Amyloid-PET and White Matter Hyperintensities Have Independent Effects on Baseline Cognitive Function and Synergistic Effects on Longitudinal Executive Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Imaging Analysis
2.2.1. White Matter Hyperintensity Quantification Method
2.2.2. Calculation of Florbetapir Cortical Summary Values
2.3. Composite Measures of Executive Function and Memory: ADNI_EF and ADNI_Memory
2.4. Statistical Analyses
3. Results
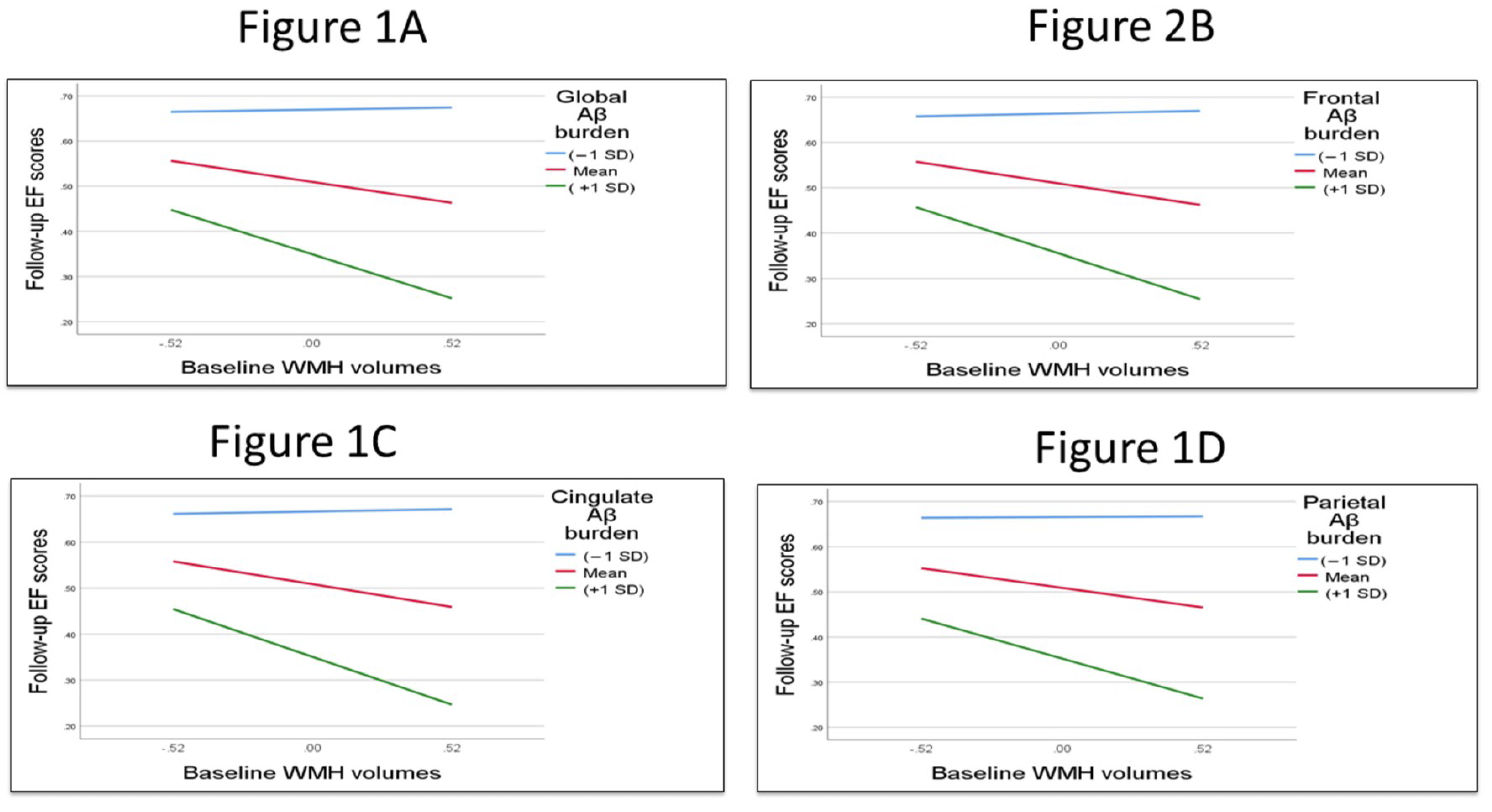
3.1. Testing the Interaction Term and the Main Effect of Global and Regional Aβ Burden and WMHs with Cognition
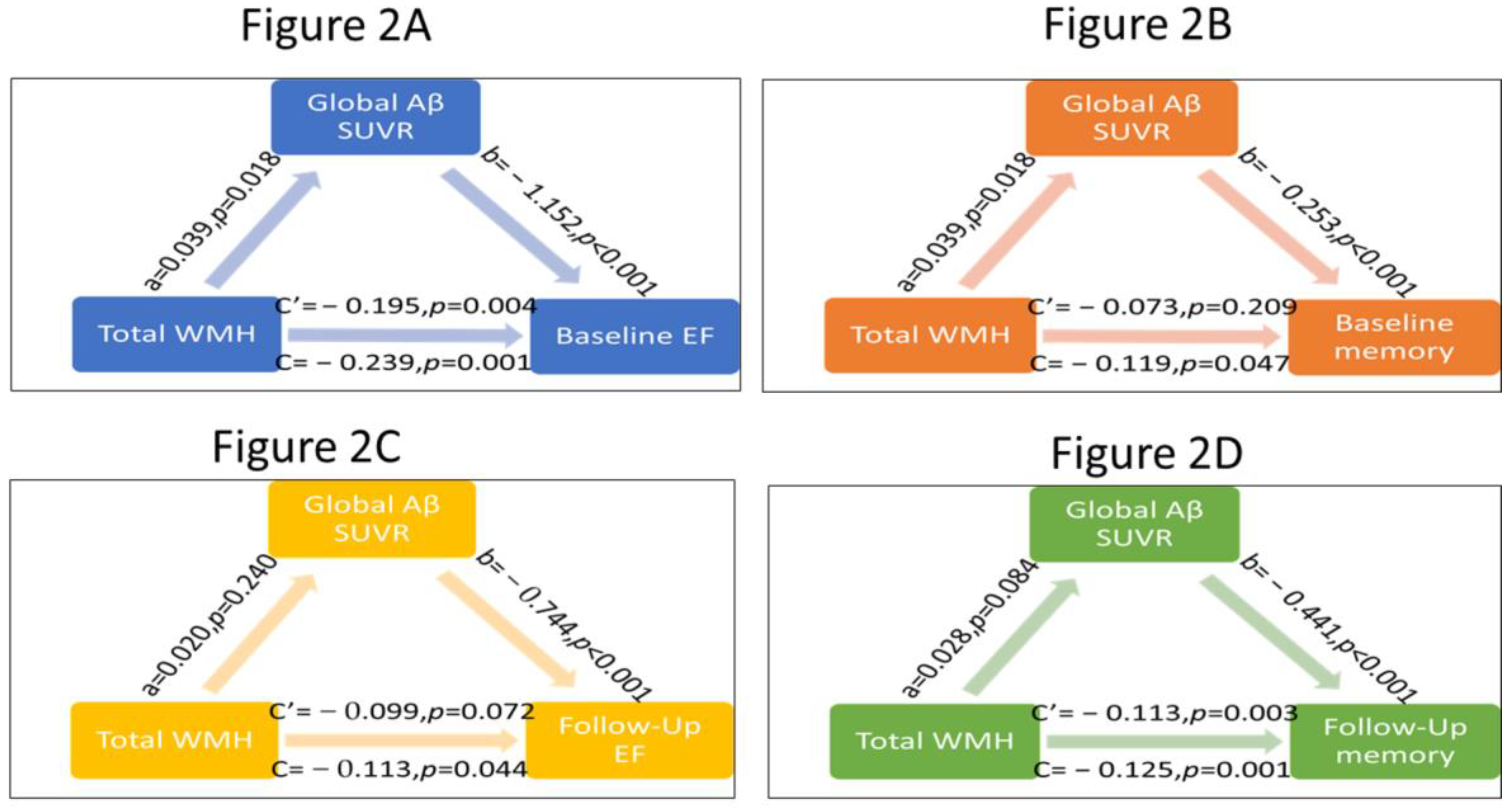
3.2. Testing the Mediation Effects of Global and Regional Aβ Burden on WMH-Related Cognitive Performance
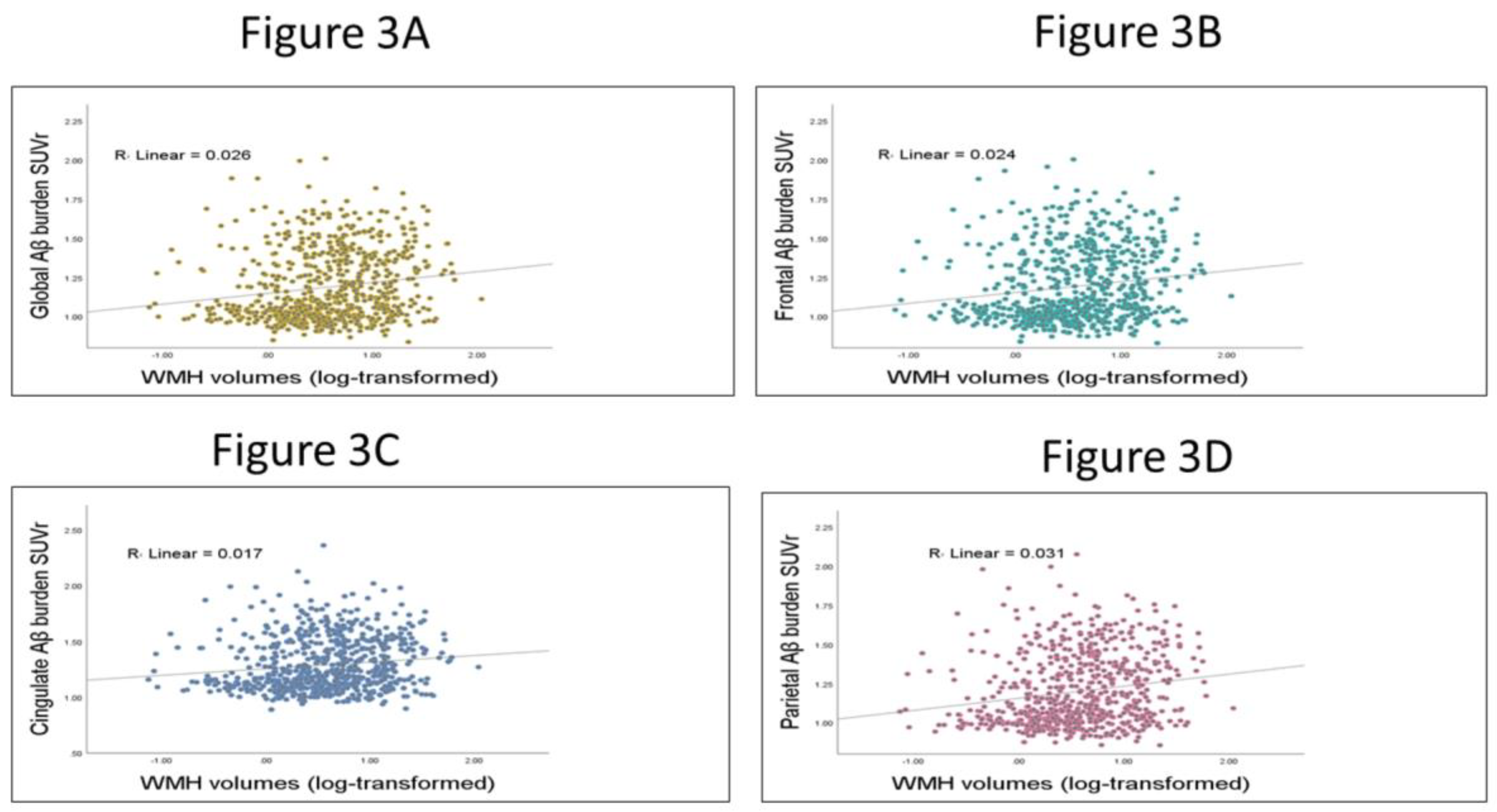
3.3. Testing the Relation between WMHs and Global and Regional Aβ SUVr
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chui, H.C.; Ramirez-Gomez, L. Clinical and imaging features of mixed Alzheimer and vascular pathologies. Alzheimer’s Res. Ther. 2015, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.W.; Hong, J.; Jeon, J. Cerebral small vessel disease and Alzheimer’s disease: A review. Front. Neurol. 2020, 11, 927. [Google Scholar] [CrossRef]
- Sato, N.; Morishita, R. The roles of lipid and glucose metabolism in modulation of β-amyloid, tau, and neurodegeneration in the pathogenesis of Alzheimer disease. Front. Aging Neurosci. 2015, 7, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, J.A.; Arvanitakis, Z.; Bang, W.; Bennett, D.A. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007, 69, 2197–2204. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010, 120, 287–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vemuri, P.; Lesnick, T.G.; Przybelski, S.A.; Knopman, D.S.; Preboske, G.M.; Kantarci, K.; Raman, M.R.; Machulda, M.M.; Mielke, M.M.; Lowe, V.J.; et al. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain 2015, 138, 761–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedden, T.; Mormino, E.C.; Amariglio, R.E.; Younger, A.P.; Schultz, A.; Becker, J.A.; Buckner, R.L.; Johnson, K.A.; Sperling, R.A.; Rentz, D.M. Cognitive profile of amyloid burden and white matter hyperintensities in cognitively normal older adults. J. Neurosci. 2012, 32, 16233–16242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roseborough, A.; Ramirez, J.; Black, S.E.; Edwards, J.D. Associations between amyloid β and white matter hyperintensities: A systematic review. Alzheimer’s Dement. 2017, 13, 1154–1167. [Google Scholar] [CrossRef]
- Marchant, N.L.; Reed, B.R.; DeCarli, C.S.; Madison, C.M.; Weiner, M.W.; Chui, H.C.; Jagust, W.J. Cerebrovascular disease, beta-amyloid, and cognition in aging. Neurobiol. Aging 2012, 33, 1006.e25–1006.e36. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-H.; Seo, S.W.; Kim, C.; Kim, S.H.; Kim, G.H.; Kim, S.T.; Jeon, S.; Lee, J.M.; Oh, S.J.; Kim, J.S.; et al. Effects of cerebrovascular disease and amyloid beta burden on cognition in subjects with subcortical vascular cognitive impairment. Neurobiol. Aging 2014, 35, 254–260. [Google Scholar] [CrossRef]
- Han, J.W.; Maillard, P.; Harvey, D.; Fletcher, E.; Martinez, O.; Johnson, D.K.; Olichney, J.M.; Farias, S.T.; Villeneuve, S.; Jagust, W.; et al. Association of vascular brain injury, neurodegeneration, amyloid, and cognitive trajectory. Neurology 2020, 95, e2622–e2634. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Im, K.; Kwon, H.; Lee, J.-M.; Kim, C.; Kim, Y.J.; Jung, N.-Y.; Cho, H.; Ye, B.S.; Noh, Y.; et al. Clinical effect of white matter network disruption related to amyloid and small vessel disease. Neurology 2015, 85, 63–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupont, P.S.; Bocti, C.; Joannette, M.; Lavallée, M.M.; Nikelski, J.; Vallet, G.T.; Chertkow, H.; Joubert, S. Amyloid burden and white matter hyperintensities mediate age-related cognitive differences. Neurobiol. Aging 2020, 86, 16–26. [Google Scholar]
- Lee, M.J.; Seo, S.W.; Na, D.L.; Kim, C.; Park, J.H.; Kim, G.H.; Kim, C.H.; Noh, Y.; Cho, H.; Kim, H.J.; et al. Synergistic effects of ischemia and β-amyloid burden on cognitive decline in patients with subcortical vascular mild cognitive impairment. JAMA Psychiatry 2014, 71, 412–422. [Google Scholar] [CrossRef]
- Brugulat-Serrat, A.; Salvadó, G.; Sudre, C.H.; Grau-Rivera, O.; Suárez-Calvet, M.; Falcon, C.; Sánchez-Benavides, G.; Gramunt, N.; Fauria, K.; Cardoso, M.J.; et al. Patterns of white matter hyperintensities associated with cognition in middle-aged cognitively healthy individuals. Brain Imaging Behav. 2020, 14, 2012–2023. [Google Scholar] [CrossRef] [Green Version]
- Garnier-Crussard, A.; Bougacha, S.; Wirth, M.; André, C.; Delarue, M.; Landeau, B.; Mézenge, F.; Kuhn, E.; Gonneaud, J.; Chocat, A.; et al. White matter hyperintensities across the adult lifespan: Relation to age, Aβ load, and cognition. Alzheimers Res. Ther. 2020, 12, 127. [Google Scholar] [CrossRef]
- Tullberg, M.; Fletcher, E.; DeCarli, C.; Mungas, D.; Reed, B.R.; Harvey, D.J.; Weiner, M.W.; Chui, H.C.; Jagust, W.J. White matter lesions impair frontal lobe function regardless of their location. Neurology 2004, 63, 246–253. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Knopman, D.S.; Jagust, W.J.; Petersen, R.C.; Weiner, M.W.; Aisen, P.S.; Shaw, L.M.; Vemuri, P.; Wiste, H.J.; Weigand, S.D.; et al. Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013, 12, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Viqar, F.; Zimmerman, M.E.; Narkhede, A.; Tosto, G.; Benzinger, T.L.; Marcus, D.S.; Fagan, A.M.; Goate, A.; Fox, N.C.; et al. White matter hyperintensities are a core feature of Alzheimer’s disease: Evidence from the dominantly inherited Alzheimer network. Ann. Neurol. 2016, 79, 929–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iturria-Medina, Y.I.; Sotero, R.C.; Toussaint, P.J.; Mateos-Pérez, J.M.; Evans, A.C. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat. Commun. 2016, 7, 11934. [Google Scholar] [CrossRef] [Green Version]
- Moscoso, A.; Rey-Bretal, D.; Silva-Rodríguez, J.; Aldrey, J.M.; Cortes, J.; Pias-Peleteiro, J.; Ruibal, A.; Aguiar, P.; Alzheimer’s Disease Neuroimaging Initiative. White matter hyperintensities are associated with subthreshold amyloid accumulation. Neuroimage 2020, 218, 116944. [Google Scholar] [CrossRef]
- Kanaan, N.M.; Pigino, G.F.; Brady, S.T.; Lazarov, O.; Binder, L.I.; Morfini, G.A. Axonal degeneration in Alzheimer’s disease: When signaling abnormalities meet the axonal transport system. Exp. Neurol. 2013, 246, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Salvadores, N.; Gerónimo-Olvera, C.; Court, F.A. Axonal Degeneration in AD: The Contribution of Aβ and Tau. Front. Aging Neurosci. 2020, 12, 581767. [Google Scholar] [CrossRef] [PubMed]
- Lorenzini, L.; Ansems, L.T.; Alves, I.L.; Ingala, S.; García, D.V.; Tomassen, J.; Sudre, C.; Salvadó, G.; Shekari, M.; Operto, G.; et al. Regional associations of white matter hyperintensities and early cortical amyloid pathology. Brain Commun. 2022, 4, fcac150. [Google Scholar] [CrossRef] [PubMed]
- Pålhaugen, L.; Sudre, C.H.; Tecelao, S.; Nakling, A.; Almdahl, I.S.; Kalheim, L.F.; Cardoso, M.J.; Johnsen, S.H.; Rongve, A.; Aarsland, D.; et al. Brain amyloid and vascular risk are related to distinct white matter hyperintensity patterns. J. Cereb. Blood Flow Metab. 2021, 41, 1162–1174. [Google Scholar] [CrossRef] [PubMed]
- Weaver, N.A.; Doeven, T.; Barkhof, F.; Biesbroek, J.M.; Groeneveld, O.N.; Kuijf, H.J.; Prins, N.D.; Scheltens, P.; Teunissen, C.E.; van der Flier, W.M.; et al. Cerebral amyloid burden is associated with white matter hyperintensity location in specific posterior white matter regions. Neurobiol. Aging 2019, 84, 225–234. [Google Scholar] [CrossRef]
- Mueller, S.G.; Weiner, M.W.; Thal, L.J.; Petersen, R.C.; Jack, C.R.; Jagust, W.; Trojanowski, J.Q.; Toga, A.W.; Beckett, L. Ways toward an early diagnosis in Alzheimer’s disease: The Alzheimer’s Disease Neuroimaging Initiative (ADNI). Alzheimer’s Dement. 2005, 1, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Jack, C.R., Jr.; Bernstein, M.A.; Fox, N.C.; Thompson, P.; Alexander, G.; Harvey, D.; Borowski, B.; Britson, P.J.; LWhitwell, J.; Ward, C.; et al. The Alzheimer’s disease neuroimaging initiative (ADNI): MRI methods. J. Magn. Reson. Imaging 2008, 27, 685–691. [Google Scholar] [CrossRef] [Green Version]
- Petersen, R.C.; Aisen, P.S.; Beckett, L.A.; Donohue, M.C.; Gamst, A.C.; Harvey, D.J.; Jack, C.R.; Jagust, W.J.; Shaw, L.M.; Toga, A.W.; et al. Alzheimer’s disease neuroimaging initiative (ADNI): Clinical characterization. Neurology 2010, 74, 201–209. [Google Scholar] [CrossRef] [Green Version]
- DeCarli, C.; Maillard, P.; Fletcher, E. Four Tissue Segmentation in ADNI II. Alzheimer’s Disease Neuroimaging Initiative. Department of Neurology and Neurology and Center for Neuroscience, University of California at Davis. 2013. Available online: https://www.alz.washington.edu/WEB/adni_proto.pdf (accessed on 18 December 2013).
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.; Woolrich, M.W.; Smith, S.M. Fsl. Neuroimage 2012, 62, 782–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.; Johansen-Berg, H.; Bannister, P.R.; De Luca, M.; Drobnjak, I.; Flitney, D.E.; et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004, 23, S208–S219. [Google Scholar] [CrossRef] [PubMed]
- Jagust, W.J.; Landau, S.M.; Koeppe, R.A.; Reiman, E.M.; Chen, K.; Mathis, C.A.; Price, J.C.; Foster, N.L.; Wang, A.Y. The Alzheimer’s disease neuroimaging initiative 2 PET core: 2015. Alzheimer’s Dement. 2015, 11, 757–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischl, B.; Sereno, M.; Dale, A. Cortical surface-based analysis: II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 1999, 9, 195–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischl, B.; Salat, D.H.; Busa, E.; Albert, M.; Dieterich, M.; Haselgrove, C.; van der Kouwe, A.; Killiany, R.; Kennedy, D.; Klaveness, S.; et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 2002, 33, 341–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowrangi, M.A.; Lyketsos, C.; Rao, V.; Munro, C.A. Systematic review of neuroimaging correlates of executive functioning: Converging evidence from different clinical populations. J. Neuropsychiatry Clin. Neurosci. 2014, 26, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Cordón, A.; Monté-Rubio, G.; Sanabria, A.; Rodriguez-Gomez, O.; Valero, S.; Abdelnour, C.; Marquié, M.; Espinosa, A.; Ortega, G.; Hernandez, I.; et al. Subtle executive deficits are associated with higher brain amyloid burden and lower cortical volume in subjective cognitive decline: The FACEHBI cohort. Sci. Rep. 2020, 10, 17721. [Google Scholar] [CrossRef]
- Ranasinghe, K.G.; Hinkley, L.B.; Beagle, A.J.; Mizuiri, D.; Dowling, A.F.; Honma, S.M.; Finucane, M.M.; Scherling, C.; Miller, B.L.; Nagarajan, S.S.; et al. Regional functional connectivity predicts distinct cognitive impairments in Alzheimer’s disease spectrum. Neuroimage Clin. 2014, 5, 385–395. [Google Scholar] [CrossRef] [Green Version]
- Ali, D.G.; Bahrani, A.A.; Barber, J.M.; El Khouli, R.H.; Gold, B.T.; Harp, J.P.; Jiang, Y.; Wilcock, D.M.; Jicha, G.A. Amyloid-PET Levels in the Precuneus and Posterior Cingulate Cortices Are Associated with Executive Function Scores in Preclinical Alzheimer’s Disease Prior to Overt Global Amyloid Positivity. J. Alzheimer’s Dis. 2022, 88, 1127–1135. [Google Scholar] [CrossRef]
- Gibbons, L.E.; Carle, A.C.; Mackin, R.S.; Mukherjee, S.; Insel, P.; Curtis, S.M. Composite measures of executive function and memory: ADNI_EF and ADNI_Mem. Alzheimer’s Dis. Neuroimaging Initiat. 2012, 6, 517–527. [Google Scholar]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach; Guilford Publications: New York, NY, USA, 2017. [Google Scholar]
- Marchant, N.L.; Reed, B.R.; Sanossian, N.; Madison, C.M.; Kriger, S.; Dhada, R.; Mack, W.J.; DeCarli, C.; Weiner, M.W.; Mungas, D.M.; et al. The aging brain and cognition: Contribution of vascular injury and aβ to mild cognitive dysfunction. JAMA Neurol. 2013, 70, 488–495. [Google Scholar] [CrossRef] [Green Version]
- Carmichael, O.; Mungas, D.; Beckett, L.; Harvey, D.; Farias, S.T.; Reed, B.; Olichney, J.; Miller, J.; DeCarli, C. MRI predictors of cognitive change in a diverse and carefully characterized elderly population. Neurobiol. Aging 2012, 33, 83–95.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.; Ly, M.; Karim, H.T.; Wei, W.; Snitz, B.E.; Klunk, W.E.; Aizenstein, H.J. The effect of amyloid deposition on longitudinal resting-state functional connectivity in cognitively normal older adults. Alzheimers Res. Ther. 2020, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Williams, V.J.; Stephens, K.A.; Kim, C.; Bai, L.; Zhang, M.; Salat, D.H. The effect of white matter signal abnormalities on default mode network connectivity in mild cognitive impairment. Hum. Brain Mapp. 2020, 41, 1237–1248. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Vipin, A.; Wong, B.; Ng, K.P.; Kandiah, N. Differential Effects of Confluent and Nonconfluent White Matter Hyperintensities on Functional Connectivity in Mild Cognitive Impairment. Brain Connect. 2020, 10, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Maillard, P.; Carmichael, O.; Fletcher, E.; Reed, B.; Mungas, D.; DeCarli, C. Coevolution of white matter hyperintensities and cognition in the elderly. Neurology 2012, 79, 442–448. [Google Scholar] [CrossRef] [Green Version]
- Carey, C.L.; Kramer, J.H.; Josephson, S.A.; Mungas, D.; Reed, B.R.; Schuff, N.; Weiner, M.W.; Chui, H.C. Subcortical lacunes are associated with executive dysfunction in cognitively normal elderly. Stroke 2008, 39, 397–402. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Yatawara, C.; Wang, B.; Wong, B.; Tan, Y.J.; Zailan, F.Z.; Ng, K.P.; Kandiah, N. APOE4 and Confluent White Matter Hyperintensities Have a Synergistic Effect on Episodic Memory Impairment in Prodromal Dementia. J. Alzheimer’s Dis. 2022, 87, 1103–1114. [Google Scholar] [CrossRef]
- Ottoy, J.; Ozzoude, M.; Zukotynski, K.; Adamo, S.; Scott, C.; Gaudet, V.; Ramirez, J.; Swardfager, W.; Cogo-Moreira, H.; Lam, B.; et al. Vascular burden and cognition: Mediating roles of neurodegeneration and amyloid PET. Alzheimers Dement. 2022. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef]
- Park, M.; Moon, Y.; Han, S.-H.; Kim, H.K.; Moon, W.-J. Myelin loss in white matter hyperintensities and normal-appearing white matter of cognitively impaired patients: A quantitative synthetic magnetic resonance imaging study. Eur. Radiol. 2019, 29, 4914–4921. [Google Scholar] [CrossRef]
- Bartzokis, G. Alzheimer’s disease as homeostatic responses to age-related myelin breakdown. Neurobiol. Aging 2011, 32, 1341–1371. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Range | Mean (SD) | Sample Size |
|---|---|---|---|
| Age (years) | 55–96 | 73.13 (7.41) | 714 |
| Education (years) | 10–20 | 16.31 (2.6) | 714 |
| Global Aβ burden (SUVr) | 0.84–2.01 | 1.19 (0.22) | 714 |
| Frontal Aβ burden (SUVr) | 0.83–2.01 | 1.19 (0.23) | 714 |
| Cingulate Aβ burden (SUVr) | 0.89–2.36 | 1.29 (0.22) | 714 |
| Parietal Aβ burden (SUVr) | 0.87–2.08 | 1.20 (0.22) | 714 |
| WMH volumes in cubic centimeters | 0.07–61.02 | 6.94 (8.54) | 714 |
| WMH volumes (log-transformed) | −1.13 to 1.79 | 0.57 (0.52) | 714 |
| Total intracranial volumes | 1084.29 to 1861.82 | 411.88 (135.97) | 714 |
| ADNI-EF (baseline) | −3.01 to 2.99 | 0.54 (0.94) | 714 |
| ADNI-EF (follow-up) | −3.01 to 2.99 | 0.50 (1.06) | 661 |
| ADNI-memory (baseline) | −2.8 to 3.14 | 0.56 (0.81) | 714 |
| ADNI-memory (follow-up) | −2.62 to 3.06 | 0.52 (0.94) | 661 |
| Time between test (years) | 1 to 3 | 1.86 (0.49) | 661 |
| APOE e4 allele N(%) copy 1 | 2 to 4 | 58 (8.1) | 714 |
| APOE e4 allele N(%) copy 2 | 3 to 4 | 300 (42.1) | 714 |
| Sex male N (%) | 388 (54.3) | 714 |
| Path (a) Coefficient β (p-Value) | Path (b) Coefficient β (p-Value) | Direct Effect of WMH Path (c’) Coefficient β (p-Value) | Total Effect Path (c) Coefficient β (p-Value) | CI for Indirect Effect | ||
|---|---|---|---|---|---|---|
| Cognitive Function | Aβ Burden | |||||
| Baseline EF | Global Aβ SUVr | 0.039 (0.018) | −1.152 (<0.001) | −0.195 (0.004) | −0.239 (0.001) | −0.09 and −0.01 |
| Frontal Aβ SUVr | 0.037 (0.032) | −1.093 (<0.001) | −0.199 (0.003) | −0.239 (0.001) | −0.008 and −0.005 | |
| Cingulate Aβ SUVr | 0.031 (0.085) | −0.979 (<0.001) | −0.216 (0.002) | −0.239 (0.001) | −0.057 and 0.02 | |
| Parietal Aβ SUVr | 0.052 (0.007) | −1.114 (<0.001) | −0.182 (0.007) | −0.239 (0.001) | −0.11 and −0.02 | |
| Baseline memory | Global Aβ SUVr | 0.039 (0.018) | −1.253 (<0.001) | −0.073 (0.209) | −0.119 (0.047) | −0.01 and −0.006 |
| Frontal Aβ SUVr | 0.037 (0.033) | −1.148 (<0.001) | −0.077 (0.178) | −0.119 (0.047) | −0.11 and −0.01 | |
| Cingulate Aβ SUVr | 0.03 (0.085) | −1.013 (0.001) | −0.089 (0.121) | −0.119 (0.047) | −0.07 and 0.004 | |
| Parietal Aβ SUVr | 0.052 (0.002) | −1.174 (<0.001) | −0.059 (0.302) | −0.119 (0.047) | −0.11 and −0.02 | |
| Follow-up EF scores | Global Aβ SUVr | 0.02 (0.24) | −0.744 (<0.001) | −0.099 (0.072) | −0.113 (0.044) | −0.045 and 0.009 |
| Frontal Aβ SUVr | 0.018 (0.314) | −0.689 (<0.001) | −0.102 (0.061) | −0.113 (0.044) | −0.045 and 0.009 | |
| Cingulate Aβ SUVr | 0.016 (0.385) | −0.685 (<0.001) | −0.101 (0.065) | −0.113 (0.044) | −0.04 and 0.014 | |
| Parietal Aβ SUVr | 0.029 (0.088) | −0.717 (<0.001) | −0.092 (0.093) | −0.113 (0.044) | −0.05 and 0.01 | |
| Follow-up memory scores | Global Aβ SUVr | 0.028 (0.084) | −0.441 (<0.001) | −0.113 (0.003) | −0.125 (0.001) | −0.031 and 0.002 |
| Frontal Aβ SUVr | 0.026 (0.127) | −0.418 (<0.001) | −0.114 (0.004) | −0.125 (0.001) | −0.028 and 0.04 | |
| Cingulate Aβ SUVr | 0.021 (0.238) | −0.373 (<0.001) | −0.117 (0.002) | −0.125 (0.001) | −0.024 and 0.05 | |
| Parietal Aβ SUVr | 0.039 (0.019) | −0.408 (<0.001) | −0.109 (0.004) | −0.125 (0.001) | −0.035 and 0.002 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, D.G.; Abner, E.L.; Bahrani, A.A.; El Khouli, R.; Gold, B.T.; Jiang, Y.; Wilcock, D.M.; Jicha, G.A. Amyloid-PET and White Matter Hyperintensities Have Independent Effects on Baseline Cognitive Function and Synergistic Effects on Longitudinal Executive Function. Brain Sci. 2023, 13, 218. https://doi.org/10.3390/brainsci13020218
Ali DG, Abner EL, Bahrani AA, El Khouli R, Gold BT, Jiang Y, Wilcock DM, Jicha GA. Amyloid-PET and White Matter Hyperintensities Have Independent Effects on Baseline Cognitive Function and Synergistic Effects on Longitudinal Executive Function. Brain Sciences. 2023; 13(2):218. https://doi.org/10.3390/brainsci13020218
Chicago/Turabian StyleAli, Doaa G., Erin L. Abner, Ahmed A. Bahrani, Riham El Khouli, Brian T. Gold, Yang Jiang, Donna M. Wilcock, and Gregory A. Jicha. 2023. "Amyloid-PET and White Matter Hyperintensities Have Independent Effects on Baseline Cognitive Function and Synergistic Effects on Longitudinal Executive Function" Brain Sciences 13, no. 2: 218. https://doi.org/10.3390/brainsci13020218
APA StyleAli, D. G., Abner, E. L., Bahrani, A. A., El Khouli, R., Gold, B. T., Jiang, Y., Wilcock, D. M., & Jicha, G. A. (2023). Amyloid-PET and White Matter Hyperintensities Have Independent Effects on Baseline Cognitive Function and Synergistic Effects on Longitudinal Executive Function. Brain Sciences, 13(2), 218. https://doi.org/10.3390/brainsci13020218





