Abstract
An inflammatory response after an aneurysmal subarachnoid hemorrhage (aSAH) has always been in the spotlight. However, few studies have compared the prognostic impact of inflammatory biomarkers. Moreover, why these inflammatory biomarkers contribute to a poor prognosis is also unclear. We retrospectively reviewed aSAH patients admitted to our institution between January 2015 and December 2020. The 90-day unfavorable functional outcome was defined as a modified Rankin scale (mRS) of ≥ 3. Independent inflammatory biomarker-related risk factors associated with 90-day unfavorable outcomes were derived from a forward stepwise multivariate analysis. Receiver operating characteristic curve analysis was conducted to identify the best cut-off value of inflammatory biomarkers. Then, patients were divided into two groups according to each biomarker’s cut-off value. To eliminate the imbalances in baseline characteristics, propensity score matching (PSM) was carried out to assess the impact of each biomarker on in-hospital complications. A total of 543 patients were enrolled in this study and 96 (17.7%) patients had unfavorable 90-day outcomes. A multivariate analysis showed that the white blood cell (WBC) count, the systemic inflammation response index, the neutrophil count, the neutrophil-to-albumin ratio, the monocyte count, and the monocyte-to-lymphocyte ratio were independently associated with 90-day unfavorable outcomes. The WBC count showed the best predictive ability (area under the curve (AUC) = 0.710, 95% CI = 0.652–0.769, p < 0.001). After PSM, almost all abnormal levels of inflammatory biomarkers were associated with a higher incidence of pneumonia during hospitalization. The WBC count had the strongest association with poor outcomes. Similar to nearly all other inflammatory biomarkers, the cause of poor prognosis may be the higher incidence of in-hospital pneumonia.
1. Introduction
Aneurysmal subarachnoid hemorrhages (aSAH) are one of the most common neurological emergencies with a high mortality rate of 22–50%, and even patients who receive optimal medical care may experience a long-term disability and cognitive impairment [1,2]. There are many reasons for this unfavorable outcome, of which researchers generally believe that early brain injuries (EBI) may be the most important cause of poor patient prognoses. EBI is a complex concept used to explain the pathophysiological events in the brain within 72 h after aneurysm rupture, including 0energy failure, ionic changes, increased endothelin-1 levels, depletion of nitric oxide, inflammation, oxidative stress, etc. [3,4,5,6]. Among these, inflammation after a hemorrhage is an important factor affecting the outcomes of aSAH patients, since some changes are associated with the development of cerebral vasospasm (CVS) and delayed cerebral ischemia (DCI) [7,8,9,10].
Recently, many studies have revealed the relationship between inflammatory biomarkers and a poor prognosis of patients with aSAH, such as white blood cells (WBC), neutrophils (NEUT), and monocytes (MONO) [11,12,13]. In addition, some derived inflammatory biomarkers have already been reported to be associated with unfavorable outcomes in patients with aSAH or ischemic stroke, including the neutrophil-to-lymphocyte ratio (NLR), the neutrophil-to-albumin ratio (NAR), the lymphocyte-to-monocyte ratio (LMR), the monocyte-to-lymphocyte ratio (MLR), the platelet-to-white blood cell ratio (PWR), the platelet-to-neutrophil ratio (PNR), the platelet-to-lymphocyte ratio (PLR), the mean platelet volume-to-platelet count ratio (MPV/PLT), the systemic inflammation response index (SIRI, neutrophil count × monocyte count/lymphocyte count), and the systemic immune-inflammation index (SII, platelet count × neutrophil count/lymphocyte count) [14,15,16,17,18,19,20,21]. Although they all perform well in predicting poor outcomes according to the previous studies, unfortunately, most of these biomarkers have been reported separately, which means few studies have compared their predictive ability together in aSAH patients. Moreover, the reasons that these biomarkers lead to poor prognoses have also not been widely recognized. Finding out the best inflammatory biomarker and exploring the causes of poor prognosis have great significance, which can help clinicians better understand the role of the inflammatory response in the prognoses of patients with aSAH.
Therefore, the objectives of this study were to analyze the associations between inflammatory biomarkers and outcomes of aSAH patients, compare the ability of these inflammatory biomarkers to predict outcomes, and try to find out potential reasons for these inflammatory biomarkers leading to unfavorable outcomes.
2. Materials and Methods
2.1. Study Design
We retrospectively analyzed the clinical data of aSAH patients hospitalized in the neurosurgery department of Beijing Tiantan Hospital between January 2015 and December 2020. All patients’ data were derived from the Long-term Prognosis of Emergency Aneurysmal Subarachnoid Hemorrhage (LongTEAM) Registry study (Registration No. NCT04785976). In the present study, all patients had angiographically documented aneurysms with subarachnoid hemorrhage (SAH), confirmed by either computed tomography (CT) or lumbar puncture. The inclusion criteria were as follows: (1) age ≥ 18 years old; (2) emergency admission; (3) less than 72 h from the rupture to the admission and less than 72 h from the admission to treatment; (4) a single aneurysm; and (5) treated with surgical clipping or endovascular coiling. The exclusion criteria were as follows: (1) previous SAH; (2) history of neurosurgery due to any cause; (3) physical disability due to any previous disease; and (4) insufficient medical, radiological, and laboratory information.
This study was approved by the Institutional Review Board of Beijing Tiantan Hospital. Informed consent for clinical analysis was obtained from all individual participants or their authorized representatives. All the studies were performed according to the Declaration of Helsinki and the local ethics policies. All patients were managed according to the guidelines [22].
2.2. Patients’ Data
From patients’ medical records, we collected their (1) demographic information and their medical history, including age, gender, hypertension, and diabetes mellitus; (2) lifestyle risk factors, including current smoking history; (3) location of aneurysm; (4) first CT-related information on admission, including their modified Fisher scale (mFS) grade, Graeb score, Subarachnoid Hemorrhage Early Brain Edema Score (SEBES) [23], and acute hydrocephalus; (5) World Federation of Neurological Societies (WFNS) grade on admission; (6) first inflammation-related laboratory examination on admission, including their WBC count (normal range: (3.5–9.5) × 109/L), MONO count (normal range: (0.10–0.60) × 109/L), lymphocyte count (LY, normal range: (1.10–3.2) × 109/L), NEUT count (normal range: (1.80–6.30) × 109/L), PLT count (normal range: (125–350) × 109/L), NLR, NAR, SIRI, SII, MLR, LMR, PWR, PLR, MPV/PLT, and PNR; (7) treatment modality, including surgical clipping and endovascular coiling; and (8) in-hospital complications, including DCI, intracranial infection, stress ulcer bleeding, hypoproteinemia, pneumonia, and deep vein thrombosis.
2.3. Outcome Measurement
The primary outcome was the functional neurological outcome, measured by the modified Rankin scale (mRS) 90 days after discharge (the neurosurgeon followed up with patients via telephone or an outpatient appointment). Patients were divided into an unfavorable outcome group with mRS ≥ 3. The second outcome was the occurrence of in-hospital complications.
2.4. Statistical Analysis
The analysis was performed with SPSS Statistics 26.0 (IBM, Armonk, New York, NY, USA) and GraphPad PRISM 8.3.0. (GraphPad Software Inc., San Diego, CA, USA). Significance was set at p < 0.05.
Continuous variables were summarized as means ± standard deviation or medians with interquartile range (IQR). Categorical variables were summarized as frequency (percentage). After testing for normality, continuous variables were analyzed using the independent Student t-test (normal distribution), the Mann–Whitney U test or the Kruskal–Wallis H test. The Pearson chi-square test or Fisher’s exact test were performed to test the dichotomized variables. Due to the lack of daily biomarkers data, the point at which each person’s blood was first drawn was included in the analysis. The time the blood sample of inflammatory biomarkers was taken was classified into three groups (Day 1 (rupture to admission interval between 0 and 24 h), Day 2 (rupture to admission interval between 25 and 48 h), and Day 3 (rupture to admission interval between 49 and 72 h)) to find out whether there were different levels in the predefined groups over time.
Only variables with p < 0.05 in univariate analysis were entered in a forward stepwise likelihood ratio multivariate logistic model to identify the inflammation-related independent risk factors associated with 90-day unfavorable outcomes. The odds ratios (OR) and 95% confidence intervals (CI) of variables were calculated. Interaction terms were used to investigate whether the association between inflammatory biomarkers and the 90-day unfavorable outcomes differed according to the WFNS grade group or treatment modality. Afterward, subgroup analysis was conducted in the same manner as for the primary outcome in patients in the same WFNS grade and treatment modality group.
Receiver operating characteristic (ROC) analysis was performed to evaluate associations between the inflammatory biomarkers on the admission and 90-day outcomes to determine the best cut-off value for the prediction. Furthermore, we used the area under the curve (AUC) in ROC analysis to compare the predictive ability of different inflammatory biomarkers. To observe the impact of inflammatory biomarkers on in-hospital complications, we performed propensity score matching (PSM) with a match tolerance of 0.02 and a ratio of 1:1 to adjust for imbalances of baseline characteristics such as age, hypertension, WFNS grade, mFS grade, Graeb score, acute hydrocephalus, and surgical clipping between the two outcome groups. After PSM, according to each inflammatory biomarker’s cut-off value, we divided the patients into two groups to observe the relationship between various inflammatory biomarkers and in-hospital complications.
3. Results
A total of 543 patients were enrolled in this study. A total of 96 (17.7%) patients had unfavorable 90-day outcomes and 447 (82.3%) patients had favorable 90-day outcomes. Compared with patients with favorable outcomes, patients who had unfavorable 90-day outcomes were older (58.7 ± 10.8 vs. 53.9 ± 11.0, p < 0.001), more likely to choose surgical clipping, had a higher incidence of hypertension history (71/96 (74.0%) vs. 255/447 (57.0), p = 0.002), had a higher proportion of WFNS grade 4–5 on admission (56/96 (58.3%) vs. 73/447 (16.3%), p < 0.001), had a higher proportion of mFS grade 3–4 on admission (89/96 (92.7%) vs. 339/447 (75.8%), p < 0.001), had a higher proportion of Graeb score 4–5 on admission (22/96 (22.9%) vs. 26/447 (5.8%), p < 0.001), and had a higher proportion of acute hydrocephalus on admission (50/96 (52.1%) vs. 175/447 (39.1%), p = 0.020). In addition, patients who had 90-day unfavorable outcomes had a significantly higher WBC count (median (IQR) 15.26 (12.44–18.54) vs. 11.86 (9.40–14.68), p < 0.001), MONO count (median (IQR) 0.57 (0.40–0.81) vs. 0.39 (0.26–0.54), p < 0.001), NEUT count (median (IQR) 13.56 (11.03–16.34) vs. 10.51 (7.97–13.27), p < 0.001), NLR (median (IQR) 15.21 (8.99–21.00) vs. 11.06 (6.97–15.70), p < 0.001), NAR (median (IQR) 0.32 (0.25–0.39) vs. 0.25 (0.19–0.31), p < 0.001), SIRI (median (IQR) 7.06 (4.13–11.70) vs. 3.68 (2.36–6.24), p < 0.001), SII (median (IQR) 3412 (2089–5002) vs. 2470 (1535–3548), p < 0.001), and MLR (median (IQR) 0.57 (0.36–0.79) vs. 0.37 (0.26–0.54), p < 0.001), but lower LMR (median (IQR) 1.77 (1.26–2.79) vs. 2.70 (1.87–3.79), p < 0.001), PWR (median (IQR) 15.26 (12.13–19.80) vs. 19.00 (14.96–23.51), p < 0.001), and PNR (median (IQR) 17.67 (13.29–21.10) vs. 21.69 (16.60–27.92), p < 0.001) (Table 1).

Table 1.
Baseline characteristics.
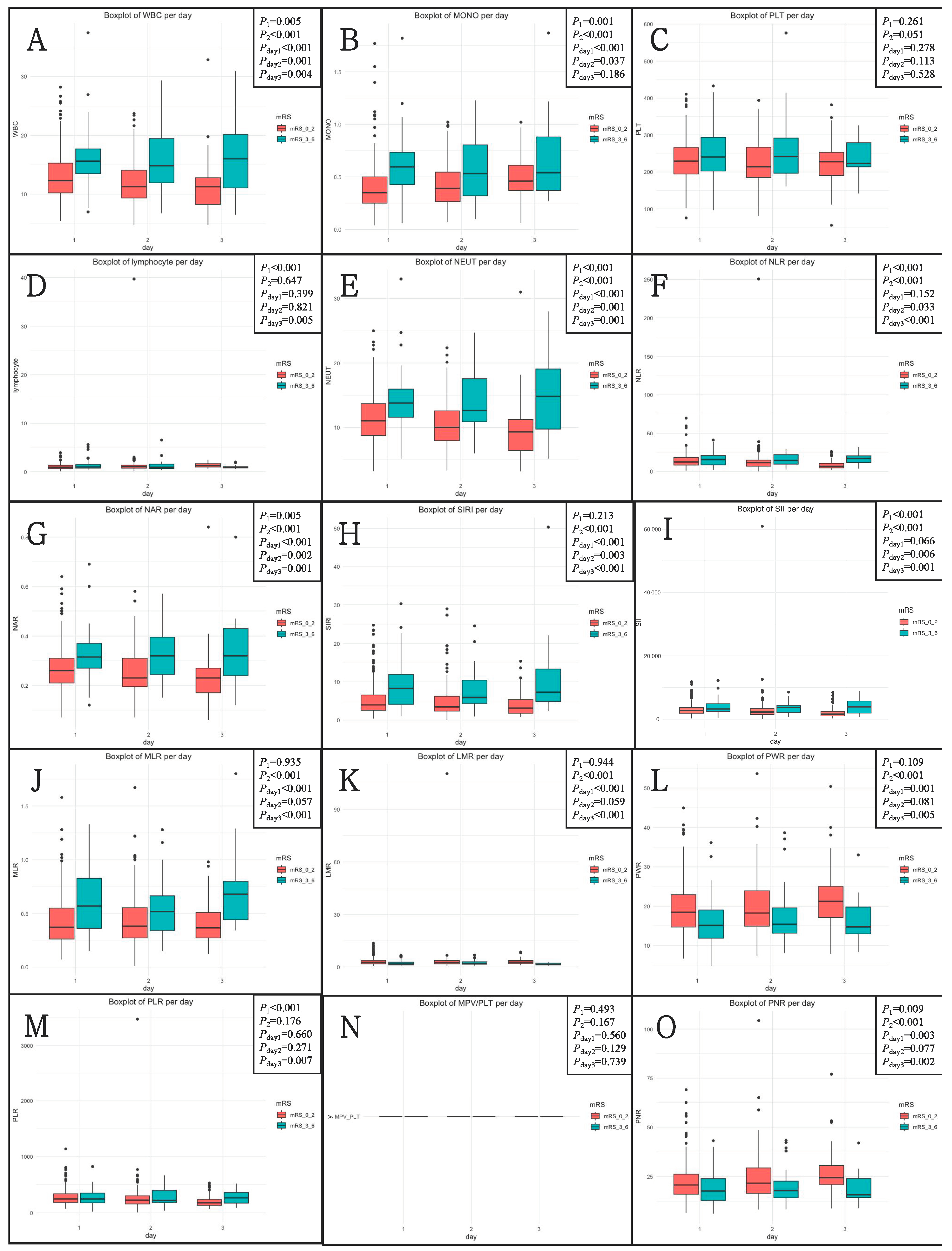
After the rupture event occurred, the levels of inflammatory biomarkers were analyzed. A total of 298 patients (54.9%) received tests on post-hemorrhagic day 1, 158 (29.1%) on day 2, and 87 (16.0%) on day 3. The levels of inflammatory biomarkers according to three blood sample timings are shown in Figure 1. On Day 1, Day 2, and Day 3, the levels of 10, 7, and 12 inflammatory biomarkers, respectively, were different across the 90-day unfavorable outcomes.
Figure 1.
The levels of inflammatory biomarkers according to three blood sample drawn timings (Day 1, Day 2, and Day 3) across 90-day functional outcomes. (A) WBC; (B) MONO; (C) PLT; (D) lymphocyte; (E) NEUT; (F) NLR; (G) NLR; (H) SIRI; (I) SII; (J) MLR; (K) LMR; (L) PWR; (M) PLR; (N) MPV/PLT; (O) PNR. mRS, modified Rankin scale; NLR, neutrophil-to-lymphocyte ratio; NAR, neutrophil-to-albumin ratio; SIRI, systemic inflammation response index; SII, systemic immune-inflammation index; MLR, monocyte-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; PWR, platelet-to-white blood cell ratio; PLR, platelet-to-lymphocyte ratio; MPV/PLT, mean platelet volume-to-platelet count ratio; PNR, platelet-to-neutrophil ratio.
P1 was determined by the Kruskal–Wallis H test for different levels of inflammatory biomarkers in three groups. P2 was determined by the Mann–Whitney U test for different levels of inflammatory biomarkers between 90-day favorable and unfavorable outcome groups. Pday1, Pday2, and Pday3 were determined by the Mann–Whitney U test for different levels of inflammatory biomarkers according to three groups between 90-day favorable and unfavorable outcome groups.
3.1. Inflammatory Biomarker-Related Risk Factors Associated with 90-Day Unfavorable Outcomes
The inflammatory biomarkers of significance (p < 0.05) in univariate analysis were successively analyzed by multivariate analysis. After adjusting for age, hypertension, WFNS grade 4–5, mFS grade 3–4, a Graeb score of 5–12, acute hydrocephalus, and treatment modality, the multivariate analysis showed that the WBC count (OR = 1.15, 95% CI = 1.08–1.22, p < 0.001), SIRI (OR = 1.09, 95% CI = 1.04–1.14, p < 0.001), NEUT count (OR = 1.14, 95% CI = 1.08–1.22, p < 0.001), NAR (OR = 110.19, 95% CI = 9.42–1288.36, p < 0.001), MONO count (OR = 7.38, 95% CI = 2.75–19.76, p < 0.001), and MLR (OR = 4.70, 95% CI = 1.92–11.48, p = 0.001) were independently associated with 90-day unfavorable outcomes in aSAH patients (Table 2). In addition, the results of subgroup analyses of the WFNS grade and treatment modality on the primary outcome are shown in Table 3 and Table 4. In a subgroup analysis of the two WFNS grades, there are interactions between inflammatory biomarkers (i.e., WBC, NEUT, NAR, and MONO) and 90-day unfavorable outcomes. In a subgroup analysis of the two treatment mortality, there are interactions between inflammatory biomarkers (i.e., WBC, SIRI, NEUT, NAR, MONO, and MLR) and 90-day unfavorable outcomes.

Table 2.
Multivariate analysis results of inflammatory biomarkers after adjusting for age, hypertension, WFNS grade 4–5, mFS grade 3–4, a Graeb score of 5–12, acute hydrocephalus, and treatment modality.

Table 3.
WFNS grade subgroup analysis of 90-day unfavorable outcomes.

Table 4.
Treatment mortality subgroup analysis of 90-day unfavorable outcomes.
3.2. Associations between Inflammatory Biomarkers and WFNS Grade, mFS Grade, and Graeb Score
Except for LMR, PWR, and PNR, which showed lower levels for worse clinical grades, all other biomarkers showed higher levels for worse clinical grades (Table 5).

Table 5.
Associations between inflammatory biomarkers and WFNS grade, mFS grade, and Graeb score on admission.
3.3. Receiver Operating Characteristic Curve Analysis
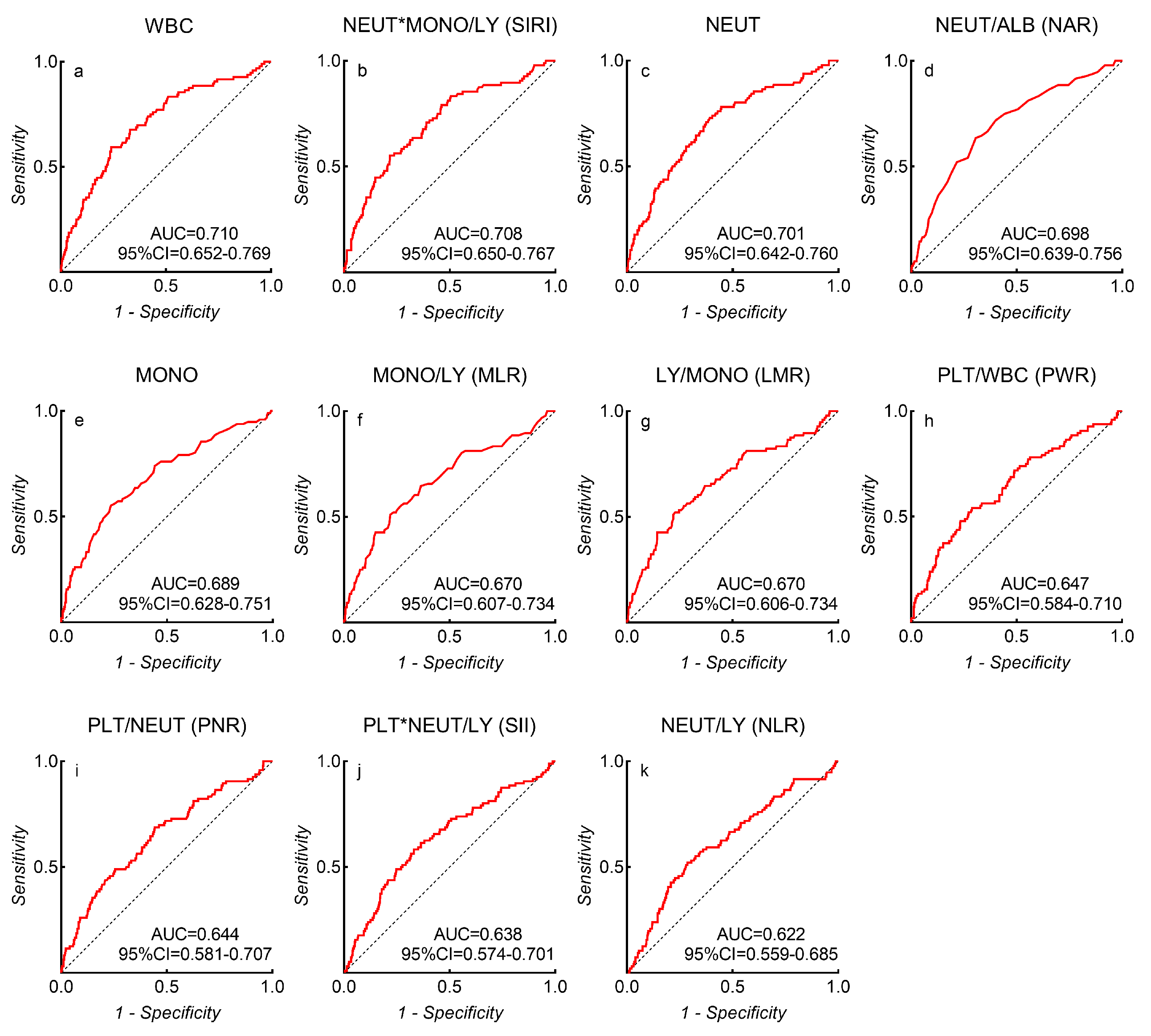
Among all inflammatory biomarkers, the three which most strongly associated with unfavorable outcomes were the WBC count (AUC = 0.710, 95% CI = 0.652–0.769, p < 0.001), SIRI (AUC = 0.708, 95% CI = 0.650–0.767, p < 0.001), and the NEUT count (AUC = 0.701, 95% CI = 0.642–0.760, p < 0.001) (Figure 2). ROC analysis identified a WBC count of 14.82 × 109/L (sensitivity = 49%, specificity = 76%, and Youden’s index = 0.35), SIRI of 6.77 (sensitivity = 55%, specificity = 78%, and Youden’s index = 0.34), NEUT count of 11.39 × 109/L (sensitivity = 73%, specificity = 61%, and Youden’s index = 0.34), NAR of 0.29 (sensitivity = 64%, specificity = 70%, and Youden’s index = 0.33), MONO count of 0.55 × 109/L (sensitivity = 55%, specificity = 77%, and Youden’s index = 0.32), LMR of 1.79 (sensitivity = 52%, specificity = 77%, and Youden’s index = 0.29), MLR of 0.56 (sensitivity = 51%, specificity = 78%, and Youden’s index = 0.29), PWR of 15.62 (sensitivity = 54%, specificity = 71%, and Youden’s index = 0.25), PNR of 20.72 (sensitivity = 69%, specificity = 56%, and Youden’s index = 0.24), SII of 3102 (sensitivity = 58%, specificity = 67%, and Youden’s index = 0.25), and NLR of 14.88 (sensitivity = 52%, specificity = 71%, and Youden’s index = 0.23) as the best cut-off values to discriminate favorable or unfavorable 90-day outcomes in aSAH patients.
Figure 2.
The AUC values of each inflammatory biomarker-related risk factor that predict 90-day unfavorable outcomes in aSAH patients. (a) WBC; (b) SIRI; (c) NEUT; (d) NAR; (e) MONO; (f) MLR; (g) LMR; (h) PWR; (i) PNR; (j) SII; (k) NLR.
3.4. Associations between Inflammatory Biomarkers and In-Hospital Complications
We adjusted for imbalances of baseline characteristics such as age, hypertension, WFNS grade, mFS grade, Graeb score, acute hydrocephalus, and surgical clipping. There was no statistical difference in baseline characteristics between the two groups after PSM (Table 6).

Table 6.
Patient characteristics and group comparisons before and after propensity score matching.
According to each inflammatory biomarker’s cut-off value, patients with a WBC count of >14.82 × 109/L had a higher incidence of in-hospital pneumonia (55/80 (68.8) vs. 38/92 (41.3%), p < 0.001) when compared with patients with a WBC count of ≤14.82 × 109/L. Patients with a SIRI of >6.77 had a higher incidence of in-hospital pneumonia (49/74 (66.2%) vs. 44/98 (44.9%), p = 0.006) when compared with patients with a SIRI of ≤6.77. Patients with an NEUT count of >11.39 × 109/L had a higher incidence of in-hospital pneumonia (63/100 (63.0%) vs. 30/72 (41.7%), p = 0.006) when compared with patients with an NEUT count of ≤11.39 × 109/L. Patients with an NAR of >0.29 had a higher incidence of in-hospital pneumonia (59/86 (68.6%) vs. 34/86 (39.5%), p < 0.001) when compared with patients with an NAR of ≤0.29. Patients with a MONO count of >0.55 × 109/L had a higher incidence of in-hospital intracranial infection (13/67 (19.4%) vs. 9/105 (8.6%), p < 0.001) when compared with patients with a MONO count of ≤0.55 × 109/L. Patients with an LMR of <1.79 had a higher incidence of in-hospital pneumonia (47/71 (66.2%) vs. 46/101 (45.5%), p = 0.008) when compared with patients with an LMR of >1.79. Patients with an MLR of >0.56 had a higher incidence of in-hospital pneumonia (45/69 (65.2%) vs. 48/103 (46.6%), p = 0.016) when compared with patients with an MLR of ≤0.56. Patients with a PWR of <15.62 had a higher incidence of in-hospital pneumonia (53/80 (66.3%) vs. 40/92 (43.5%), p = 0.003) when compared with patients with a PWR of ≥15.62. Patients with a PNR of <20.72 had a higher incidence of in-hospital pneumonia (64/102 (62.7%) vs. 29/70 (41.4%), p = 0.006) when compared with patients with a PNR of ≥20.72. Patients with an SII of >3102 had a higher incidence of in-hospital pneumonia (55/84 (65.5%) vs. 38/88 (43.2%), p = 0.003) when compared with patients with an SII of ≤3102. Patients with an NLR of >14.88 had a higher incidence of in-hospital pneumonia (47/74 (63.5%) vs. 46/98 (46.9%), p = 0.031) when compared with patients with an NLR of ≤14.88 (Table 7).

Table 7.
Associations between inflammatory biomarkers and in-hospital complications.
4. Discussion
This large retrospective study reviewed several accessible clinical inflammatory biomarkers associated with unfavorable outcomes in aSAH patients and compared their predictive ability on the prognosis. We found that almost all abnormal biomarkers related to a higher incidence of pneumonia during hospitalization, which may be an important reason for the poor prognosis of aSAH patients. Exploring the relationship between these inflammatory biomarkers and prognosis can better help clinicians understand the pathophysiological processes of aSAH and further make more active adjustments to treatment strategies.
aSAH has been shown as a state of systemic inflammation and immunosuppression [24]. It has been reported that inflammatory responses can impact the prognosis of aSAH. Still, the causes of poor prognosis are inconclusive, even though many studies have suggested that inflammation may be related to DCI, leading to unfavorable outcomes [25]. After aSAH, peripheral WBCs have been shown to migrate to the brain with activated NEUT (the most abundant type of WBC found in the circulation), causing damage to the brain and leading to a poor prognosis [11,12,26,27]. The results of this finding are in line with our previous research, showing that the WBC count remains stable in predicting 90-day outcomes [28]. In our study, WBC and NEUT both showed better predictive abilities on the prognosis of aSAH patients. To date, only a few articles focused on the MONO after aSAH. Thus, there is a very limited understanding of the role of MONO in the pathogenesis of aSAH. The possible reason is that MONO rapidly differentiates into macrophages after entering the tissue from circulation, while existing resources cannot distinguish this process. MONO can promote the inflammatory process by interacting with PLT and endothelial cells [29]. One study indicated that MONO benefits by removing subarachnoid space debris [30]. However, the other two studies showed contradictory conclusions, indicating that MONO mediates the development of cerebral vasospasm and leads to a poor prognosis [13,31]. The elevated MONO count revealed a higher incidence of in-hospital intracranial infection in our research.
Besides WBC, NEUT, and MONO, inflammation is also involved in PLT activation and thrombosis, which can concomitantly release inflammatory molecules [32]. Thus, many inflammatory biomarkers are related to PLT count [32,33]. However, there was little significant difference in PLT count between patients with favorable and unfavorable outcomes in our study. LY plays an important role in the anti-inflammatory response, but research on LY in aSAH patients is very limited [34]. One previous study indicated that T-lymphocyte inhibitors had neuroprotective effects in experimental SAH but they lacked clinical evidence [35]. In our study, there was no relationship between LY count and outcome.
In addition to the individual biomarkers such as WBC, NEUT, MONO, PLT, and LY, researchers have created some ratio-related inflammatory biomarkers to minimize the potential confounders caused by the alternation of individual blood parameters of patients. Among these inflammatory biomarkers, NLR, NAR, LMR, MLR, PWR, PNR, PLR, MPV/PLT, SIRI, and SII were associated with patient outcomes [14,15,16,17,18,19,20,21]. However, aSAH is an emergency, thus requiring clinicians to have the ability to judge the condition and make treatment decisions quickly. Among the numerous inflammatory biomarkers associated with prognosis, it is of great clinical significance to help clinicians quickly find the best indicators that may be associated with prognosis. Our study demonstrated that these biomarkers were all associated with the initial clinical status of the patient. WBC has the strongest predictive ability. Similar to almost all the other biomarkers, an elevated WBC count indicated a higher incidence of in-hospital pneumonia, which may be responsible for the poor outcomes. Our conclusion is consistent with another study, which revealed that elevated NLR was associated with higher in-hospital pneumonia after adjusting for patient baseline characteristics [14].
Unlike previous studies, which have indicated that inflammatory responses are associated with DCI [15,20], we found no relationship between abnormal inflammatory biomarkers and DCI. We speculated that although the investigators were assuming a connection between inflammation and DCI, there is currently a lack of relevant clinical evidence. The combination of complications during the patient’s hospitalization, the collection method of inflammatory biomarkers, and the confounding factors of the patient may have led the investigators to draw different conclusions.
Our previous study found that pneumonia and DCI during hospitalization may have a long-lasting impact on the prognosis of aSAH patients treated with surgical clipping and endovascular coiling [36]. It is noteworthy that nursing will become more difficult when pneumonia occurs, leading to patients staying in bed longer and increasing the risk of other severe complications. Moreover, similar to DCI, severe pneumonia would endanger the patient’s life. The clinical evidence provided by our study highlights the importance of controlling inflammation and pneumonia to improve patient outcomes.
There are various limitations of our study. First, the statistical data were retrospectively collected. Second, this study did not include some inflammatory biomarkers due to insufficient technical equipment. Third, whether these inflammatory biomarkers differ between races is unclear, so the optimal cutoff value for predicting the outcome is only of reference significance. Fourth, the AUC of 0.7 is fair and would be below the acceptance threshold for “good” predictive ability. Thus, the cutoff value of some biomarkers may not be likely to be clinically useful. Fifth, it is unclear if the patient had an infection before the onset. Sixth, not all patient biomarkers were observed at the three-time points, and the analysis is likely incomplete due to missing data. We integrated three days into one time point to use our clinical data and increase generality. We found 10, 7, and 12 inflammatory biomarkers differed across the 90-day unfavorable outcomes on Day 1, Day 2, and Day 3, respectively. Finally, subgroup-stratified analyses showed similar patterns of association as with the pooled (non-stratified) analysis in some inflammatory biomarkers groups, while others were not. The interpretation of this inconsistent finding warrants further investigations.
5. Conclusions
In this large retrospective study, we compared the relationship between different admission inflammatory markers and the prognosis of patients with aSAH. We found that they were all related to the clinical status of patients at admission. WBCs had the strongest association with poor functional outcomes in aSAH patients. Similar to all other inflammatory biomarkers, except monocytes, the occurrence of abnormal levels suggested the incidence of pneumonia during hospitalization, which may be an important reason for the poor outcomes of patients with aSAH. The clinical evidence provided by our study highlights the importance of controlling inflammation and pneumonia to improve patient outcomes.
Author Contributions
Conceptualization, Z.N. and F.L.; methodology, Z.N., F.L. and R.L.; software, Z.N. and F.L.; validation, R.L., X.C. and Y.Z.; formal analysis, Z.N. and F.L.; investigation, Z.N. and F.L.; resources, X.C. and Y.Z.; data curation, Z.N.; writing—original draft preparation, Z.N. and F.L.; writing—review and editing, R.L. and X.C.; visualization, Z.N.; supervision, X.C. and Y.Z.; project administration, Y.Z.; funding acquisition, X.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Key Research and Development Program of China (grant nos. 2021YFC2501101 and 2020YFC2004701).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Beijing Tiantan Hospital (protocol code KY 2021–008-01).
Informed Consent Statement
Informed consent for clinical analyses was obtained from all individual participants or their authorized representatives.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank all the staff and participants for their contribution to this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lovelock, C.E.; Rinkel, G.J.; Rothwell, P.M. Time trends in outcome of subarachnoid hemorrhage: Population-based study and systematic review. Neurology 2010, 74, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, T.K.; Molyneux, A.J.; Hall, N.; Yeates, D.R.; Goldacre, R.; Sneade, M.; Clarke, A.; Goldacre, M.J. The falling rates of hospital admission, case fatality, and population-based mortality for subarachnoid hemorrhage in England, 1999–2010. J. Neurosurg. 2016, 125, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Yan, J.; Rolland, W.B.; Soejima, Y.; Caner, B.; Zhang, J.H. Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl. Stroke Res. 2013, 4, 432–446. [Google Scholar] [CrossRef]
- Kusaka, G.; Ishikawa, M.; Nanda, A.; Granger, D.N.; Zhang, J.H. Signaling pathways for early brain injury after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2004, 24, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Sehba, F.A.; Friedrich, V. Early events after aneurysmal subarachnoid hemorrhage. Acta. Neurochir. Suppl. 2015, 120, 23–28. [Google Scholar] [CrossRef]
- Suzuki, H. What is early brain injury? Transl. Stroke Res. 2015, 6, 1–3. [Google Scholar] [CrossRef]
- Aronowski, J.; Zhao, X. Molecular pathophysiology of cerebral hemorrhage: Secondary brain injury. Stroke 2011, 42, 1781–1786. [Google Scholar] [CrossRef]
- Macdonald, R.L.; Schweizer, T.A. Spontaneous subarachnoid haemorrhage. Lancet 2017, 389, 655–666. [Google Scholar] [CrossRef]
- Sehba, F.A.; Pluta, R.M.; Zhang, J.H. Metamorphosis of subarachnoid hemorrhage research: From delayed vasospasm to early brain injury. Mol. Neurobiol. 2011, 43, 27–40. [Google Scholar] [CrossRef]
- Dodd, W.S.; Laurent, D.; Dumont, A.S.; Hasan, D.M.; Jabbour, P.M.; Starke, R.M.; Hosaka, K.; Polifka, A.J.; Hoh, B.L.; Chalouhi, N. Pathophysiology of Delayed Cerebral Ischemia After Subarachnoid Hemorrhage: A Review. J. Am. Heart Assoc. 2021, 10, e021845. [Google Scholar] [CrossRef]
- Rothoerl, R.D.; Axmann, C.; Pina, A.L.; Woertgen, C.; Brawanski, A. Possible role of the C-reactive protein and white blood cell count in the pathogenesis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J. Neurosurg. Anesthesiol. 2006, 18, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.H.; Feske, S.K.; Simmons, S.L.; Konigsberg, R.G.; Orzell, S.C.; Marckmann, A.; Bourget, G.; Bauer, D.J.; De Jager, P.L.; Du, R.; et al. Elevated peripheral neutrophils and matrix metalloproteinase 9 as biomarkers of functional outcome following subarachnoid hemorrhage. Transl. Stroke Res. 2011, 2, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Feghali, J.; Kim, J.; Gami, A.; Rapaport, S.; Caplan, J.M.; McDougall, C.G.; Huang, J.; Tamargo, R.J.; Jackson, C.M. Monocyte-based inflammatory indices predict outcomes following aneurysmal subarachnoid hemorrhage. Neurosurg. Rev. 2021, 44, 3499–3507. [Google Scholar] [CrossRef] [PubMed]
- Giede-Jeppe, A.; Reichl, J.; Sprugel, M.I.; Lucking, H.; Hoelter, P.; Eyupoglu, I.Y.; Kuramatsu, J.B.; Huttner, H.B.; Gerner, S.T. Neutrophil-to-lymphocyte ratio as an independent predictor for unfavorable functional outcome in aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2019, 132, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Zhang, S.; Wang, C.; Zou, C.; Li, A. Neutrophil-to-Albumin Ratio as a Biomarker of Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2021, 147, e453–e458. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.W.; Yi, H.J.; Lee, D.H.; Sung, J.H. Prognostic Significance of Various Inflammation-Based Scores in Patients with Mechanical Thrombectomy for Acute Ischemic Stroke. World Neurosurg. 2020, 141, e710–e717. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Huang, Y.; Li, S.; Lin, J.; Liu, W.; Ding, Z.; Li, X.; Chen, Y.; Pang, W.; Yang, D.; et al. Platelet-to-White Blood Cell Ratio: A Prognostic Predictor for 90-Day Outcomes in Ischemic Stroke Patients with Intravenous Thrombolysis. J. Stroke Cerebrovasc. Dis. 2016, 25, 2430–2438. [Google Scholar] [CrossRef]
- Yun, S.; Jun Yi, H.; Hoon Lee, D.; Hoon Sung, J. Clinical significance of platelet to neutrophil ratio and platelet to lymphocyte ratio in patients with aneurysmal subarachnoid hemorrhage. J. Clin. Neurosci. 2021, 92, 49–54. [Google Scholar] [CrossRef]
- Wang, Z.; Pei, W.; Chen, L.; Ning, Y.; Luo, Y. Mean Platelet Volume/Platelet Count Ratio is Associated with Poor Clinical Outcome After Aneurysmal Subarachnoid Hemorrhage. J. Stroke Cerebrovasc. Dis. 2020, 29, 105208. [Google Scholar] [CrossRef]
- Yun, S.; Yi, H.J.; Lee, D.H.; Sung, J.H. Systemic Inflammation Response Index and Systemic Immune-inflammation Index for Predicting the Prognosis of Patients with Aneurysmal Subarachnoid Hemorrhage. J. Stroke Cerebrovasc. Dis. 2021, 30, 105861. [Google Scholar] [CrossRef]
- Luo, F.; Li, Y.; Zhao, Y.; Sun, M.; He, Q.; Wen, R.; Xie, Z. Systemic immune-inflammation index predicts the outcome after aneurysmal subarachnoid hemorrhage. Neurosurg. Rev. 2022, 45, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.S., Jr.; Rabinstein, A.A.; Carhuapoma, J.R.; Derdeyn, C.P.; Dion, J.; Higashida, R.T.; Hoh, B.L.; Kirkness, C.J.; Naidech, A.M.; Ogilvy, C.S.; et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 2012, 43, 1711–1737. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Burkett, A.; Paz, A.; Savarraj, J.P.; Hinds, S.; Hergenroeder, G.; Gusdon, A.M.; Ren, X.; Hong, J.H.; Choi, H.A. Systemic inflammatory markers of persistent cerebral edema after aneurysmal subarachnoid hemorrhage. J. Neuroinflamm. 2022, 19, 199. [Google Scholar] [CrossRef] [PubMed]
- Saand, A.R.; Yu, F.; Chen, J.; Chou, S.H. Systemic inflammation in hemorrhagic strokes-A novel neurological sign and therapeutic target? J. Cereb. Blood Flow Metab. 2019, 39, 959–988. [Google Scholar] [CrossRef] [PubMed]
- Muroi, C.; Hugelshofer, M.; Seule, M.; Tastan, I.; Fujioka, M.; Mishima, K.; Keller, E. Correlation among systemic inflammatory parameter, occurrence of delayed neurological deficits, and outcome after aneurysmal subarachnoid hemorrhage. Neurosurgery 2013, 72, 367–375, discussion 375. [Google Scholar] [CrossRef] [PubMed]
- Schneider, U.C.; Schiffler, J.; Hakiy, N.; Horn, P.; Vajkoczy, P. Functional analysis of Pro-inflammatory properties within the cerebrospinal fluid after subarachnoid hemorrhage in vivo and in vitro. J. Neuroinflamm. 2012, 9, 28. [Google Scholar] [CrossRef]
- Friedrich, V.; Flores, R.; Muller, A.; Bi, W.; Peerschke, E.I.; Sehba, F.A. Reduction of neutrophil activity decreases early microvascular injury after subarachnoid haemorrhage. J. Neuroinflamm. 2011, 8, 103. [Google Scholar] [CrossRef]
- Li, R.; Lin, F.; Chen, Y.; Lu, J.; Han, H.; Ma, L.; Zhao, Y.; Yan, D.; Li, R.; Yang, J.; et al. A 90-Day Prognostic Model Based on the Early Brain Injury Indicators after Aneurysmal Subarachnoid Hemorrhage: The TAPS Score. Transl. Stroke Res. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Ren, H.; Liu, X.; Wang, L.; Gao, Y. Lymphocyte-to-Monocyte Ratio: A Novel Predictor of the Prognosis of Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2017, 26, 2595–2602. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, Z.; Zhang, R.; Chen, P.; Zhang, X.; Cheng, F.; Gou, X. Monocytes in the Peripheral Clearance of Amyloid-beta and Alzheimer’s Disease. J. Alzheimers Dis. 2019, 68, 1391–1400. [Google Scholar] [CrossRef]
- Walsh, K.B.; Sekar, P.; Langefeld, C.D.; Moomaw, C.J.; Elkind, M.S.; Boehme, A.K.; James, M.L.; Osborne, J.; Sheth, K.N.; Woo, D.; et al. Monocyte Count and 30-Day Case Fatality in Intracerebral Hemorrhage. Stroke 2015, 46, 2302–2304. [Google Scholar] [CrossRef]
- Frontera, J.A.; Provencio, J.J.; Sehba, F.A.; McIntyre, T.M.; Nowacki, A.S.; Gordon, E.; Weimer, J.M.; Aledort, L. The Role of Platelet Activation and Inflammation in Early Brain Injury Following Subarachnoid Hemorrhage. Neurocrit. Care 2017, 26, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.D.; Thomas, T.C.; Lass, E.; Ai, J.; Wan, H.; Lifshitz, J.; Baker, A.J.; Macdonald, R.L. Platelet-mediated changes to neuronal glutamate receptor expression at sites of microthrombosis following experimental subarachnoid hemorrhage. J. Neurosurg. 2014, 121, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Liu, J.; Shan, W.; Zhang, Y.; Han, C.; Wang, R.; Sun, L. Association of lymphocyte-to-monocyte ratio with total coronary plaque burden in patients with coronary artery disease. Coron. Artery Dis. 2020, 31, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Zhang, X.; Li, Q.; Zhao, H.; Qu, J.; Zhang, J.H.; Liu, X.; Feng, H.; Chen, Y. Cyclosporine A alleviated matrix metalloproteinase 9 associated blood-brain barrier disruption after subarachnoid hemorrhage in mice. Neurosci. Lett. 2017, 649, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Lin, F.; Chen, Y.; Lu, J.; Han, H.; Yan, D.; Li, R.; Yang, J.; Li, Z.; Zhang, H.; et al. In-hospital complication-related risk factors for discharge and 90-day outcomes in patients with aneurysmal subarachnoid hemorrhage after surgical clipping and endovascular coiling: A propensity score-matched analysis. J. Neurosurg. 2021, 137, 381–392. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).