Left Prefrontal tDCS during Learning Does Not Enhance Subsequent Verbal Episodic Memory in Young Adults: Results from Two Double-Blind and Sham-Controlled Experiments
Abstract
:1. Introduction
2. Experiment 1: Materials and Methods
2.1. Participants
2.1.1. Sample Size
2.1.2. Demographic Characteristics
2.2. Materials
2.2.1. Questionnaires
2.2.2. Experimental Stimuli
2.2.3. Brain Stimulator
2.3. Procedure
2.3.1. Electrode Setup
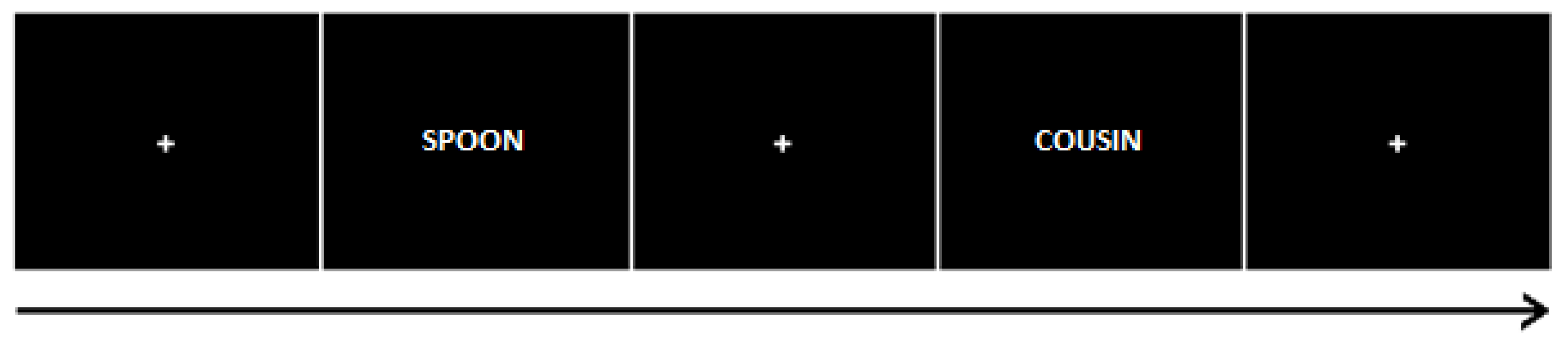
2.3.2. Incidental Learning Task
2.3.3. Recognition Task, Day 1
2.3.4. Recognition Task, Day 2
2.4. Data Analysis
3. Experiment 1: Results
3.1. Memory Performance Measure
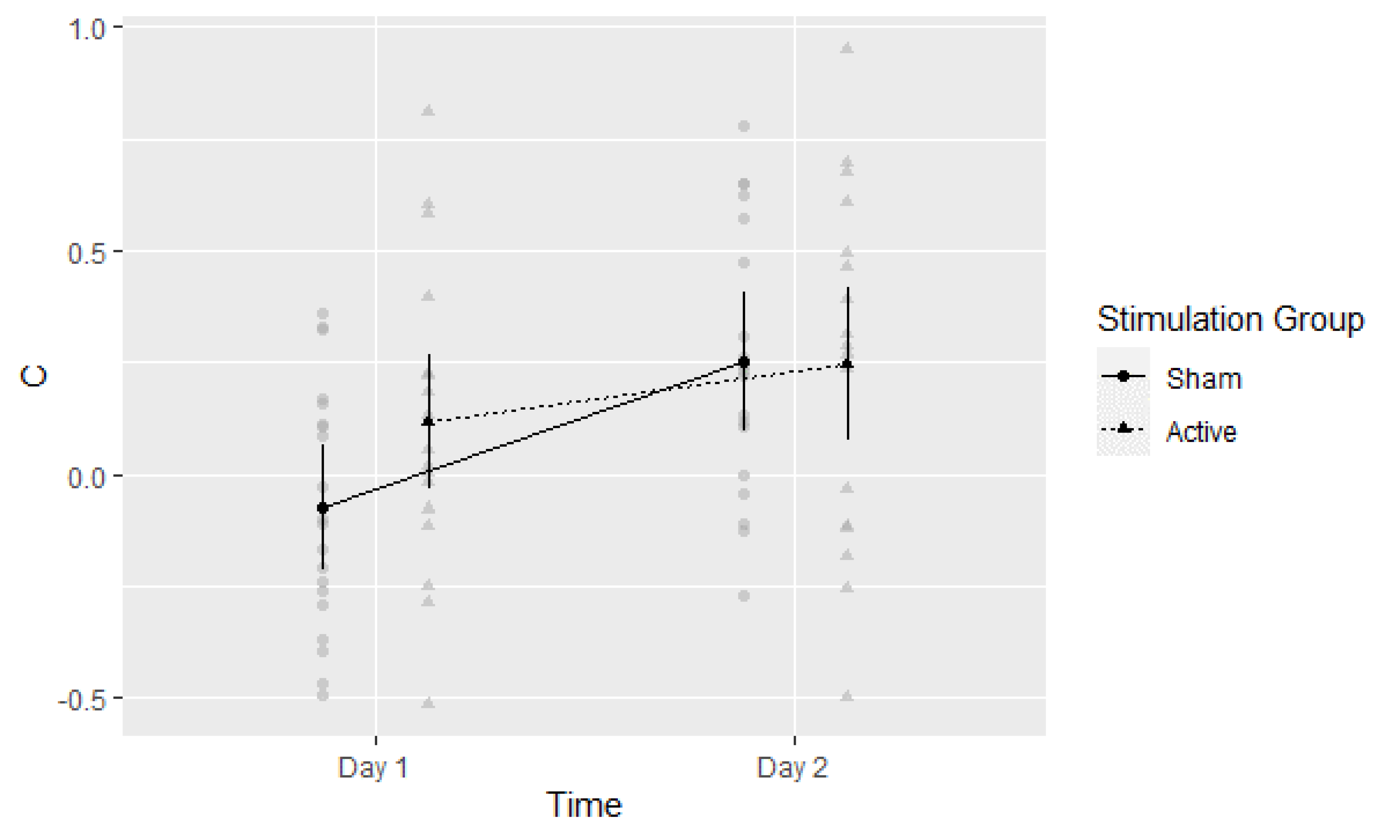
3.2. D-Prime (d′)
3.3. Hit Rates (HR)
3.4. False Alarms (FA)
3.5. Post-Hoc Exploratory Analysis of Signal Detection Threshold (c)
3.6. Survey of Sensations Related to Transcranial Electrical Stimulation Results
4. Experiment 2: Materials and Methods
4.1. Participants
4.1.1. Sample Size Calculation
4.1.2. Demographic Statistics
4.1.3. Randomisation Procedure
4.2. Materials
4.2.1. Questionnaires
4.2.2. Word Lists
4.2.3. Stimulation Equipment and Parameters
4.3. Procedure
4.3.1. Consent and Screening
4.3.2. Electrode Setup
4.3.3. Procedure Overview
4.3.4. Intentional Learning Task
4.3.5. Resting Period
4.3.6. Recall Task, Day 1 and Day 2
4.3.7. Associative Recall Task, Day 1 and Day 2
4.3.8. Word Recognition Task, Day 2
4.3.9. Colour Recognition Task, Day 2
4.3.10. Debrief
5. Experiment 2: Results
5.1. Memory Performance Measure
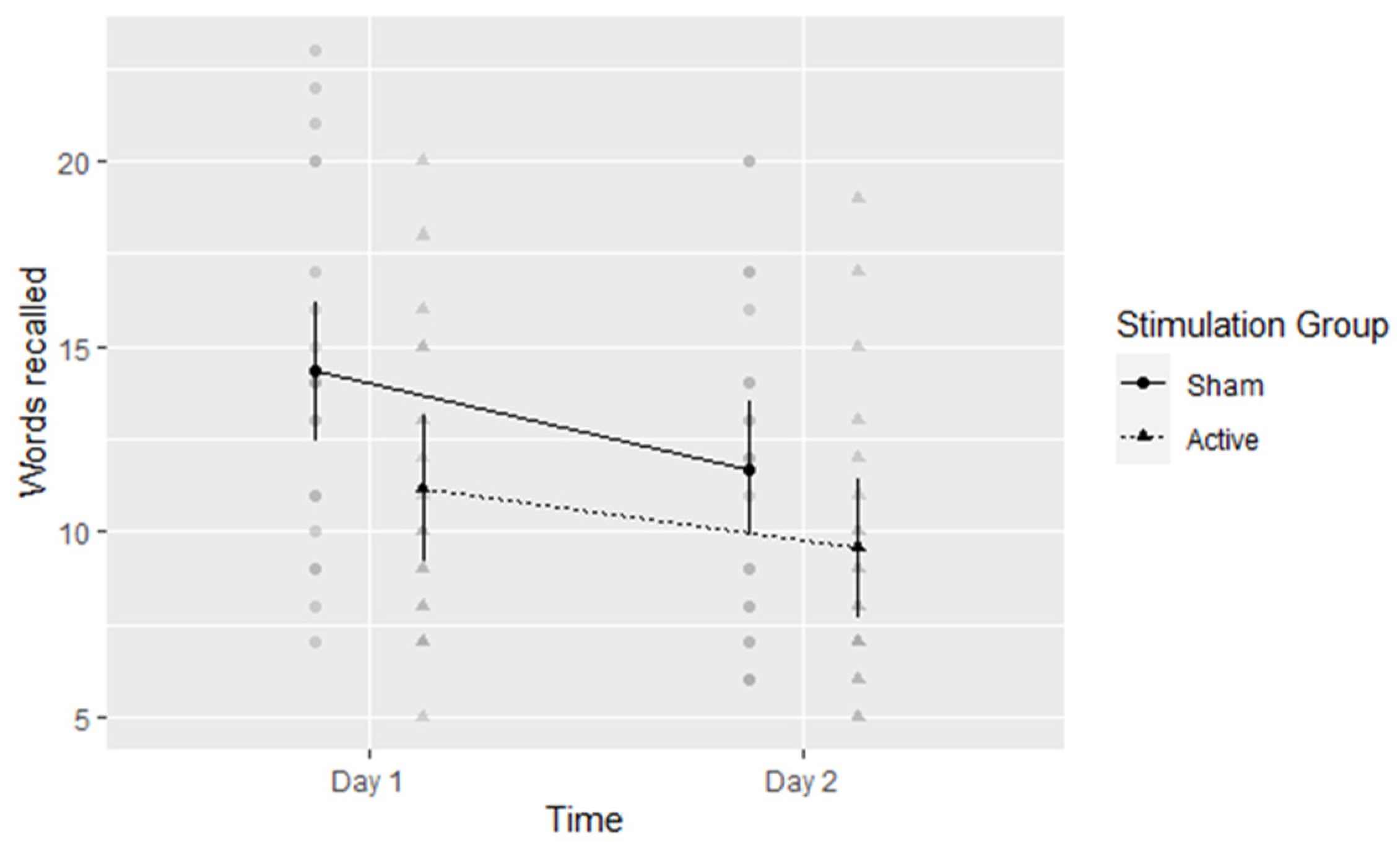
5.2. Verbal Free Recall
5.3. Word Recognition
5.4. Associative Colour Recognition
5.5. Post-Hoc Exploratory Analysis of Signal Detection Threshold (c)
5.6. Cumulative Effect Size
6. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F.; et al. Transcranial direct current stimulation: State of the art 2008. Brain. Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef]
- Dayan, E.; Censor, N.; Buch, E.R.; Sandrini, M.; Cohen, L.G. Noninvasive brain stimulation: From physiology to network dynamics and back. Nat. Neurosci. 2013, 16, 838–844. [Google Scholar] [CrossRef] [Green Version]
- Javadi, A.H.; Walsh, V. Transcranial direct current stimulation (tDCS) of the left dorsolateral prefrontal cortex modulates declarative memory. Brain Stimul. 2012, 5, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Javadi, A.H.; Cheng, P.; Walsh, V. Short duration transcranial direct current stimulation (tDCS) modulates verbal memory. Brain Stimul. 2012, 5, 468–474. [Google Scholar] [CrossRef]
- Pisoni, A.; Turi, Z.; Raithel, A.; Ambrus, G.G.; Alekseichuk, I.; Schacht, A.; Paulus, W.; Antal, A. Separating recognition processes of declarative memory via anodal tDCS: Boosting old item recognition by temporal and new item detection by parietal stimulation. PLoS ONE 2015, 10, e0123085. [Google Scholar] [CrossRef] [Green Version]
- Sandrini, M.; Manenti, R.; Brambilla, M.; Cobelli, C.; Cohen, L.G.; Cotelli, M. Older adults get episodic memory boosting from noninvasive stimulation of prefrontal cortex during learning. Neurobiol. Aging 2016, 39, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Medvedeva, A.; Materassi, M.; Neacsu, V.; Beresford-Webb, J.; Hussin, A.; Khan, N.; Newton, F.; Galli, G. Effects of Anodal Transcranial Direct Current Stimulation Over the Ventrolateral Prefrontal Cortex on Episodic Memory Formation and Retrieval. Cereb. Cortex. 2019, 29, 657–665. [Google Scholar] [CrossRef]
- Rossi, S.; Cappa, S.F.; Babiloni, C.; Pasqualetti, P.; Miniussi, C.; Carducci, F.; Babiloni, F.; Rossini, P.M. Prefrontal [correction of Prefontal] cortex in long-term memory: An "interference" approach using magnetic stimulation. Nat. Neurosci. 2001, 4, 948–952. [Google Scholar] [CrossRef]
- Sandrini, M.; Cappa, S.F.; Rossi, S.; Rossini, P.M.; Miniussi, C. The role of prefrontal cortex in verbal episodic memory: rTMS evidence. J. Cogn. Neurosci. 2003, 15, 855–861. [Google Scholar] [CrossRef] [Green Version]
- Spaniol, J.; Davidson, P.S.; Kim, A.S.; Han, H.; Moscovitch, M.; Grady, C.L. Event-related fMRI studies of episodic encoding and retrieval: Meta-analyses using activation likelihood estimation. Neuropsychologia 2009, 47, 1765–1779. [Google Scholar] [CrossRef]
- Manuel, A.L.; Schnider, A. Differential processing of immediately repeated verbal and non-verbal stimuli: An evoked-potential study. Eur. J. Neurosci. 2016, 43, 89–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, D.M.; Swets, J.A. Signal Detection Theory and Psychophysics; Wiley: New York, NY, USA, 1966. [Google Scholar]
- Leshikar, E.D.; Leach, R.C.; McCurdy, M.P.; Trumbo, M.C.; Sklenar, A.M.; Frankenstein, A.N.; Matzen, L.E. Transcranial direct current stimulation of dorsolateral prefrontal cortex during encoding improves recall but not recognition memory. Neuropsychologia 2017, 106, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Sandrini, M.; Cohen, L.G. Noninvasive brain stimulation in neurorehabilitation. Handb. Clin. Neurol. 2013, 116, 499–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolin, S.; Loo, C.K.; Bai, S.; Dokos, S.; Martin, D.M. Focalised stimulation using high definition transcranial direct current stimulation (HD-tDCS) to investigate declarative verbal learning and memory functioning. Neuroimage 2015, 117, 11–19. [Google Scholar] [CrossRef]
- Habich, A.; Kloppel, S.; Abdulkadir, A.; Scheller, E.; Nissen, C.; Peter, J. Anodal tDCS Enhances Verbal Episodic Memory in Initially Low Performers. Front. Hum. Neurosci. 2017, 11, 542. [Google Scholar] [CrossRef]
- Brunye, T.T.; Smith, A.M.; Horner, C.B.; Thomas, A.K. Verbal long-term memory is enhanced by retrieval practice but impaired by prefrontal direct current stimulation. Brain. Cogn. 2018, 128, 80–88. [Google Scholar] [CrossRef]
- Dedoncker, J.; Brunoni, A.R.; Baeken, C.; Vanderhasselt, M.A. A Systematic Review and Meta-Analysis of the Effects of Transcranial Direct Current Stimulation (tDCS) Over the Dorsolateral Prefrontal Cortex in Healthy and Neuropsychiatric Samples: Influence of Stimulation Parameters. Brain. Stimul. 2016, 9, 501–517. [Google Scholar] [CrossRef] [Green Version]
- Summers, J.J.; Kang, N.; Cauraugh, J.H. Does transcranial direct current stimulation enhance cognitive and motor functions in the ageing brain? A systematic review and meta-analysis. Ageing Res. Rev. 2016, 25, 42–54. [Google Scholar] [CrossRef]
- Huo, L.; Zhu, X.; Zheng, Z.; Ma, J.; Ma, Z.; Gui, W.; Li, J. Effects of Transcranial Direct Current Stimulation on Episodic Memory in Older Adults: A Meta-analysis. J. Gerontol. B Psychol. Sci. Soc. Sci. 2021, 76, 692–702. [Google Scholar] [CrossRef]
- Bartl, G.J.; Blackshaw, E.; Crossman, M.; Allen, P.; Sandrini, M. Systematic review and network meta-analysis of anodal tDCS effects on verbal episodic memory: Modeling heterogeneity of stimulation locations. Z. für Psychol. 2020, 228, 3. [Google Scholar] [CrossRef]
- Hill, A.T.; Fitzgerald, P.B.; Hoy, K.E. Effects of Anodal Transcranial Direct Current Stimulation on Working Memory: A Systematic Review and Meta-Analysis of Findings From Healthy and Neuropsychiatric Populations. Brain Stimul. 2016, 9, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Horvath, J.C.; Forte, J.D.; Carter, O. Quantitative Review Finds No Evidence of Cognitive Effects in Healthy Populations From Single-session Transcranial Direct Current Stimulation (tDCS). Brain Stimul. 2015, 8, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527 Pt 3, 633. [Google Scholar] [CrossRef]
- Sandrini, M.; Brambilla, M.; Manenti, R.; Rosini, S.; Cohen, L.G.; Cotelli, M. Noninvasive stimulation of prefrontal cortex strengthens existing episodic memories and reduces forgetting in the elderly. Front. Aging Neurosci. 2014, 6, 289. [Google Scholar] [CrossRef]
- Sandrini, M.; Manenti, R.; Gobbi, E.; Rusich, D.; Bartl, G.J.; Cotelli, M. Transcranial direct current stimulation applied after encoding facilitates episodic memory consolidation in older adults. Neurobiol. Learn. Mem. 2019, 163, 107037. [Google Scholar] [CrossRef]
- Peña-Gómez, C.; Sala-Lonch, R.; Junqué, C.; Clemente, I.C.; Vidal, D.; Bargalló, N.; Falcón, C.; Valls-Solé, J.; Pascual-Leone, Á.; Bartrés-Faz, D. Modulation of large-scale brain networks by transcranial direct current stimulation evidenced by resting-state functional MRI. Brain Stimul. 2012, 5, 252–263. [Google Scholar] [CrossRef] [Green Version]
- Weber, M.J.; Messing, S.B.; Rao, H.; Detre, J.A.; Thompson-Schill, S.L. Prefrontal transcranial direct current stimulation alters activation and connectivity in cortical and subcortical reward systems: A tDCS-fMRI study. Hum. Brain Mapp. 2014, 35, 3673–3686. [Google Scholar] [CrossRef] [Green Version]
- Keeser, D.; Meindl, T.; Bor, J.; Palm, U.; Pogarell, O.; Mulert, C.; Brunelin, J.; Möller, H.-J.; Reiser, M.; Padberg, F. Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. J. Neurosci. 2011, 31, 15284–15293. [Google Scholar] [CrossRef]
- Krause, M.R.; Zanos, T.P.; Csorba, B.A.; Pilly, P.K.; Choe, J.; Phillips, M.E.; Datta, A.; Pack, C.C. Transcranial direct current stimulation facilitates associative learning and alters functional connectivity in the primate brain. Curr. Biol. 2017, 27, 3086–3096. e3083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammann, C.; Lindquist, M.A.; Celnik, P.A. Response variability of different anodal transcranial direct current stimulation intensities across multiple sessions. Brain Stimul. 2017, 10, 757–763. [Google Scholar] [CrossRef]
- Shinde, A.B.; Lerud, K.D.; Munsch, F.; Alsop, D.C.; Schlaug, G. Effects of tDCS dose and electrode montage on regional cerebral blood flow and motor behavior. Neuroimage 2021, 237, 118144. [Google Scholar] [CrossRef] [PubMed]
- Jamil, A.; Batsikadze, G.; Kuo, H.-I.; Labruna, L.; Hasan, A.; Paulus, W.; Nitsche, M.A. Systematic evaluation of the impact of stimulation intensity on neuroplastic after-effects induced by transcranial direct current stimulation. J. Physiol. 2017, 595, 1273–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esmaeilpour, Z.; Marangolo, P.; Hampstead, B.M.; Bestmann, S.; Galletta, E.; Knotkova, H.; Bikson, M. Incomplete evidence that increasing current intensity of tDCS boosts outcomes. Brain Stimul. 2018, 11, 310–321. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods. 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Keel, J.C.; Smith, M.J.; Wassermann, E.M. A safety screening questionnaire for transcranial magnetic stimulation. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2001, 112, 720. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Fertonani, A.; Ferrari, C.; Miniussi, C. What do you feel if I apply transcranial electric stimulation? Safety, sensations and secondary induced effects. Clin. Neurophysiol. 2015, 126, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Coltheart, M. The MRC psycholinguistic database. Q. J. Exp. Psychol. Sect. A 1981, 33, 497–505. [Google Scholar] [CrossRef]
- Wilson, M. MRC psycholinguistic database: Machine-usable dictionary, version 2.00. Behav. Res. Methods Instrum. Comput. 1988, 20, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Bikson, M.; Grossman, P.; Thomas, C.; Zannou, A.L.; Jiang, J.; Adnan, T.; Mourdoukoutas, A.P.; Kronberg, G.; Truong, D.; Boggio, P.; et al. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimul. 2016, 9, 641–661. [Google Scholar] [CrossRef]
- Klem, G.H.; Luders, H.O.; Jasper, H.H.; Elger, C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 3–6. [Google Scholar] [PubMed]
- Herwig, U.; Satrapi, P.; Schonfeldt-Lecuona, C. Using the international 10-20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr. 2003, 16, 95–99. [Google Scholar] [CrossRef] [PubMed]
- R Foundation for Statistical Computing. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- afex: Analysis of Factorial Experiments. 2022. Available online: https://CRAN.R-project.org/package=afex (accessed on 30 January 2023).
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef] [Green Version]
- Stanislaw, H.; Todorov, N. Calculation of signal detection theory measures. Behav. Res. Methods Instrum. Comput. 1999, 31, 137–149. [Google Scholar] [CrossRef] [Green Version]
- Bang, H.; Flaherty, S.P.; Kolahi, J.; Park, J. Blinding assessment in clinical trials: A review of statistical methods and a proposal of blinding assessment protocol. Clin. Res. Regul. Aff. 2010, 27, 42–51. [Google Scholar] [CrossRef]
- Bang, H.; Ni, L.; Davis, C.E. Assessment of blinding in clinical trials. Control Clin. Trials 2004, 25, 143–156. [Google Scholar] [CrossRef]
- Galli, G.; Vadillo, M.A.; Sirota, M.; Feurra, M.; Medvedeva, A. A systematic review and meta-analysis of the effects of transcranial direct current stimulation (tDCS) on episodic memory. Brain Stimul. 2019, 12, 231–241. [Google Scholar] [CrossRef]
- Voroslakos, M.; Takeuchi, Y.; Brinyiczki, K.; Zombori, T.; Oliva, A.; Fernandez-Ruiz, A.; Kozak, G.; Kincses, Z.T.; Ivanyi, B.; Buzsaki, G.; et al. Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nat. Commun. 2018, 9, 483. [Google Scholar] [CrossRef] [Green Version]
- Jog, M.; Smith, R.X.; Jann, K.; Dunn, W.; Lafon, B.; Truong, D.; Wu, A.; Parra, L.; Bikson, M.; Wang, D.J. In-vivo imaging of magnetic fields induced by transcranial direct current stimulation (tDCS) in human brain using MRI. Sci. Rep. 2016, 6, 34385. [Google Scholar] [CrossRef]
- Villamar, M.F.; Volz, M.S.; Bikson, M.; Datta, A.; DaSilva, A.F.; Fregni, F. Technique and considerations in the use of 4x1 ring high-definition transcranial direct current stimulation (HD-tDCS). JoVE (J. Vis. Exp.) 2013, 77, e50309. [Google Scholar]
- Kim, J.; Kim, H.; Jeong, H.; Roh, D.; Kim, D.H. tACS as a promising therapeutic option for improving cognitive function in mild cognitive impairment: A direct comparison between tACS and tDCS. J. Psychiatr. Res. 2021, 141, 248–256. [Google Scholar] [CrossRef]
- Grover, S.; Wen, W.; Viswanathan, V.; Gill, C.T.; Reinhart, R.M.G. Long-lasting, dissociable improvements in working memory and long-term memory in older adults with repetitive neuromodulation. Nat. Neurosci. 2022, 25, 1237–1246. [Google Scholar] [CrossRef]
- Minarik, T.; Berger, B.; Althaus, L.; Bader, V.; Biebl, B.; Brotzeller, F.; Fusban, T.; Hegemann, J.; Jesteadt, L.; Kalweit, L. The importance of sample size for reproducibility of tDCS effects. Front. Hum. Neurosci. 2016, 10, 453. [Google Scholar] [CrossRef] [Green Version]
- Heroux, M.E.; Loo, C.K.; Taylor, J.L.; Gandevia, S.C. Questionable science and reproducibility in electrical brain stimulation research. PLoS ONE 2017, 12, e0175635. [Google Scholar] [CrossRef] [Green Version]
- Szucs, D.; Ioannidis, J.P.A. Empirical assessment of published effect sizes and power in the recent cognitive neuroscience and psychology literature. PLoS Biol. 2017, 15, e2000797. [Google Scholar] [CrossRef] [Green Version]
- Petrovskaya, A.; Kirillov, B.; Asmolova, A.; Galli, G.; Feurra, M.; Medvedeva, A. Examining the effects of transcranial direct current stimulation on human episodic memory with machine learning. PLoS ONE 2020, 15, e0235179. [Google Scholar] [CrossRef]
- Habich, A.; Slotboom, J.; Peter, J.; Wiest, R.; Kloppel, S. No Effect of Anodal tDCS on Verbal Episodic Memory Performance and Neurotransmitter Levels in Young and Elderly Participants. Neural. Plast. 2020, 2020, 8896791. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.D. Registered reports: A new publishing initiative at Cortex. Cortex 2013, 49, 609–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmons, P.; Nelson, J.D.; Simonsohn, U. Pre-registration: Why and how. J. Consum. Psychol. 2021, 31, 151–162. [Google Scholar] [CrossRef]
- Yarkoni, T.; Poldrack, R.A.; Van Essen, D.C.; Wager, T.D. Cognitive neuroscience 2.0: Building a cumulative science of human brain function. Trends Cogn. Sci. 2010, 14, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, S.; Fishman, J.R.; McGowan, M.L.; Juengst, E.T. Big data, open science and the brain: Lessons learned from genomics. Front. Hum. Neurosci. 2014, 8, 239. [Google Scholar] [CrossRef] [PubMed]




| Allocation Guess | Active Group | Sham Group |
|---|---|---|
| ‘Active’ | 10 | 8 |
| ‘Sham’ | 5 | 5 |
| ‘Don’t know’ | 0 | 1 |
| BBI [95% CI] | 0.333 [0.192 to 0.474] | 0.214 [0.064 to 0.365] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartl, G.; Allen, P.; Sandrini, M. Left Prefrontal tDCS during Learning Does Not Enhance Subsequent Verbal Episodic Memory in Young Adults: Results from Two Double-Blind and Sham-Controlled Experiments. Brain Sci. 2023, 13, 241. https://doi.org/10.3390/brainsci13020241
Bartl G, Allen P, Sandrini M. Left Prefrontal tDCS during Learning Does Not Enhance Subsequent Verbal Episodic Memory in Young Adults: Results from Two Double-Blind and Sham-Controlled Experiments. Brain Sciences. 2023; 13(2):241. https://doi.org/10.3390/brainsci13020241
Chicago/Turabian StyleBartl, Gergely, Paul Allen, and Marco Sandrini. 2023. "Left Prefrontal tDCS during Learning Does Not Enhance Subsequent Verbal Episodic Memory in Young Adults: Results from Two Double-Blind and Sham-Controlled Experiments" Brain Sciences 13, no. 2: 241. https://doi.org/10.3390/brainsci13020241




