Novel Drug Delivery
2.4. Oral Traditional Dosage Form
2.4.1. Donepezil
2.4.2. Galantamine
2.4.3. Physostigmine
2.4.4. Memantine
2.5. Transdermal Drug Delivery
2.5.1. Cholinesterase Inhibitors
Physostigmine
Tacrine
Rivastigmine
Donepezil
Phenserine
Galantamine
2.5.2. Noncompetitive N-Methyl-D-Aspartate
Memantine
2.6. Neurological Preservative
Allopregnanolone
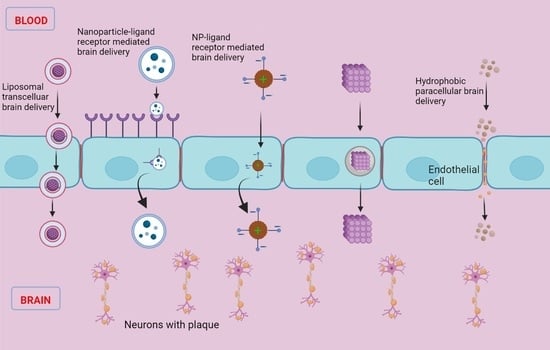
2.7. Nanotechnology
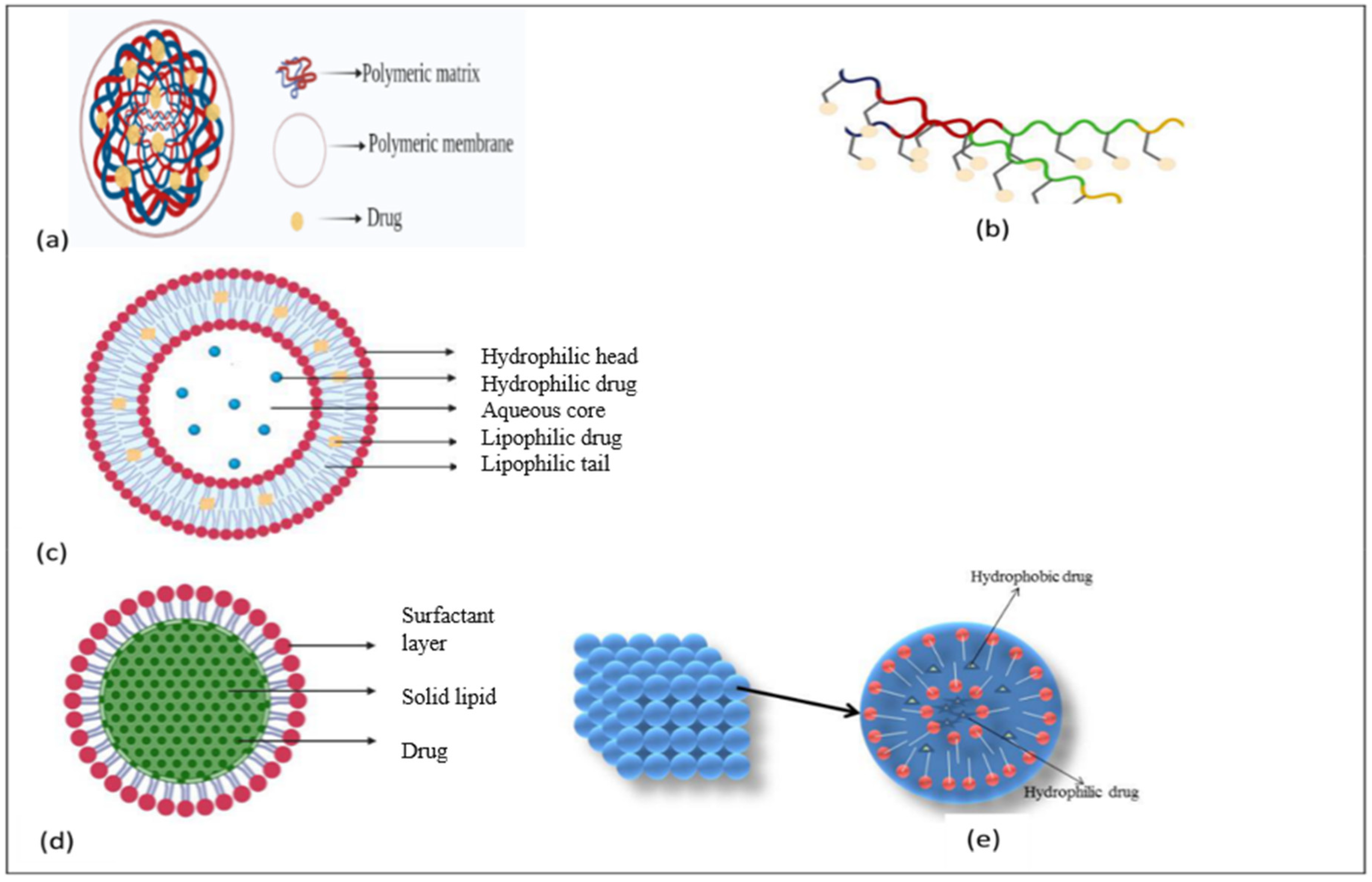
2.7.1. Polymeric Nanoparticles
2.7.2. Aptamers
RNA Aptamers
DNA Aptamers
Peptide Aptamers
2.7.3. Liposomes
2.7.4. Lipid Nanoparticles
Lipoprotein-Based Nanoparticles
2.7.5. Nanoparticle–Biomolecule Conjugates
Curcumin-Loaded Nanoparticles
Antibody-Tethered Nanoparticle
2.7.6. Optical Imaging
2.7.7. Cubosomes
2.7.8. Magnetic Nanoparticles
2.7.9. Inorganic Nanoparticles
Nanocomposites
2.7.10. Dendrimers—Macromolecular Drug Carriers
2.7.11. Nanoemulsions—Binary Drug Vehicular Systems
2.8. Limitation of Existing Routes for AD Treatment
3. Methodology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hider, R.C.; Ma, Y.; Molina-Holgado, F.; Gaeta, A.; Roy, S. Iron chelation as a potential therapy for neurodegenerative disease. Biochem. Soc. Trans. 2008, 36, 1304–1308. [Google Scholar] [CrossRef] [PubMed]
- Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 7 October 2021).
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, A.L.F.; Chien, Y.W.; Jin Lin, S. Transdermal Delivery of Treatment for Alzheimer’s Disease: Development, Clinical Performance and Future Prospects. Drugs Aging 2008, 25, 761–775. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Tam, K. Pathological mechanisms and therapeutic strategies for Alzheimer’s disease. Neural Regen. Res. 2022, 17, 543. [Google Scholar] [PubMed]
- Hossain, M.; Jhee, S.S.; Shiovitz, T.; McDonald, C.; Sedek, G.; Pommier, F.; Cutler, N.R. Estimation of the Absolute Bioavailability of Rivastigmine in Patients with Mild to Moderate Dementia of the Alzheimer’s Type. Clin. Pharmacokinet. 2002, 41, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid β-peptide. Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Wickner, R.B.; Bezsonov, E.E.; Son, M.; Ducatez, M.; DeWilde, M.; Edskes, H.K. Anti-Prion Systems in Yeast and Inositol Polyphosphates. Biochemistry 2018, 57, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Wickner, R.B.; Edskes, H.K.; Gorkovskiy, A.; Bezsonov, E.E.; Stroobant, E.E. Yeast and Fungal Prions: Amyloid-Handling Systems, Amyloid Structure, and Prion Biology. Adv. Genet. 2016, 93, 191–236. [Google Scholar]
- Wilkinson, G.F.; Pritchard, K. In Vitro Screening for Drug Repositioning. J. Biomol. Screen. 2015, 20, 167–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobinick, E.L. The value of drug repositioning in the current pharmaceutical market. Drug News Perspect. 2009, 22, 119. [Google Scholar] [CrossRef] [PubMed]
- Ashburn, T.T.; Thor, K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Corbett, A.; Pickett, J.; Burns, A.; Corcoran, J.; Dunnett, S.B.; Edison, P.; Hagan, J.J.; Holmes, C.; Jones, E.; Katona, C.; et al. Drug repositioning for Alzheimer’s disease. Nat. Rev. Drug Discov. 2012, 11, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Cramer, P.E.; Cirrito, J.R.; Wesson, D.W.; Lee, C.Y.D.; Karlo, J.C.; Zinn, A.E.; Casali, B.T.; Restivo, J.L.; Goebel, W.D.; James, M.J.; et al. ApoE-Directed Therapeutics Rapidly Clear β-Amyloid and Reverse Deficits in AD Mouse Models. Science 2012, 335, 1503–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aicardi, G. New Hope from an Old Drug: Fighting Alzheimer’s Disease with the Cancer Drug Bexarotene (Targretin)? Rejuvenation Res. 2013, 16, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Baruch, K.; Deczkowska, A.; Rosenzweig, N.; Tsitsou-Kampeli, A.; Sharif, A.M.; Matcovitch-Natan, O.; Kertser, A.; David, E.; Amit, I.; Schwartz, M. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer’s disease. Nat. Med. 2016, 22, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Vostrov, A.A.; Taheny, M.J.; Izkhakov, N.; Quitschke, W.W. A nuclear factor-binding domain in the 5’-untranslated region of the amyloid precursor protein promoter: Implications for the regulation of gene expression. BMC Res. Notes 2010, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Grossi, C.; Francese, S.; Casini, A.; Rosi, M.C.; Luccarini, I.; Fiorentini, A.; Gabbiani, C.; Messori, L.; Moneti, G.; Casamenti, F. Clioquinol Decreases Amyloid-β Burden and Reduces Working Memory Impairment in a Transgenic Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2009, 17, 423–440. [Google Scholar] [CrossRef]
- De Felice, F.G.; Vieira, M.N.N.; Bomfim, T.R.; Decker, H.; Velasco, P.T.; Lambert, M.P.; Viola, K.L.; Zhao, W.Q.; Ferreira, S.T.; Klein, W.L.; et al. Protection of synapses against Alzheimer’s-linked toxins: Insulin signaling prevents the pathogenic binding of Aβ oligomers. Proc. Natl. Acad. Sci. USA 2009, 106, 1971–1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Rivera, J.; Denner, L.; Dineley, K.T. Rosiglitazone reversal of Tg2576 cognitive deficits is independent of peripheral gluco-regulatory status. Behav. Brain Res. 2011, 216, 255–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheline, Y.I.; West, T.; Yarasheski, K.; Swarm, R.; Jasielec, M.S.; Fisher, J.R.; Ficker, W.D.; Yan, P.; Xiong, C.; Frederiksen, C.; et al. An Antidepressant Decreases CSF Aβ Production in Healthy Individuals and in Transgenic AD Mice. Sci. Transl. Med. 2014, 6, 236re4. Available online: https://www.science.org/doi/10.1126/scitranslmed.3008169 (accessed on 22 November 2021). [CrossRef] [PubMed] [Green Version]
- Jenner, P. Preclinical evidence for neuroprotection with monoamine oxidase-B inhibitors in Parkinson’s disease. Neurology 2004, 63 (Suppl. S2), S13–S22. [Google Scholar] [CrossRef] [PubMed]
- McClean, P.L.; Gault, V.A.; Harriott, P.; Hölscher, C. Glucagon-like peptide-1 analogues enhance synaptic plasticity in the brain: A link between diabetes and Alzheimer’s disease. Eur. J. Pharmacol. 2010, 630, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Breitner, J.C.; Baker, L.D.; Montine, T.J.; Meinert, C.L.; Lyketsos, C.G.; Ashe, K.H.; Brandt, J.; Craft, S.; Evans, D.E.; Green, R.C.; et al. Extended results of the Alzheimer’s disease anti-inflammatory prevention trial. Alzheimer’s Dement. 2011, 7, 402–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anekonda, T.S.; Quinn, J.F.; Harris, C.; Frahler, K.; Wadsworth, T.L.; Woltjer, R.L. L-type voltage-gated calcium channel blockade with isradipine as a therapeutic strategy for Alzheimer’s disease. Neurobiol. Dis. 2011, 41, 62–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- in ’t Veld, B.A.; Ruitenberg, A.; Hofman, A.; Launer, L.J.; van Duijn, C.M.; Stijnen, T.; Breteler, M.M.; Stricker, B.H. Nonsteroidal Antiinflammatory Drugs and the Risk of Alzheimer’s Disease. N. Engl. J. Med. 2001, 345, 1515–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paris, D.; Quadros, A.; Humphrey, J.; Patel, N.; Crescentini, R.; Crawford, F.; Mullan, M. Nilvadipine antagonizes both Aβ vasoactivity in isolated arteries, and the reduced cerebral blood flow in APPsw transgenic mice. Brain Res. 2004, 999, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.; Guo, Z.; Halagappa, V.; Pearson, M.; Gray, A.; Matsuoka, Y.; Brown, M.; Martin, B.; Iyun, T.; Maudsley, S.; et al. Prophylactic treatment with paroxetine ameliorates behavioral deficits and retards the development of amyloid and tau pathologies in 3xTgAD mice. Exp. Neurol. 2007, 205, 166–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Stefano, A.; Iannitelli, A.; Laserra, S.; Sozio, P. Drug delivery strategies for Alzheimer’s disease treatment. Expert Opin. Drug Deliv. 2011, 8, 581–603. [Google Scholar] [CrossRef]
- Clark, C.M.; Karlawish, J.H.T. Alzheimer Disease: Current Concepts and Emerging Diagnostic and Therapeutic Strategies. Ann. Intern. Med. 2003, 138, 400. [Google Scholar] [CrossRef] [PubMed]
- Patocka, J.; Jun, D.; Kuca, K. Possible Role of Hydroxylated Metabolites of Tacrine in Drug Toxicity and Therapy of Alzheimers Disease. Curr. Drug Metab. 2008, 9, 332–335. [Google Scholar] [CrossRef]
- Danysz, W.; Parsons, C.G. The NMDA receptor antagonist memantine as a symptomatological and neuroprotective treatment for Alzheimer’s disease: Preclinical evidence. Int. J. Geriat. Psychiatry 2003, 18 (Suppl. S1), S23–S32. [Google Scholar] [CrossRef]
- Seltzer, B. Donepezil: An update. Expert Opin. Pharmacother. 2007, 8, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S. Cholinergic Adverse Effects of Cholinesterase Inhibitors in Alzheimer’s Disease: Epidemiology and Management. Drugs Aging 2001, 18, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, B. Galantamine-ER for the treatment of mild-to-moderate Alzheimer’s disease. Clin. Interv. Aging 2010, 5, 1–6. [Google Scholar] [PubMed]
- Lee, S.H.; Kim, S.H.; Noh, Y.H.; Choi, B.M.; Noh, G.J.; Park, W.D.; Kim, E.J.; Cho, I.H.; Bae, C.S. Pharmacokinetics of Memantine after a Single and Multiple Dose of Oral and Patch Administration in Rats. Basic Clin. Pharmacol. Toxicol. 2016, 118, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Abetz, L.; Rofail, D.; Mertzanis, P.; Heelis, R.; Rosa, K.; Tellefsen, C.; de Climens, A.R.; McBurney, C.; Thomas, S. Alzheimer’s disease treatment: Assessing caregiver preferences for mode of treatment delivery. Adv. Ther. 2009, 26, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Claxton, A.J.; Cramer, J.; Pierce, C. A systematic review of the associations between dose regimens and medication compliance. Clin. Ther. 2001, 23, 1296–1310. [Google Scholar] [CrossRef]
- Muramatsu, R.S.; Litzinger, M.H.J.; Fisher, E.; Takeshita, J. Alternative formulations, delivery methods, and administration options for psychotropic medications in elderly patients with behavioral and psychological symptoms of dementia. Am. J. Geriatr. Pharmacother. 2010, 8, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Farlow, M.R.; Salloway, S.; Tariot, P.N.; Yardley, J.; Moline, M.L.; Wang, Q.; Brand-Schieber, E.; Zou, H.; Hsu, T.; Satlin, A. Effectiveness and tolerability of high-dose (23 mg/d) versus standard-dose (10 mg/d) donepezil in moderate to severe Alzheimer’s disease: A 24-week, randomized, double-blind study. Clin. Ther. 2010, 32, 1234–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.D.; Woo, J.S.; Kang, J.H.; Yong, C.S.; Choi, H.G. Preparation and Evaluation of Taste-Masked Donepezil Hydrochloride Orally Disintegrating Tablets. Biol. Pharm. Bull. 2010, 33, 1364–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, D.M.; Plosker, G.L. Galantamine Extended Release. CNS Drugs 2006, 20, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Brodaty, H.; Corey-Bloom, J.; Potocnik, F.C.V.; Truyen, L.; Gold, M.; Damaraju, C.R.V. Galantamine Prolonged-Release Formulation in the Treatment of Mild to Moderate Alzheimer’s Disease. Dement. Geriatr. Cogn. Disord. 2005, 20, 120–132. [Google Scholar] [CrossRef]
- Scholz, O.A.; Wolff, A.; Schumacher, A.; Giannola, L.I.; Campisi, G.; Ciach, T.; Velten, T. Drug delivery from the oral cavity: Focus on a novel mechatronic delivery device. Drug Discov. Today 2008, 13, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Whelpton, R.; Hurst, P. Bioavailability of oral physostigmine. N. Engl. J. Med. 1985, 313, 1293–1294. [Google Scholar] [PubMed]
- Bolourchian, N.; Hadidi, N.; Foroutan, S.; Shafaghi, B. Development and optimization of a sublingual tablet formulation for physostigmine salicylate. Acta Pharm. 2009, 59, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Bassil, N.; Thaipisuttikul, P.; Grossberg, G.T. Memantine ER, a once-daily formulation for the treatment of Alzheimer’s disease. Expert Opin. Pharmacother. 2010, 11, 1765–1771. [Google Scholar] [CrossRef] [PubMed]
- Potyk, D. Treatments for Alzheimer disease. South. Med. J. 2005, 98, 628–635. [Google Scholar] [CrossRef]
- Imbimbo, B.P. Pharmacodynamic-tolerability relationships of cholinesterase inhibitors for Alzheimer’s disease. CNS Drugs 2001, 15, 375–390. [Google Scholar] [CrossRef]
- Levy, D.; Glikfeld, P.; Grunfeld, Y.; Grunwald, J.; Kushnir, M.; Levy, A.; Meshulam, Y.; Spiegelstein, M.; Zehavi, D.; Fisher, A. A Novel Transdermal Therapeutic System as a Potential Treatment for Alzheimer’s Disease. In Alzheimer’s and Parkinson’s Disease; Fisher, A., Hanin, I., Lachman, C., Eds.; Advances in Behavioral Biology; Springer: Boston, MA, USA, 1986; Volume 29, pp. 557–563. Available online: http://link.springer.com/10.1007/978-1-4613-2179-8_63 (accessed on 17 January 2023).
- Walter, K.; Müller, M.; Barkworth, M.F.; Nieciecki, A.V.; Stanislaus, F. Pharmacokinetics of physostigmine in man following a single application of a transdermal system. Br. J. Clin. Pharmacol. 1995, 39, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Guy, R.H.; Kalia, Y.N.; Delgado-Charro, M.B.; Merino, V.; López, A.; Marro, D. Iontophoresis: Electrorepulsion and electroosmosis. J. Control. Release 2000, 64, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Upasani, R.S.; Banga, A.K. Response surface methodology to investigate the iontophoretic delivery of tacrine hydrochloride. Pharm. Res. 2004, 21, 2293–2299. [Google Scholar] [CrossRef] [PubMed]
- Kankkunen, T.; Sulkava, R.; Vuorio, M.; Kontturi, K.; Hirvonen, J. Transdermal iontophoresis of tacrine in vivo. Pharm. Res. 2002, 19, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Jaskari, T.; Vuorio, M.; Kontturi, K.; Urtti, A.; Manzanares, J.A.; Hirvonen, J. Controlled transdermal iontophoresis by ion-exchange fiber. J. Control. Release 2000, 67, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, S.; Gale, R. Skin adhesives and skin adhesion. 1. Transdermal drug delivery systems. Biomaterials 1998, 19, 1119–1136. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Giau, V.V.; Vo, T.K. Current advances in transdermal delivery of drugs for Alzheimer’s disease. Indian J. Pharmacol. 2017, 49, 145–154. [Google Scholar] [PubMed]
- Tezel, A.; Sens, A.; Mitragotri, S. A theoretical analysis of low-frequency sonophoresis: Dependence of transdermal transport pathways on frequency and energy density. Pharm. Res. 2002, 19, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Khanmohammadi, M.; Elmizadeh, H.; Ghasemi, K. Investigation of Size and Morphology of Chitosan Nanoparticles Used in Drug Delivery System Employing Chemometric Technique. Iran. J. Pharm. Res. 2015, 14, 665–675. [Google Scholar]
- Heydorn, W.E. Donepezil (E2020): A new acetylcholinesterase inhibitor. Review of its pharmacology, pharmacokinetics, and utility in the treatment of Alzheimer’s disease. Expert Opin. Investig. Drugs 1997, 6, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Valia, K.H.; Ramaraju, V.S. Transdermal Methods and Systems for Treating Alzheimer’s Disease. U.S. Patent 20080044461A1, 21 February 2008. [Google Scholar]
- Kim, K.H.; Gwak, H.S. Effects of vehicles on the percutaneous absorption of donepezil hydrochloride across the excised hairless mouse skin. Drug Dev. Ind. Pharm. 2011, 37, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Saluja, S.; Kasha, P.C.; Paturi, J.; Anderson, C.; Morris, R.; Banga, A.K. A novel electronic skin patch for delivery and pharmacokinetic evaluation of donepezil following transdermal iontophoresis. Int. J. Pharm. 2013, 453, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Small, G.; Dubois, B. A review of compliance to treatment in Alzheimer’s disease: Potential benefits of a transdermal patch. Curr. Med. Res. Opin. 2007, 23, 2705–2713. [Google Scholar] [CrossRef] [PubMed]
- Prvulovic, D.; Hampel, H.; Pantel, J. Galantamine for Alzheimer’s disease. Expert Opin. Drug Metab. Toxicol. 2010, 6, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Park, C.W.; Son, D.D.; Kim, J.Y.; Oh, T.O.; Ha, J.M.; Rhee, Y.S.; Park, E.S. Investigation of formulation factors affecting in vitro and in vivo characteristics of a galantamine transdermal system. Int. J. Pharm. 2012, 436, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Woo, F.Y.; Basri, M.; Masoumi, H.R.F.; Ahmad, M.B.; Ismail, M. Formulation optimization of galantamine hydrobromide loaded gel drug reservoirs in transdermal patch for Alzheimer’s disease. Int. J. Nanomed. 2015, 10, 3879–3886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKeage, K. Spotlight on memantine in moderate to severe Alzheimer’s disease. Drugs Aging 2010, 27, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Del Rio-Sancho, S.; Serna-Jiménez, C.E.; Calatayud-Pascual, M.A.; Balaguer-Fernández, C.; Femenía-Font, A.; Merino, V.; López-Castellano, A. Transdermal absorption of memantin—Effect of chemical enhancers, iontophoresis, and role of enhancer lipophilicity. Eur. J. Pharm. Biopharm. 2012, 82, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Singh, C.; Liu, L.; Irwin, R.W.; Chen, S.; Chung, E.J.; Thompson, R.F.; Brinton, R.D. Allopregnanolone reverses neurogenic and cognitive deficits in mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2010, 107, 6498–6503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharm. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Irwin, R.W.; Solinsky, C.M.; Loya, C.M.; Salituro, F.G.; Rodgers, K.E.; Bauer, G.; Rogawski, M.A.; Brinton, R.D. Allopregnanolone preclinical acute pharmacokinetic and pharmacodynamic studies to predict tolerability and efficacy for Alzheimer’s disease. PLoS ONE 2015, 10, e0128313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Singh, M.; Gambhir, I.S. Nanotechnology for Alzheimer’s disease detection. Dig. J. Nanomater. Biosyst. 2008, 3, 75–79. [Google Scholar]
- Vestergaard, M.; Kerman, K.; Kim, D.K.; Ha, M.H.; Tamiya, E. Detection of Alzheimer’s tau protein using localised surface plasmon resonance-based immunochip. Talanta 2008, 74, 1038–1042. [Google Scholar] [CrossRef]
- Sun, D.; Li, N.; Zhang, W.; Zhao, Z.; Mou, Z.; Huang, D.; Liu, J.; Wang, W. Design of PLGA-functionalized quercetin nanoparticles for potential use in Alzheimer’s disease. Colloids Surf. B Biointerfaces 2016, 148, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Zhang, Y.; Jin, X.; Li, Y.; Zhang, L. A novel synthesis of selenium nanoparticles encapsulated PLGA nanospheres with curcumin molecules for the inhibition of amyloid β aggregation in Alzheimer’s disease. J. Photochem. Photobiol. B Biol. 2019, 190, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Vilella, A.; Belletti, D.; Sauer, A.K.; Hagmeyer, S.; Sarowar, T.; Masoni, M.; Stasiak, N.; Mulvihill, J.J.E.; Ruozi, B.; Forni, F.; et al. Reduced plaque size and inflammation in the APP23 mouse model for Alzheimer’s disease after chronic application of polymeric nanoparticles for CNS targeted zinc delivery. J. Trace Elem. Med. Biol. 2018, 49, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.T.; Harroun, S.G.; Wu, C.W.; Mao, J.Y.; Chang, H.T.; Huang, C.C. Satellite-like Gold Nanocomposites for Targeted Mass Spectrometry Imaging of Tumor Tissues. Nanotheranostics 2017, 1, 141–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ylera, F.; Lurz, R.; Erdmann, V.A.; Fürste, J.P. Selection of RNA Aptamers to the Alzheimer’s Disease Amyloid Peptide. Biochem. Biophys. Res. Commun. 2002, 290, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- Findeis, M.A. The role of amyloid β peptide 42 in Alzheimer’s disease. Pharmacol. Ther. 2007, 116, 266–286. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Tada, K.; Mihara, H. RNA aptamers selected against amyloid β-peptide (Aβ) inhibit the aggregation of Aβ. Mol. BioSyst. 2009, 5, 986. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, F.; Murakami, K.; Summers, J.L.; Chen, C.H.B.; Bitan, G. RNA aptamers generated against oligomeric Abeta40 recognize common amyloid aptatopes with low specificity but high sensitivity. PLoS ONE 2009, 4, e7694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, K.; Obata, Y.; Sekikawa, A.; Ueda, H.; Izuo, N.; Awano, T.; Takabe, K.; Shimizu, T.; Irie, K. An RNA aptamer with potent affinity for a toxic dimer of amyloid β42 has potential utility for histochemical studies of Alzheimer’s disease. J. Biol. Chem. 2020, 295, 4870–4880. [Google Scholar] [CrossRef] [PubMed]
- Tsukakoshi, K.; Abe, K.; Sode, K.; Ikebukuro, K. Selection of DNA Aptamers That Recognize α-Synuclein Oligomers Using a Competitive Screening Method. Anal. Chem. 2012, 84, 5542–5547. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, M.; AlShamaileh, H.; Huang, H.; Tannenberg, R.K.; Chen, S.; Worrall, S.; Dodd, P.R.; Veedu, R.N. Development of DNA aptamers targeting low-molecular-weight amyloid-β peptide aggregates in vitro. Chem. Commun. 2018, 54, 4593–4596. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, L.; Zhao, J.; Li, L.; Wang, M.; Gao, P.; Wang, Q.; Zhang, X.; Wang, W. Advances in aptamers against Aβ and applications in Aβ detection and regulation for Alzheimer’s disease. Theranostics 2022, 12, 2095–2114. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, Q.; Yang, X.; Nie, W.; Zou, L.; Liu, X.; Wang, K. Aptamer as a Tool for Investigating the Effects of Electric Field on Aβ 40 Monomer and Aggregates Using Single-Molecule Force Spectroscopy. Anal. Chem. 2019, 91, 1954–1961. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mourtas, S.; Lazar, A.N.; Markoutsa, E.; Duyckaerts, C.; Antimisiaris, S.G. Multifunctional nanoliposomes with curcumin–lipid derivative and brain targeting functionality with potential applications for Alzheimer disease. Eur. J. Med. Chem. 2014, 80, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Bernardi, A.; Frozza, R.L.; Meneghetti, A.; Hoppe, J.B.; Battastini, A.M.O.; Pohlmann, A.R.; Guterres, S.S.; Salbego, C.G. Indomethacin-loaded lipid-core nanocapsules reduce the damage triggered by Aβ1-42 in Alzheimer’s disease models. Int. J. Nanomed. 2012, 7, 4927–4942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Q.; Huang, M.; Yao, L.; Wang, X.; Gu, X.; Chen, J.; Chen, J.; Huang, J.; Hu, Q.; Kang, T.; et al. Lipoprotein-Based Nanoparticles Rescue the Memory Loss of Mice with Alzheimer’s Disease by Accelerating the Clearance of Amyloid-Beta. ACS Nano 2014, 8, 2345–2359. [Google Scholar] [CrossRef] [PubMed]
- Muntimadugu, E.; Dhommati, R.; Jain, A.; Challa, V.G.S.; Shaheen, M.; Khan, W. Intranasal delivery of nanoparticle encapsulated tarenflurbil: A potential brain targeting strategy for Alzheimer’s disease. Eur. J. Pharm. Sci. 2016, 92, 224–234. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, X.; Wan, X.; Shao, X.; Liu, Q.; Zhang, Z.; Zhang, Q. The potential use of H102 peptide-loaded dual-functional nanoparticles in the treatment of Alzheimer’s disease. J. Control. Release 2014, 192, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Akhter, S.; Rizwanullah, M.; Khan, M.A.; Pigeon, L.; Addo, R.T.; Greig, N.H.; Midoux, P.; Pichon, C.; Kamal, M.A. Nanotechnology Based Theranostic Approaches in Alzheimer’s Disease Management: Current Status and Future Perspective. Curr. Alzheimer Res. 2017, 14, 1164–1181. [Google Scholar] [CrossRef] [Green Version]
- Brambilla, D.; Verpillot, R.; Le Droumaguet, B.; Nicolas, J.; Taverna, M.; Kóňa, J.; Lettiero, B.; Hashemi, S.H.; De Kimpe, L.; Canovi, M.; et al. PEGylated nanoparticles bind to and alter amyloid-beta peptide conformation: Toward engineering of functional nanomedicines for Alzheimer’s disease. ACS Nano 2012, 6, 5897–5908. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.; Gangalum, P.R.; Wagner, S.; Portilla-Arias, J.; Ding, H.; Rekechenetskiy, A.; Konda, B.; Inoue, S.; Black, K.L.; Ljubimova, J.Y.; et al. Curcumin Targeted, Polymalic Acid-Based MRI Contrast Agent for the Detection of Aβ Plaques in Alzheimer’s Disease: Curcumin Targeted, Polymalic Acid-Based MRI Contrast. Macromol. Biosci. 2015, 15, 1212–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arruebo, M.; Valladares, M.; González-Fernández, Á. Antibody-Conjugated Nanoparticles for Biomedical Applications. J. Nanomater. 2009, 2009, 439389. [Google Scholar] [CrossRef] [Green Version]
- Tamba, B.I.; Streinu, V.; Foltea, G.; Neagu, A.N.; Dodi, G.; Zlei, M.; Tijani, A.; Stefanescu, C. Tailored surface silica nanoparticles for blood-brain barrier penetration: Preparation and in vivo investigation. Arab. J. Chem. 2018, 11, 981–990. [Google Scholar] [CrossRef]
- Karami, Z.; Hamidi, M. Cubosomes: Remarkable drug delivery potential. Drug Discov. Today 2016, 21, 789–801. [Google Scholar] [CrossRef]
- Elnaggar, Y.; Etman, S.; Abdelmonsif, D.; Abdallah, O. Novel piperine-loaded Tween-integrated monoolein cubosomes as brain-targeted oral nanomedicine in Alzheimer’s disease: Pharmaceutical, biological, and toxicological studies. Int. J. Nanomed. 2015, 10, 5459. [Google Scholar]
- Do, T.D.; Amin, F.U.; Noh, Y.; Kim, M.O.; Yoon, J. Guidance of Magnetic Nanocontainers for Treating Alzheimer’s Disease Using an Electromagnetic, Targeted Drug-Delivery Actuator. J. Biomed. Nanotechnol. 2016, 12, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Poduslo, J.F.; Wengenack, T.M.; Curran, G.L.; Wisniewski, T.; Sigurdsson, E.M.; Macura, S.I.; Borowski, B.J.; Jack, C.R., Jr. Molecular Targeting of Alzheimer’s Amyloid Plaques for Contrast-Enhanced Magnetic Resonance Imaging. Neurobiol. Dis. 2002, 11, 315–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amiri, H.; Saeidi, K.; Borhani, P.; Manafirad, A.; Ghavami, M.; Zerbi, V. Alzheimer’s Disease: Pathophysiology and Applications of Magnetic Nanoparticles as MRI Theranostic Agents. ACS Chem. Neurosci. 2013, 4, 1417–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivasankarapillai, V.S.; Jose, J.; Shanavas, M.S.; Marathakam, A.; Uddin, M.d.S.; Mathew, B. Silicon Quantum Dots: Promising Theranostic Probes for the Future. Curr. Drug Targets 2019, 20, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Sachdeva, B.; Sachdeva, P.; Chaudhary, V.; Rani, G.M.; Sinha, J.K. Graphene quantum dots as a potential diagnostic and therapeutic tool for the management of Alzheimer’s disease. Carbon Lett. 2022, 32, 1381–1394. [Google Scholar] [CrossRef]
- Kamigaito, O. What can be improved by nanometer composites? J. Jpn. Soc. Powder Powder Metall. 1991, 38, 315–321. [Google Scholar] [CrossRef]
- Chen, Q.; Du, Y.; Zhang, K.; Liang, Z.; Li, J.; Yu, H.; Ren, R.; Feng, J.; Jin, Z.; Li, F.; et al. Tau-Targeted Multifunctional Nanocomposite for Combinational Therapy of Alzheimer’s Disease. ACS Nano 2018, 12, 1321–1338. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Charyulu, R.N. Prolonged drug delivery system of an antifungal drug by association with polyamidoamine dendrimers. Int. J. Pharm. Investig. 2016, 6, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aso, E.; Martinsson, I.; Appelhans, D.; Effenberg, C.; Benseny-Cases, N.; Cladera, J.; Gouras, G.; Ferrer, I.; Klementieva, O. Poly(propylene imine) dendrimers with histidine-maltose shell as novel type of nanoparticles for synapse and memory protection. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Sood, S.; Jain, K.; Gowthamarajan, K. Optimization of curcumin nanoemulsion for intranasal delivery using design of experiment and its toxicity assessment. Colloids Surf. B Biointerfaces 2014, 113, 330–337. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Cervi, V.F.; Gehrcke, M.; da Silveira, E.F.; Azambuja, J.H.; Braganhol, E.; Sari, M.H.; Zborowski, V.A.; Nogueira, C.W.; Cruz, L. Ketoprofen-loaded pomegranate seed oil nanoemulsion stabilized by pullulan: Selective antiglioma formulation for intravenous administration. Colloids Surf. B Biointerfaces 2015, 130, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Nigam, K.; Srivastava, S.; Tyagi, A.; Dang, S. Memantine nanoemulsion: A new approach to treat Alzheimer’s disease. J. Microencapsul. 2020, 37, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Castellani, R.J.; Perry, G. Pathogenesis and Disease-modifying Therapy in Alzheimer’s Disease: The Flat Line of Progress. Arch. Med. Res. 2012, 43, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Tonda-Turo, C.; Origlia, N.; Mattu, C.; Accorroni, A.; Chiono, V. Current Limitations in the Treatment of Parkinson’s and Alzheimer’s Diseases: State-of-the-Art and Future Perspective of Polymeric Carriers. Curr. Med. Chem. 2019, 25, 5755–5771. [Google Scholar] [CrossRef] [PubMed]
- Neganova, M.E.; Aleksandrova, Y.R.; Sukocheva, O.A.; Klochkov, S.G. Benefits and limitations of nanomedicine treatment of brain cancers and age-dependent neurodegenerative disorders. Semin. Cancer Biol. 2022, 86, 805–833. [Google Scholar] [PubMed]
- Agrawal, M.; Ajazuddin; Tripathi, D.K.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Mourtas, S.; Hammarlund-Udenaes, M.; Alexander, A. Recent advancements in liposomes targeting strategies to cross blood-brain barrier (BBB) for the treatment of Alzheimer’s disease. J. Control. Release 2017, 260, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Passeri, E.; Elkhoury, K.; Morsink, M.; Broersen, K.; Linder, M.; Tamayol, A.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Alzheimer’s Disease: Treatment Strategies and Their Limitations. Int. J. Mol. Sci. 2022, 23, 13954. [Google Scholar] [CrossRef]

| Drug | Present Activity | Mol. wt. (g/mol) | LogP | Anti-Alzheimer’s Effect |
|---|---|---|---|---|
| Citalopram | Antidepressant | 324.39 | 3.76 | Lowers the levels of Aβ in brain interstitial fluid [22] |
| Deprenyl | Anti-Parkinson | 187.28 | 3.24 | APP cleavage assistance [23] |
| Exendin–4 | Antidiabetic | 4186.66 | 3.58 | Improves cognitive function and increases synaptic plasticity [24] |
| Ibuprofen | Anti-inflammatory | 206.3 | 3.97 | Alters APP processing, neuroprotective effects [25] |
| Isradipine | Antihypertensive | 371.38 | 4.28 | Neuroprotective effects [26] |
| Naproxen | Anti-inflammatory | 230.26 | 3.29 | Neuroprotective action [27] |
| Nilvadipine | Antihypertensive | 385.4 | 2.97 | Decreases neurotoxicity and Aβ burden [28] |
| Paroxetine | Antidepressant | 329.37 | 3.1 | Reduces Aβ and tau accumulation [29] |
| Drug | LogP | Clearance | Half Life | Adverse Effect | Dosage Form |
|---|---|---|---|---|---|
| Donepezil | 4.14 | 10.5 ± 2 L/h | 80 h | Drowsiness, weakness, trouble sleeping, tremor, muscle cramps | Tablet |
| Rivastigmine | 2.45 | 2.5 ± 0.2 L/h | 1.5 h | Stomach pain, loss of appetite, weight loss | Patch and capsule |
| Galantamine | 1.16 | 18 L/h | 7 h | Intravenous atropine, hallucinations | Tablet and capsule |
| Memantine | 3.3 | 1.2 L/h | 60–80 h | Swelling of face, arrhythmia, weight gain | Tablet |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Sudevan, S.T.; Nair, A.S.; Singh, A.K.; Kumar, S.; Jose, J.; Behl, T.; Mangalathillam, S.; Mathew, B.; Kim, H. Current and Future Nano-Carrier-Based Approaches in the Treatment of Alzheimer’s Disease. Brain Sci. 2023, 13, 213. https://doi.org/10.3390/brainsci13020213
Kumar A, Sudevan ST, Nair AS, Singh AK, Kumar S, Jose J, Behl T, Mangalathillam S, Mathew B, Kim H. Current and Future Nano-Carrier-Based Approaches in the Treatment of Alzheimer’s Disease. Brain Sciences. 2023; 13(2):213. https://doi.org/10.3390/brainsci13020213
Chicago/Turabian StyleKumar, Astik, Sachithra Thazhathuveedu Sudevan, Aathira Sujathan Nair, Ashutosh Kumar Singh, Sunil Kumar, Jobin Jose, Tapan Behl, Sabitha Mangalathillam, Bijo Mathew, and Hoon Kim. 2023. "Current and Future Nano-Carrier-Based Approaches in the Treatment of Alzheimer’s Disease" Brain Sciences 13, no. 2: 213. https://doi.org/10.3390/brainsci13020213
APA StyleKumar, A., Sudevan, S. T., Nair, A. S., Singh, A. K., Kumar, S., Jose, J., Behl, T., Mangalathillam, S., Mathew, B., & Kim, H. (2023). Current and Future Nano-Carrier-Based Approaches in the Treatment of Alzheimer’s Disease. Brain Sciences, 13(2), 213. https://doi.org/10.3390/brainsci13020213