Double Blast Wave Primary Effect on Synaptic, Glymphatic, Myelin, Neuronal and Neurovascular Markers
Abstract
:1. Introduction
1.1. Synaptic Markers
1.2. Glymphatic Markers
1.3. Myelin Markers
1.4. Neuronal Markers
1.5. Neurovascular/Blood-Brain-Barrier (BBB) Markers
1.6. Genomic Activation/Repair Markers
2. Materials and Methods
2.1. Animals
2.2. Study Time Course
2.3. Blast Exposure
2.4. Blood and Tissue Collection
2.5. Protein Extraction and Western Blot (WB) Procedures
2.6. Enzyme Linked Immunosorbent Assays (ELISA)
2.7. Immunofluorescence (IF)
2.8. Immunofluorescent (IF) Intensity Quantification
2.9. Statistics
3. Results
3.1. Molecular Outcomes
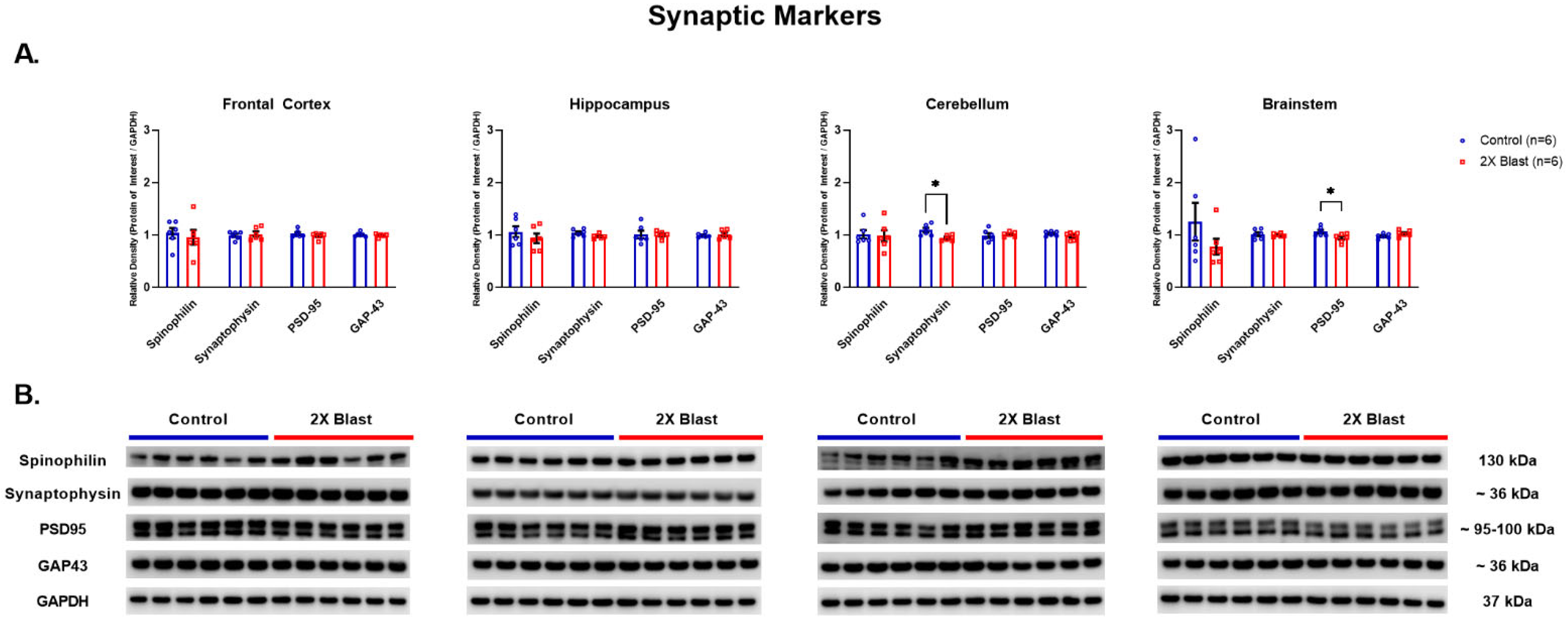
3.1.1. Effects of Explosive-Driven Double Blast Exposure on Synaptic-Markers
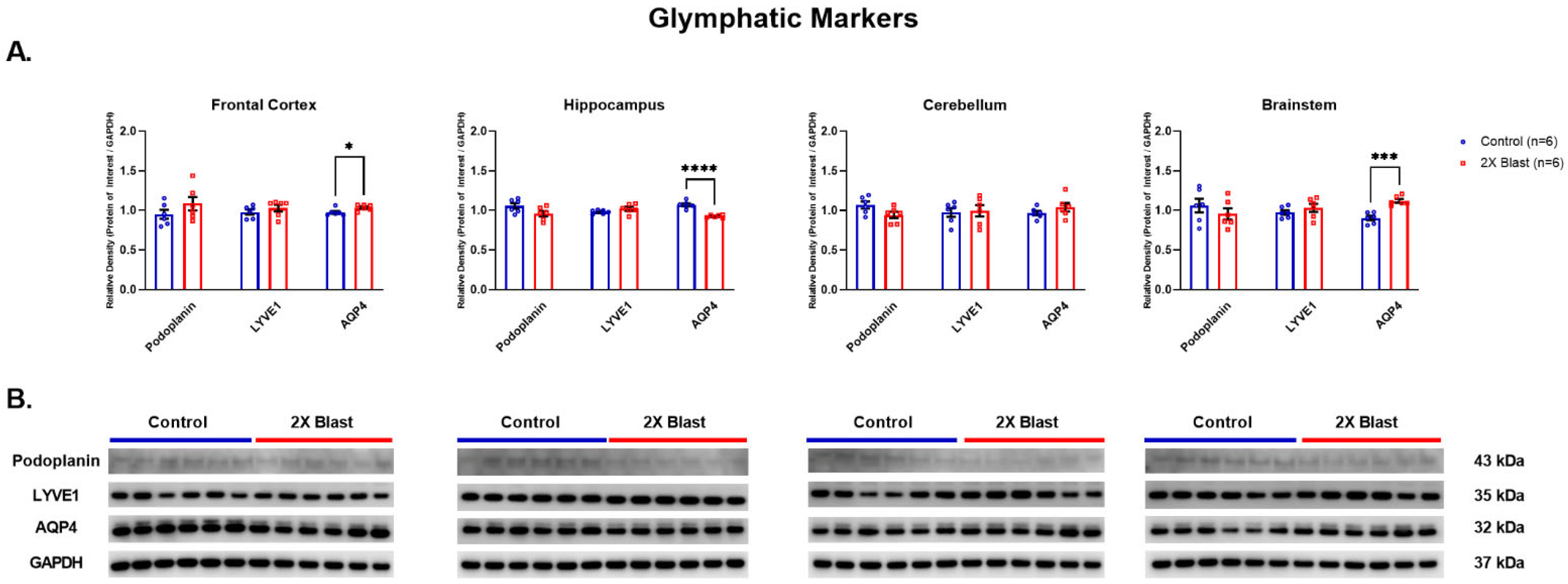
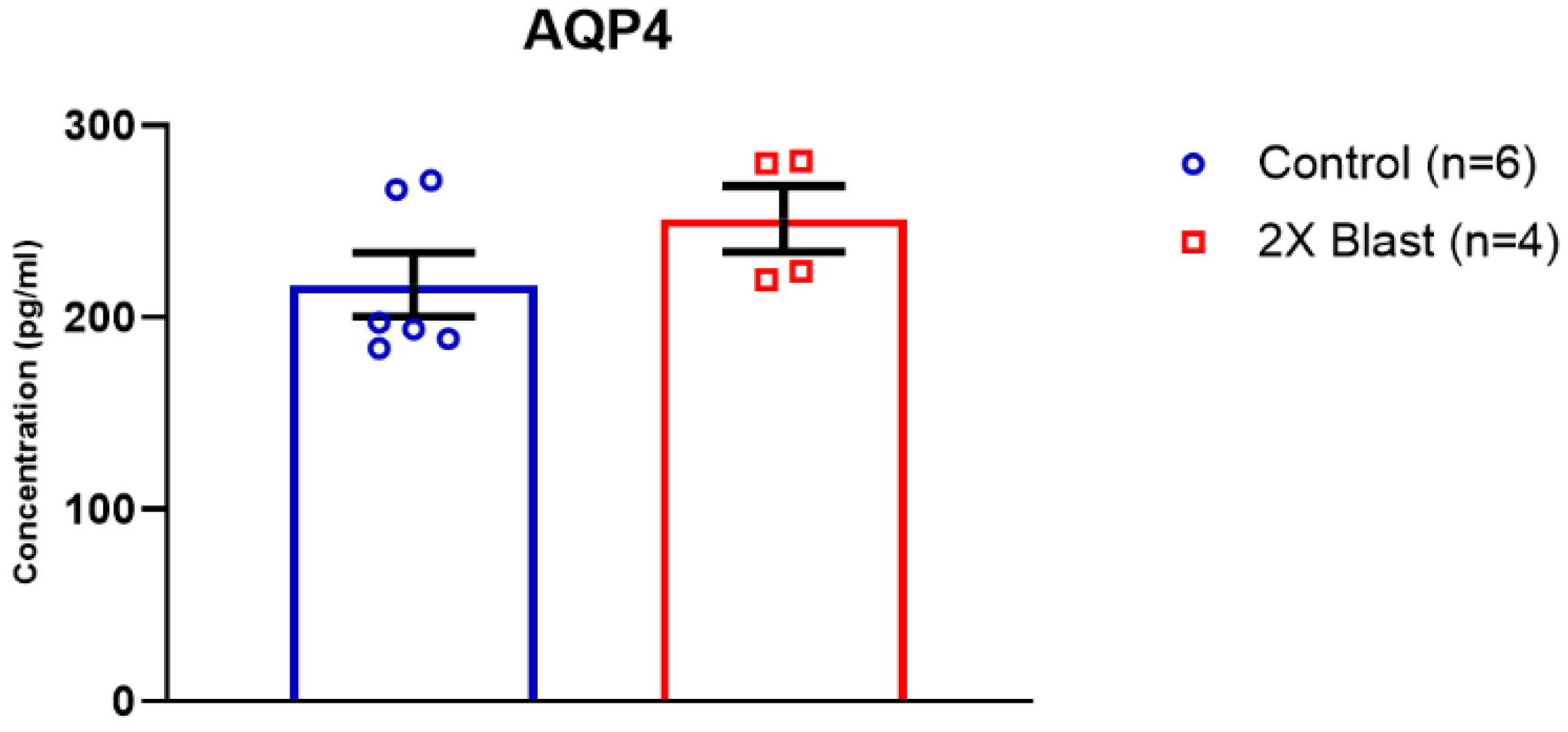
3.1.2. Effects of Explosive-Driven Double Blast Exposure on Glymphatic-Markers
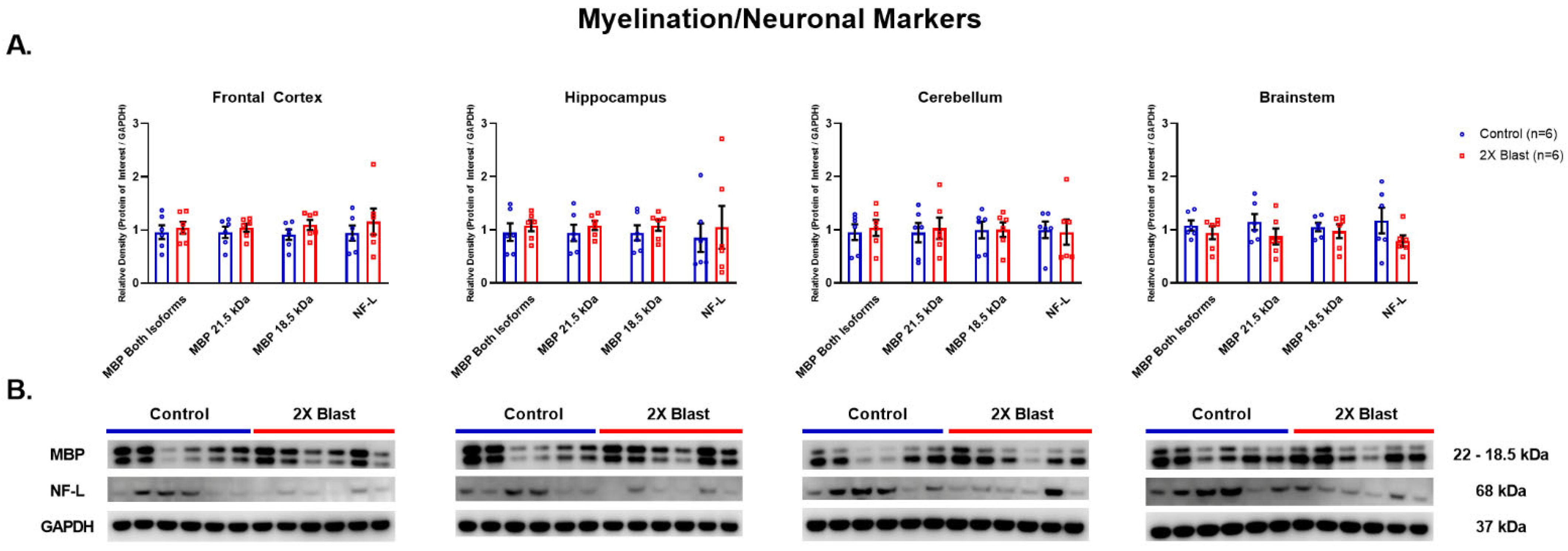
3.1.3. Effects of Explosive-Driven Double Blast Exposure on Neuronal and Myelination-Markers
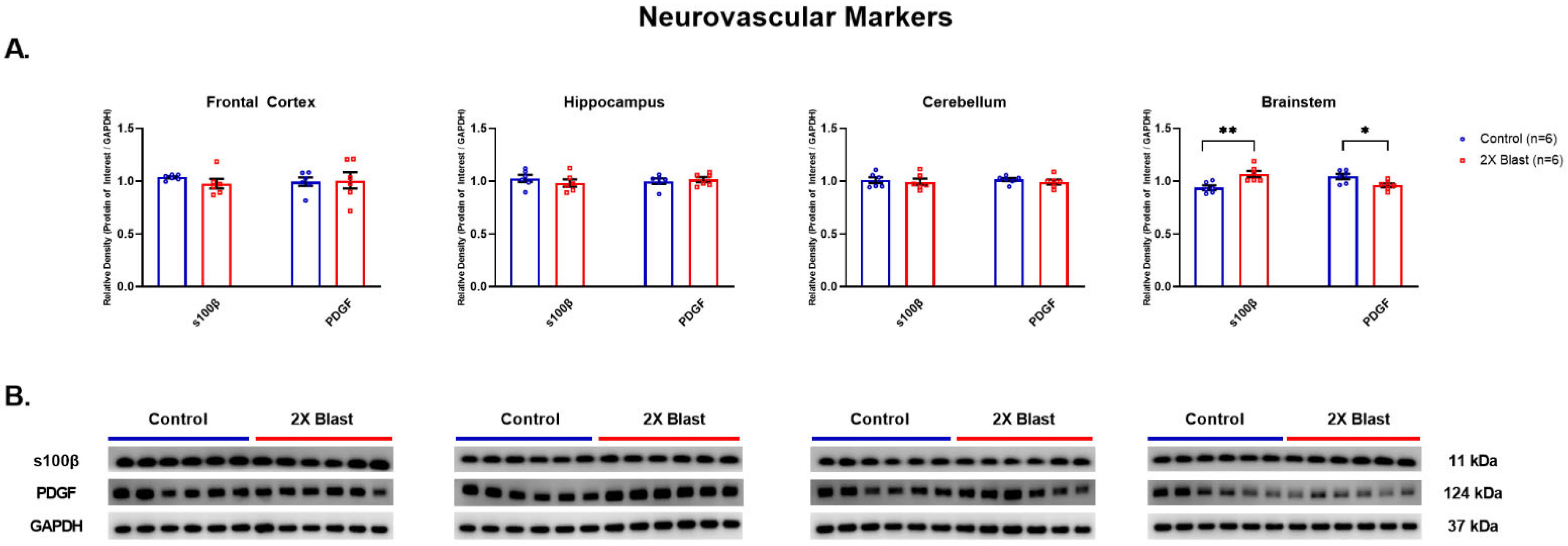
3.1.4. Effects of Explosive-Driven Double Blast Exposure on Neurovascular Markers
3.1.5. Effects of Explosive-Driven Double Blast Exposure on Genomic Activation/Repair Markers
3.2. Immunofluorescence Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoge, C.; McGurk, D.; Thomas, J.; Cox, A.; Engel, C.; Castro, C. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N. Engl. J. Med. 2008, 358, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D. Improvised explosive devices and traumatic brain injury: The military experience in Iraq and Afghanistan. Australas. Psychiatry 2009, 17, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Elder, G.; Mitsis, E.; Ahlers, S.; Cristian, A. Blast-induced mild traumatic brain injury. Psychiatr. Clin. N. Am. 2010, 33, 757–781. [Google Scholar] [CrossRef] [PubMed]
- Elder, G.A.; Stone, J.R.; Ahlers, S.T. Effects of low-level blast exposure on the nervous system: Is there really a controversy? Front. Neurol. 2014, 5, 269. [Google Scholar] [CrossRef]
- Nabity, P.S.; Jaramillo, C.A.; Resick, P.A.; McGeary, C.A.; Eapen, B.C.; Straud, C.L.; Hale, W.J.; Houle, T.T.; Litz, B.T.; Mintz, J.; et al. Consortium to Alleviate PTSD. Persistent posttraumatic headaches and functioning in veterans, Injury type can matter. Headache 2021, 61, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Haran, F.J.; Handy, J.D.; Servatius, R.J.; Rhea, C.K.; Tsao, J.W. Acute neurocognitive deficits in active duty service members following subconcussive blast exposure. Appl. Neuropsychol. Adult 2021, 28, 297–309. [Google Scholar] [CrossRef]
- Ozturk, E.D.; Chanfreau-Coffinier, C.; Sakamoto, M.S.; Delano-Wood, L.; Merritt, V.C. VA Million Veteran Program. Characterization of Million Veteran Program (MVP) enrollees with Comprehensive Traumatic Brain Injury Evaluation (CTBIE) data, An analysis of neurobehavioral symptoms. J. Psychiatr. Res. 2021, 145, 230–242. [Google Scholar] [CrossRef]
- Nelson, N.W.; Davenport, N.D.; Sponheim, S.R.; Anderson, C.R. Blast-Related Mild Traumatic Brain Injury: Neuropsychological Evaluation and Findings. In Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects; Kobeissy, F.H., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Milton Park, UK, 2015; Chapter 32. [Google Scholar]
- DePalma, R.G. Combat TBI: History, Epidemiology, and Injury Modes. In Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects; Kobeissy, F.H., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Milton Park, UK, 2015; Chapter 2. [Google Scholar]
- Miller, D.R.; Hayes, J.P.; Lafleche, G.; Salat, D.H.; Verfaellie, M. White matter abnormalities are associated with chronic postconcussion symptoms in blast-related mild traumatic brain injury. Hum. Brain Mapp. 2016, 37, 220–229. [Google Scholar] [CrossRef]
- O’Neil, M.E.; Callahan, M.; Carlson, K.F.; Roost, M.; Laman-Maharg, B.; Twamley, E.W.; Iverson, G.L.; Storzbach, D. Postconcussion symptoms reported by Operation Enduring Freedom/Operation Iraqi Freedom veterans with and without blast exposure, mild traumatic brain injury, and posttraumatic stress disorder. J. Clin. Exp. Neuropsychol. 2017, 39, 449–458. [Google Scholar] [CrossRef]
- Donald, C.L.M.; Barber, J.; Patterson, J.; Johnson, A.M.; Dikmen, S.; Fann, J.R.; Temkin, N. Association Between 5-Year Clinical Outcome in Patients with Nonmedically Evacuated Mild Blast Traumatic Brain Injury and Clinical Measures Collected within 7 Days Postinjury in Combat. JAMA Netw. Open 2019, 2, e186676. [Google Scholar] [CrossRef]
- Walker, W.C.; Franke, L.M.; McDonald, S.D.; Sima, A.P.; Keyser-Marcus, L. Prevalence of mental health conditions after military blast exposure, their co-occurrence, and their relation to mild traumatic brain injury. Brain Inj. 2015, 29, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Phipps, H.; Mondello, S.; Wilson, A.; Dittmer, T.; Rohde, N.; Schroeder, P.; Nichols, J.; McGirt, C.; Hoffman, J.; Tanksley, K.; et al. Characteristics and Impact of U.S. Military Blast-Related Mild Traumatic Brain Injury, A Systematic Review. Front. Neurol. 2020, 11, 559318. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, J.; McFarlane, A.; Bragge, P.; Armonda, R.; Grimes, J.; Ling, G. Blast-related traumatic brain injury. Lancet Neurol. 2013, 12, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Naifeh, J.A.; Mash, H.B.H.; Stein, M.B.; Fullerton, C.S.; Kessler, R.C.; Ursano, R.J. The Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS): Progress toward understanding suicide among soldiers. Mol. Psychiatry 2019, 24, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Campbell-Sills, L.; Stein, M.B.; Liu, H.; Agtarap, S.; Heeringa, S.G.; Nock, M.K.; Ursano, R.J.; Kessler, R.C. Associations of Lifetime Traumatic Brain Injury Characteristics with Prospective Suicide Attempt among Deployed US Army Soldiers. J. Head Trauma Rehabil. 2020, 35, 14–26. [Google Scholar] [CrossRef]
- Bullman, T.; Schneiderman, A. Risk of suicide among U.S. veterans who deployed as part of Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn. Inj. Epidemiol. 2021, 8, 40. [Google Scholar] [CrossRef]
- Wang, C.; Pahk, J.B.; Balaban, C.D.; Miller, M.C.; Wood, A.R.; Vipperman, J.S. Computational study of human head response to primary blast waves of five levels from three directions. PLoS ONE 2014, 9, e113264. [Google Scholar] [CrossRef]
- Przekwas, A.; Garimella, H.T.; Tan, X.G.; Chen, Z.J.; Miao, Y.; Harrand, V.; Kraft, R.H.; Gupta, R.K. Biomechanics of Blast TBI with Time-Resolved Consecutive Primary, Secondary, and Tertiary Loads. Mil. Med. 2019, 184 (Suppl. 1), 195–205. [Google Scholar] [CrossRef]
- Phillips, Y.Y. Primary blast injuries. Ann. Emerg. Med. 1986, 15, 1446–1450. [Google Scholar] [CrossRef]
- Abbotts, R.; Harrison, S.; Cooper, G. Primary blast injuries to the eye: A review of the evidence. BMJ Mil. Health 2007, 153, 119–123. [Google Scholar] [CrossRef]
- Wolf, S.; Bebarta, V.; Bonnett, C.; Pons, P.; Cantrill, S. Blast injuries. Lancet 2009, 374, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Cernak, I.; Noble-Haeusslein, L. Traumatic brain injury: An overview of pathobiology with emphasis on military populations. J. Cereb. Blood Flow Metab. 2010, 30, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Cernak, I. Understanding blast-induced neurotrauma: How far have we come? Concussion 2017, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, A.J.; Zouris, J.M.; Watrous, J.R.; McCabe, C.T.; Dougherty, A.L.; Galarneau, M.R.; Fraser, J. Multimorbidity and quality of life after blast-related injury among US military personnel: A cluster analysis of retrospective data. BMC Public Health 2020, 20, 578. [Google Scholar] [CrossRef]
- Bauman, R.; Ling, G.; Tong, L.; Januszkiewicz, A.; Agoston, D.; Delanerolle, N.; Kim, Y.; Ritzel, D.; Bell, R.; Ecklund, J.; et al. An introductory characterization of a combat-casualty-care relevant swine model of closed head injury resulting from exposure to explosive blast. J. Neurotrauma 2009, 26, 841–860. [Google Scholar] [CrossRef]
- Agoston, D. Modeling the long-term consequences of repeated blast-induced mild traumatic brain injuries. J. Neurotrauma 2017, 34, S44–S52. [Google Scholar] [CrossRef]
- Skotak, M.; Townsend, M.T.; Ramarao, K.V.; Chandra, N. A Comprehensive Review of Experimental Rodent Models of Repeated Blast TBI. Front. Neurol. 2019, 10, 1015. [Google Scholar] [CrossRef]
- Cernak, I. Blast Injuries and Blast-Induced Neurotrauma: Overview of Pathophysiology and Experimental Knowledge Models and Findings. In Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects; Kobeissy, F.H., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Milton Park, UK, 2015; Chapter 45. [Google Scholar]
- De Lanerolle, N.; Bandak, F.; Kang, D.; Li, A.; Du, F.; Swager, P.; Parks, S.; Ling, G.; Kim, J.H. Characteristics of an explosive blast-induced brain injury in an experimental model. J. Neuropathol. Exp. Neurol. 2011, 70, 1046–1057. [Google Scholar] [CrossRef]
- Pun, P.; Kan, E.; Salim, A.; Li, Z.; Ng, K.; Moochhala, S.; Ling, E.-A.; Tan, M.H.; Lu, J. Low level primary blast injury in rodent brain. Front. Neurol. 2011, 2, 19. [Google Scholar] [CrossRef]
- Risling, M.; Plantman, S.; Angeria, M.; Rostami, E.; Bellander, B.; Kirkekaard, M.; Arborelius, U.; Davidsson, J. Mechanisms of blast induced brain injuries, experimental studies in rats. NeuroImage 2011, 54, S89–S97. [Google Scholar] [CrossRef]
- Rubovitch, V.; Ten-Bosch, M.; Zohar, O.; Harrison, C.R.; Tempel-Brami, C.; Stein, E.; Hoffer, B.J.; Balaban, C.D.; Schreiber, S.; Chiu, W.-T.; et al. A mouse model of blast-induced mild traumatic brain injury. Exp. Neurol. 2011, 232, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ng, K.; Ling, G.; Wu, J.; Poon, D.; Kan, E.; Tan, M.H.; Wu, Y.J.; Li, P.; Moochhala, S.; et al. Effect of blast exposure on the brain structure and cognition in macaca fascicularis. J. Neurotrauma 2012, 29, 1434–1454. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Zhang, L.; Jin, X.; Chen, C.; Kallakuri, S.; Saif, T.; Cavanaugh, J.; King, A. Biomechanical responses of the brain in swine subject to free-field blasts. Front. Neurol. 2016, 7, 179. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Cui, J.; Simonyi, A.; Johnson, C.; Hubler, G.; DePalma, R.; Gu, Z. Linking blast physics to biological outcomes in mild traumatic brain injury: Narrative review and preliminary report of an open field blast model. Behav. Brain Res. 2018, 340, 147–158. [Google Scholar] [CrossRef]
- Kawa, L.; Kamnaksh, A.; Long, J.; Arborelius, U.; Hokfelt, T.; Agoston, D.; Risling, M. A comparative study of two blast-induced traumatic brain injury models: Changes in monoamine and galanin systems following single and repeated exposure. Front. Neurol. 2018, 9, 479. [Google Scholar] [CrossRef]
- Konan, L.; Song, H.; Pentecost, G.; Fogwe, D.; Ndam, T.; Cui, J.; Johnson, C.E.; Grant, D.; White, T.; Chen, M.; et al. Multi-focal neuronal ultrastructural abnormalities and synaptic alterations in mice after low-intensity blast exposure. J. Neurotrauma 2019, 36, 2117–2128. [Google Scholar] [CrossRef]
- Murphy, E.K.; Iacono, D.; Pan, H.; Grimes, J.B.; Parks, S.; Raiciulescu, S.; Leonessa, F.; Perl, D.P. Explosive-driven double-blast exposure: Molecular, histopathological, and behavioral consequences. Sci. Rep. 2020, 10, 17446. [Google Scholar] [CrossRef]
- Rutter, B.; Song, H.; DePalma, R.G.; Hubler, G.; Cui, J.; Gu, Z.; Johnson, C.E. Shock Wave Physics as Related to Primary Non-Impact Blast-Induced Traumatic Brain Injury. Mil. Med. 2021, 186 (Suppl. 1), 601–609. [Google Scholar] [CrossRef]
- Siedhoff, H.R.; Chen, S.; Song, H.; Cui, J.; Cernak, I.; Cifu, D.X.; DePalma, R.G.; Gu, Z. Perspectives on Primary Blast Injury of the Brain: Translational Insights into Non-inertial Low-Intensity Blast Injury. Front. Neurol. 2022, 12, 818169. [Google Scholar] [CrossRef]
- Agoston, D.V.; McCullough, J.; Aniceto, R.; Lin, I.H.; Kamnaksh, A.; Eklund, M.; GravesIII, W.M.; Dunbar, C.; Engall, J.; Schneider, E.B.; et al. Blood-Based Biomarkers of Repetitive, Subconcussive Blast Overpressure Exposure in the Training Environment: A Pilot Study. Neurotrauma Rep. 2022, 3, 479–490. [Google Scholar] [CrossRef]
- Iacono, D.; Lee, P.; Edlow, B.L.; Gray, N.; Fischl, B.; Kenney, K.; Lew, H.L.; Lozanoff, S.; Liacouras, P.; Lichtenberger, J.; et al. Early-Onset Dementia in War Veterans: Brain Polypathology and Clinicopathologic Complexity. J. Neuropathol. Exp. Neurol. 2020, 79, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Cifu, D.X. Clinical research findings from the long-term impact of military-relevant brain injury consortium-Chronic Effects of Neurotrauma Consortium (LIMBIC-CENC) 2013–2021. Brain Inj. 2022, 36, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhang, R.; Hu, S.; Lai, J. Military traumatic brain injury: A challenge straddling neurology and psychiatry. Mil. Med. Res. 2022, 9, 2. [Google Scholar] [CrossRef]
- Jose, R. Mapping the Mental Health of Residents After the 2013 Boston Marathon Bombings. J. Trauma. Stress 2018, 31, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Garfin, D.R.; Holman, E.A.; Silver, R.C. Exposure to prior negative life events and responses to the Boston marathon bombings. Psychol. Trauma 2020, 12, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, F.T.; Haidar, R.; Mansour, F.; Elbejjani, M.; Khoury, J.E.; Khoury, B.; Ghandour, L.A. Anxiety, depression and PTSD in children and adolescents following the Beirut port explosion. J. Affect. Disord. 2022, 302, 58–65. [Google Scholar] [CrossRef]
- Adams, D.J.; Arthur, C.P.; Stowell, M.H. Architecture of the Synaptophysin/Synaptobrevin Complex: Structural Evidence for an Entropic Clustering Function at the Synapse. Sci. Rep. 2015, 5, 13659. [Google Scholar] [CrossRef]
- Coley, A.A.; Gao, W.J. PSD-95 deficiency disrupts PFC-associated function and behavior during neurodevelopment. Sci. Rep. 2019, 9, 9486. [Google Scholar] [CrossRef]
- Sarrouilhe, D.; di Tommaso, A.; Métayé, T.; Ladeveze, V. Spinophilin: From partners to functions. Biochimie 2006, 88, 1099–1113. [Google Scholar] [CrossRef]
- Holahan, M.R. A Shift from a Pivotal to Supporting Role for the Growth-Associated Protein (GAP-43) in the Coordination of Axonal Structural and Functional Plasticity. Front. Cell. Neurosci. 2017, 11, 266. [Google Scholar] [CrossRef]
- Johnson, L.A.; Banerji, S.; Lawrance, W.; Gileadi, U.; Prota, G.; Holder, K.A.; Roshorm, Y.M.; Hanke, T.; Cerundolo, V.; Gale, N.W.; et al. Dendritic cells enter lymph vessels by hyaluronan-mediated docking to the endothelial receptor LYVE-1. Nat. Immunol. 2017, 18, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Astarita, J.L.; Acton, S.E.; Turley, S.J. Podoplanin: Emerging functions in development, the immune system, and cancer. Front. Immunol. 2012, 3, 283. [Google Scholar] [CrossRef] [PubMed]
- Mader, S.; Brimberg, L. Aquaporin-4 Water Channel in the Brain and Its Implication for Health and Disease. Cells 2019, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Bauer, N.M.; Schäfer, I.; White, R. Making myelin basic protein -from mRNA transport to localized translation. Front. Cell. Neurosci. 2013, 7, 169. [Google Scholar] [CrossRef]
- Fyfe, I. Neurofilament light chain-new potential for prediction and prognosis. Nat. Rev. Neurol. 2019, 15, 557. [Google Scholar] [CrossRef]
- Heizmann, C.W. S100 proteins: Diagnostic and prognostic biomarkers in laboratory medicine. Biochim. Biophys. Acta Mol. Cell. Res. 2019, 1866, 1197–1206. [Google Scholar] [CrossRef]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef]
- Beard, W.A.; Wilson, S.H. Structure and mechanism of DNA polymerase β. Biochemistry 2014, 53, 2768–2780. [Google Scholar] [CrossRef]
- Schier, A.C.; Taatjes, D.J. Structure and mechanism of the RNA polymerase II transcription machinery. Genes Dev. 2020, 34, 465–488. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. Paxinos and Watson’s: The Rat Brain in Stereotaxic Coordinates, 7th ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Shihan, M.H.; Novo, S.G.; Le Marchand, S.J.; Wang, Y.; Duncan, M.K. A simple method for quantitating confocal fluorescent images. Biochem. Biophys. Rep. 2021, 25, 100916. [Google Scholar] [CrossRef]
- Martindale, S.L.; Ord, A.S.; Rule, L.G.; Rowland, J.A. Effects of blast exposure on psychiatric and health symptoms in combat veterans. J. Psychiatr. Res. 2021, 143, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Seif, A.; Shea, C.; Schmid, S.; Stevenson, R.A. A Systematic Review of Brainstem Contributions to Autism Spectrum Disorder. Front. Integr. Neurosci. 2021, 15, 760116. [Google Scholar] [CrossRef] [PubMed]
- Benghanem, S.; Mazeraud, A.; Azabou, E.; Chhor, V.; Shinotsuka, C.R.; Claassen, J.; Rohaut, B.; Sharshar, T. Brainstem dysfunction in critically ill patients. Crit. Care 2020, 24, 5. [Google Scholar] [CrossRef]
- Sykora, P.; Misiak, M.; Wang, Y.; Ghosh, S.; Leandro, G.S.; Liu, D.; Tian, J.; Baptiste, B.A.; Cong, W.-N.; Brenerman, B.M.; et al. DNA polymerase β deficiency leads to neurodegeneration and exacerbates Alzheimer disease phenotypes. Nucleic Acids Res. 2015, 43, 943–959. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.S.B.; Chen, M.; Kochanek, P.M.; Watkins, S.C.; Jin, K.L.; Draviam, R.; Nathaniel, P.D.; Pinto, R.; Marion, D.W.; Graham, S.H. Detection of single- and double-strand DNA breaks after traumatic brain injury in rats: Comparison of in situ labeling techniques using DNA polymerase I, the Klenow fragment of DNA polymerase I, and terminal deoxynucleotidyl transferase. J. Neurotrauma 2001, 18, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.K.; Vemuganti, R. DNA damage and repair following traumatic brain injury. Neurobiol. Dis. 2021, 147, 105143. [Google Scholar] [CrossRef]
- Dassan, P.; Keir, G.; Brown, M.M. Criteria for a clinically informative serum biomarker in acute ischaemic stroke: A review of S100B. Cerebrovasc. Dis. 2009, 27, 295–302. [Google Scholar] [CrossRef]
- Foerch, C.; Singer, O.C.; Neumann-Haefelin, T.; du Mesnil de Rochemont, R.; Steinmetz, H.; Sitzer, M. Evaluation of serum S100B as a surrogate marker for long-term outcome and infarct volume in acute middle cerebral artery infarction. Arch. Neurol. 2005, 62, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Baecker, J.; Wartchow, K.; Sehm, T.; Ghoochani, A.; Buchfelder, M.; Kleindienst, A. Treatment with the Neurotrophic Protein S100B Increases Synaptogenesis after Traumatic Brain Injury. J. Neurotrauma 2020, 37, 1097–1107. [Google Scholar] [CrossRef]
- Mastandrea, P.; Mengozzi, S.; Bernardini, S. Systematic review and cumulative meta-analysis of the diagnostic accuracy of glial fibrillary acidic protein vs. S100 calcium binding protein B as blood biomarkers in observational studies of patients with mild or moderate acute traumatic brain injury. Diagnosis 2021, 9, 18–27. [Google Scholar] [CrossRef]
- Seidenfaden, S.C.; Kjerulff, J.L.; Juul, N.; Kirkegaard, H.; Møller, M.F.; Münster, A.B.; Bøtker, M.T. Diagnostic accuracy of prehospital serum S100B and GFAP in patients with mild traumatic brain injury: A prospective observational multicenter cohort study-“the PreTBI I study”. Scand. J. Trauma Resusc. Emerg. Med. 2021, 29, 75. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Gyorgy, A.; Kamnaksh, A.; Ling, G.; Tong, L.; Parks, S.; Agoston, D. Time-dependent changes of protein biomarker levels in the cerebrospinal fluid after blast traumatic brain injury. Electrophoresis 2012, 33, 3705–3711. [Google Scholar] [CrossRef] [PubMed]
- Janigro, D.; Mondello, S.; Posti, J.P.; Unden, J. GFAP and S100B: What You Always Wanted to Know and Never Dared to Ask. Front. Neurol. 2022, 13, 835597. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.R.; Boltz, A.J.; DeCicco, J.P.; Chandran, A.; DeLellis, S.M.; Healy, M.L.; Kane, S.F.; Lynch, J.H.; Means, G.E.; Hackney, A.C.; et al. Neuroinflammatory Biomarkers Associated with Mild Traumatic Brain Injury History in Special Operations Forces Combat Soldiers. J. Head Trauma Rehabil. 2020, 35, 300–307. [Google Scholar] [CrossRef]
- Gyorgy, A.; Ling, G.; Wingo, D.; Walker, J.; Tong, L.; Parks, S.; Januszkiewicz, A.; Baumann, R.; Agoston, D.V. Time-dependent changes in serum biomarker levels after blast traumatic brain injury. J. Neurotrauma 2011, 28, 1121–1126. [Google Scholar] [CrossRef]
- Haselmann, V.; Schamberger, C.; Trifonova, F.; Ast, V.; Froelich, M.F.; Strauss, M.; Kittel, M.; Jaruschewski, S.; Eschmann, D.; Neumaier, M.; et al. Plasma-based S100B testing for management of traumatic brain injury in emergency setting. Pr. Lab. Med. 2021, 26, e00236. [Google Scholar] [CrossRef]
- Sclocco, R.; Beissner, F.; Bianciardi, M.; Polimeni, J.R.; Napadow, V. Challenges and opportunities for brainstem neuroimaging with ultrahigh field MRI. Neuroimage 2018, 168, 412–426. [Google Scholar] [CrossRef]
- Artero-Guerrero, J.; Pernas-Sánchez, J.; Teixeira-Dias, F. Blast wave dynamics: The influence of the shape of the explosive. J. Hazard. Mater. 2017, 331, 189–199. [Google Scholar] [CrossRef]
- Szu, J.I.; Binder, D.K. The Role of Astrocytic Aquaporin-4 in Synaptic Plasticity and Learning and Memory. Front. Integr. Neurosci. 2016, 10, 8. [Google Scholar] [CrossRef]
- Salman, M.M.; Kitchen, P.; Halsey, A.; Wang, M.X.; Törnroth-Horsefield, S.; Conner, A.C.; Badaut, J.; Iliff, J.J.; Bill, R.M. Emerging roles for dynamic aquaporin-4 subcellular relocalization in CNS water homeostasis. Brain 2022, 145, 64–75. [Google Scholar] [CrossRef]
- Wu, J.; Carlock, C.; JMoreno-Gonzalez, I.S.; Glass, W.; Ross, A.; Barichello, T.; Quevedo, J.; Lou, Y. Requirement of brain interleukin33 for aquaporin4 expression in astrocytes and glymphatic drainage of abnormal tau. Mol. Psychiatry 2021, 26, 5912–5924. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.; Silva, J.; Ferreira, R.; Trigo, D. Glymphatic system, AQP-4, and their implications in Alzheimer’s disease. Neurol. Res. Pract. 2021, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Yamada, K.; Nishiyama, R.; Hashimoto, T.; Nishida, I.; Abe, Y.; Yasui, M.; Iwatsubo, T. Glymphatic system clears extracellular tau and protects from tau aggregation and neurodegeneration. J. Exp. Med. 2022, 219, e20211275. [Google Scholar] [CrossRef] [PubMed]
- Kawoos, U.; Abutarboush, R.; Gu, M.; Chen, Y.; Statz, J.K.; Goodrich, S.Y.; Ahlers, S.T. Blast-induced temporal alterations in blood-brain barrier properties in a rodent model. Sci. Rep. 2021, 11, 5906. [Google Scholar] [CrossRef] [PubMed]
- Sosa, M.A.G.; De Gasperi, R.; Pryor, D.; Garcia, G.S.P.; Perez, G.M.; Abutarboush, R.; Kawoos, U.; Hogg, S.; Ache, B.; Janssen, W.G.; et al. Low-level blast exposure induces chronic vascular remodeling, perivascular astrocytic degeneration and vascular-associated neuroinflammation. Acta Neuropathol. Commun. 2021, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Rainey-Smith, S.R.; Mazzucchelli, G.N.; Villemagne, V.L.; Brown, B.M.; Porter, T.; Weinborn, M.; Bucks, R.S.; Milicic, L.; Sohrabi, H.R.; Taddei, K.; et al. AIBL Research Group. Genetic variation in Aquaporin-4 moderates the relationship between sleep and brain Aβ-amyloid burden. Transl. Psychiatry 2018, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Dai, S.; Jin, C.; Si, X.; Gu, L.; Song, Z.; Gao, T.; Chen, Y.; Yan, Y.; Yin, X.; et al. Aquaporin-4 Polymorphisms Are Associated with Cognitive Performance in Parkinson’s Disease. Front. Aging Neurosci. 2022, 13, 740491. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, C.; Guo, Q.; Chu, H. Aquaporin-4 and Cognitive Disorders. Aging Dis. 2022, 13, 61–72. [Google Scholar] [CrossRef]
- Hsu, J.L.; Wei, Y.C.; Toh, C.H.; Hsiao, I.T.; Lin, K.J.; Yen, T.C.; Liao, M.; Ro, L. Magnetic Resonance Images Implicate that Glymphatic Alterations Mediate Cognitive Dysfunction in Alzheimer Disease. Ann. Neurol. 2022, 93, 164–174. [Google Scholar] [CrossRef]
- Goyal, A.; Failla, M.; Niyonkuru, C.; Amin, K.; Fabio, A.; Berger, R.; Wagner, A.K.; Ercole, A.; Thelin, E.P.; Holst, A.; et al. S100b as a prognostic biomarker in outcome prediction for patients with severe traumatic brain injury. J. Neurotrauma 2013, 30, 946–957. [Google Scholar] [CrossRef]
- Smyth, L.C.D.; Highet, B.; Jansson, D.; Wu, J.; Rustenhoven, J.; Aalderink, M.; Tan, A.; Li, S.; Johnson, R.; Coppieters, N.; et al. Characterisation of PDGF-BB:PDGFRβ signaling pathways in human brain pericytes: Evidence of disruption in Alzheimer’s disease. Commun. Biol. 2022, 5, 235. [Google Scholar] [CrossRef] [PubMed]
- Armonda, R.A.; Bell, R.S.; Vo, A.H.; Ling, G.; DeGraba, T.J.; Crandall, B.; Ecklund, J.; Campbell, W.W. Wartime traumatic cerebral vasospasm: Recent review of combat casualties. Neurosurgery 2006, 59, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Abutarboush, R.; Gu, M.; Kawoos, U.; Mullah, S.; Chen, Y.; Goodrich, S.; Lashof-Sullivan, M.; McCarron, R.M.; Statz, J.K.; Bell, R.S.; et al. Exposure to Blast Overpressure Impairs Cerebral Microvascular Responses and Alters Vascular and Astrocytic Structure. J. Neurotrauma 2019, 36, 3138–3157. [Google Scholar] [CrossRef]
- Muoio, V.; Persson, P.B.; Sendeski, M.M. The neurovascular unit-concept review. Acta Physiol. 2014, 210, 790–798. [Google Scholar] [CrossRef]
- Schaeffer, S.; Iadecola, C. Revisiting the neurovascular unit. Nat. Neurosci. 2021, 24, 1198–1209. [Google Scholar] [CrossRef] [PubMed]
- Kanner, A.A.; Marchi, N.; Fazio, V.; Mayberg, M.R.; Koltz, M.T.; Siomin, V.; Stevens, G.H.J.; Masaryk, T.; Ayumar, B.; Vogelbaum, M.A.; et al. Serum S100beta: A noninvasive marker of blood-brain barrier function and brain lesions. Cancer 2003, 97, 2806–2813. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.D.; Tharakan, B.; Lomas, A.; Wiggins-Dohlvik, K.; Alluri, H.; Shaji, C.A.; Jupiter, D.; Isbell, C.L. Exploring blood-brain barrier hyperpermeability and potential biomarkers in traumatic brain injury. Bayl. Univ. Med. Cent. Proc. 2020, 33, 199–204. [Google Scholar] [CrossRef]
- Nguyen, Q.L.; Okuno, N.; Hamashima, T.; Dang, S.T.; Fujikawa, M.; Ishii, Y.; Enomoto, A.; Maki, T.; Nguyen, H.N.; Nguyen, V.T.; et al. Vascular PDGFR-alpha protects against BBB dysfunction after stroke in mice. Angiogenesis 2021, 24, 35–46. [Google Scholar] [CrossRef]
- Uzunalli, G.; Herr, S.; Dieterly, A.M.; Shi, R.; Lyle, L.T. Structural disruption of the blood-brain barrier in repetitive primary blast injury. Fluids Barriers CNS 2021, 18, 2. [Google Scholar] [CrossRef]
- Bloniecki, V.; Zetterberg, H.; Aarsland, D.; Vannini, P.; Kvartsberg, H.; Winblad, B.; Blennow, K.; Freund-Levi, Y. Are neuropsychiatric symptoms in dementia linked to CSF biomarkers of synaptic and axonal degeneration? Alzheimers Res. Ther. 2020, 12, 153. [Google Scholar] [CrossRef]
- Carriel, V.; Garzón, I.; Campos, A.; Cornelissen, M.; Alaminos, M. Differential expression of GAP-43 and neurofilament during peripheral nerve regeneration through bio-artificial conduits. J. Tissue Eng. Regen. Med. 2017, 11, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Eastwood, S.L.; Harrison, P.J. Synaptic pathology in the anterior cingulate cortex in schizophrenia and mood disorders. A review and a Western blot study of synaptophysin, GAP-43 and the complexins. Brain Res. Bull. 2001, 55, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, A.K.; Thotakura, B.; Priyadarshini, S.C.; Patil, S.; Poojari, M.S.; Subramanian, M. Paternal stress alters synaptic density and expression of GAP-43, GRIN1, M1 and SYP genes in the hippocampus and cortex of offspring of stress-induced male rats. Morphologie 2022, 107, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Jamjoom, A.A.B.; Rhodes, J.; Andrews, P.J.D.; Grant, S.G.N. The synapse in traumatic brain injury. Brain 2021, 144, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Hulsebosch, C.E.; DeWitt, D.S.; Jenkins, L.W.; Prough, D.S. Traumatic brain injury in rats results in increased expression of Gap-43 that correlates with behavioral recovery. Neurosci. Lett. 1998, 255, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Kochanek, P.M.; Dixon, C.E.; Shellington, D.K.; Shin, S.S.; Bayır, H.; Jackson, E.K.; Kagan, V.E.; Yan, H.Q.; Swauger, P.V.; Parks, S.A.; et al. Screening of biochemical and molecular mechanisms of secondary injury and repair in the brain after experimental blast-induced traumatic brain injury in rats. J. Neurotrauma 2013, 30, 920–937. [Google Scholar] [CrossRef]


| Antibody | Protein Concentration Loaded | Antibody Concentration | Cat. # | Source | |
|---|---|---|---|---|---|
| Synaptic | Spinophilin | 10 μg | 1:1000 | 14136 | Cell Signaling, Danvers, MA, USA |
| Synaptophysin | 10 μg | 1:2000 | ab8049 | Abcam, Cambridge, MA, USA | |
| PSD-95 | 10 μg | 1:1000 | 75-028 | Antibodies, Inc., Davis, CA, USA | |
| GAP-43 | 10 μg | 1:40,000 | ab75810 | Abcam, Cambridge, MA, USA | |
| Glymphatic | Podoplanin | 20 μg | 1:1000 | 131216 | Abcam, Cambridge, MA, USA |
| LYVE1 | 10 μg | 1:1000 | ab183501 | Abcam, Cambridge, MA, USA | |
| Aquaporin-4 (B5) | 10 μg | 1:500 | sc-390488 | Santa Cruz Biotechnology, Dallas, TX, USA | |
| Axonal/Myelin | MBP | 10 μg | 1:4000 | ab62631 | Abcam, Cambridge, MA, USA |
| Neurofilament Light | 20 μg | 1:1000 | MCA-DA2 | Encor, Gainesville, FL, USA | |
| Neurovascular | S100β | 10 μg | 1:5000 | ab52642 | Abcam, Cambridge, MA, USA |
| PDGF | 10 μg | 1:5000 | ab32570 | Abcam, Cambridge, MA, USA | |
| Genomic | DNA Polymerase β | 10 μg | 1:1000 | ab26343 | Abcam, Cambridge, MA, USA |
| RNA Polymerase II | 10 μg | 1:500 | 05-623Z | Millipore-Sigma, Burlington, MA, USA |
| Antibody | Cat. # | Source |
|---|---|---|
| GAP-43 | EKL61628 | Biomatik USA, Wilmington, DE, USA |
| AQP4 | abx061777 | Abbexa, Houston, TX, USA |
| S100β | abx256298 | Abbexa, Houston, TX, USA |
| PDGF | KE10057 | Proteintech, Rosemont, IL, USA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacono, D.; Murphy, E.K.; Stimpson, C.D.; Leonessa, F.; Perl, D.P. Double Blast Wave Primary Effect on Synaptic, Glymphatic, Myelin, Neuronal and Neurovascular Markers. Brain Sci. 2023, 13, 286. https://doi.org/10.3390/brainsci13020286
Iacono D, Murphy EK, Stimpson CD, Leonessa F, Perl DP. Double Blast Wave Primary Effect on Synaptic, Glymphatic, Myelin, Neuronal and Neurovascular Markers. Brain Sciences. 2023; 13(2):286. https://doi.org/10.3390/brainsci13020286
Chicago/Turabian StyleIacono, Diego, Erin K. Murphy, Cheryl D. Stimpson, Fabio Leonessa, and Daniel P. Perl. 2023. "Double Blast Wave Primary Effect on Synaptic, Glymphatic, Myelin, Neuronal and Neurovascular Markers" Brain Sciences 13, no. 2: 286. https://doi.org/10.3390/brainsci13020286
APA StyleIacono, D., Murphy, E. K., Stimpson, C. D., Leonessa, F., & Perl, D. P. (2023). Double Blast Wave Primary Effect on Synaptic, Glymphatic, Myelin, Neuronal and Neurovascular Markers. Brain Sciences, 13(2), 286. https://doi.org/10.3390/brainsci13020286







