Endocannabinoid System and Exogenous Cannabinoids in Depression and Anxiety: A Review
Abstract
1. Introduction
2. Constituents of Cannabis sativa
3. Cannabinoid Receptors
4. Additional Receptors for Cannabinoids
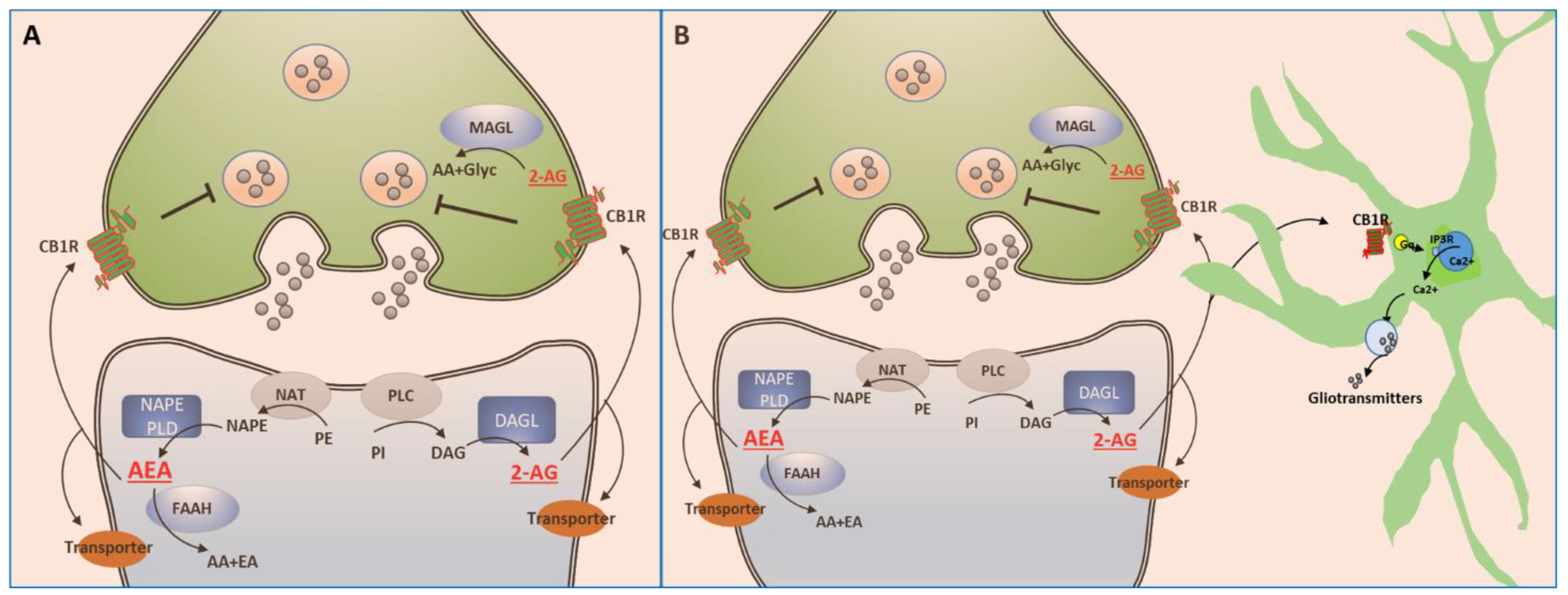
5. The Endocannabinoid System
5.1. Endocannabinoids
5.2. Enzymes Responsible for the Synthesis and Degradation of eCBs
6. Signaling of the ECS
7. Reuptake of eCBs
8. Cannabinoids in Depression and Anxiety
8.1. Cannabinoids and Depression-like Effects in Animal Studies
8.2. Evidence for the Involvement of the ECS in Depression, Anxiety, and Stress
8.3. Perturbations of the ECS in Humans with Depression
9. Clinical Observations Obtained from Long-Time Cannabis Users
10. Clinical Use of Cannabinoids to Treat Depression, Anxiety, and Stress
10.1. Δ9-Tetrahydrocannabinol
10.2. Cannabidiol
10.3. Combinations of THC and CBD
10.4. Synthetic Cannabinoids
11. Discussion, Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- ElSohly, M.A.; Slade, D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci. 2005, 78, 539–548. [Google Scholar] [CrossRef]
- Gaoni, Y.; Mechoulam, R. Isolation, structure and partialsynthesis of anactive constituent of hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- World Health Organization, Office on Drugs and Crime (UNDOC), Report 2022. Available online: https://www.unodc.org/unodc/en/press/releases/2022/June/unodc-world-drug-report-2022 (accessed on 20 July 2022).
- IsHak, W.W.; Wen, R.Y.; Naghdechi, L.; Vanle, B.; Dang, J.; Knosp, M.; Dascal, J.; Marcia, L.; Gohar, Y.; Louy, C.; et al. Pain and depression: A systematic review. Harv. Rev. Psychiatry 2018, 26, 352–363. [Google Scholar] [CrossRef]
- Huang, W.; Chen, W.; Zhang, X. Endocannabinoid system: Role in depression, reward and pain control. Mol. Med. Rep. 2016, 14, 2899–2903. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. Prog. Chem. Org. Nat. Prod. 2017, 103, 1–36. [Google Scholar] [CrossRef]
- Adams, R.; Hunt, M.; Clark, J.H. Structure of cannabidiol, a product isolated from the marihuana extract of Minnesota wild hemp. I. J. Am. Chem. Soc. 1940, 62, 196–200. [Google Scholar] [CrossRef]
- Casadio, P.; Fernandes, C.; Murray, R.M.; Di Forti, M. Cannabis use in young people: The risk for schizophrenia. Neurosci. Biobehav. Rev. 2011, 35, 1779–1787. [Google Scholar] [CrossRef]
- Pacher, P.; Bátkai, S.; Kunos, G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar] [CrossRef]
- Micale, V.; Di Marzo, V.; Sulcova, A.; Wotjak, C.T.; Drago, F. Endocannabinoid system and mood disorders: Priming a target for new therapies. Pharmacol. Ther. 2013, 138, 18–37. [Google Scholar] [CrossRef]
- Madras, B.K. Cannabinoid and Marijuana Neurobiology. In Cannabis in Medicine; Springer: Cham, Switzerland, 2020; pp. 25–47. [Google Scholar]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Di Marzo, V.; Bifulco, M.; De Petrocellis, L. The endocannabinoid system and its therapeutic exploitation. Nat. Rev. Drug Discov. 2004, 3, 771–784. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.H.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB1 and CB2. Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef]
- Herkenham, M.; Lynn, A.B.; Little, M.D.; Johnson, M.R.; Melvin, L.S.; de Costa, B.R.; Rice, K.C. Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. USA 1990, 87, 1932–1936. [Google Scholar] [CrossRef]
- Herkenham, M.; Lynn, A.B.; de Costa, B.R.; Richfield, E.K. Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Res. 1991, 547, 267–274. [Google Scholar] [CrossRef]
- Hohmann, A.G.; Herkenham, M. Localization of cannabinoid CB(1) receptor mRNA in neuronal subpopulations of rat striatum: A double-label in situ hybridization study. Synapse 2000, 7, 71–80. [Google Scholar] [CrossRef]
- Sun, Y.; Alexander, S.P.; Kendall, D.A.; Bennett, A.J. Cannabinoids and PPARalpha signalling. Biochem. Soc. Trans. 2006, 34, 1095–1097. [Google Scholar] [CrossRef]
- Sawzdargo, M.; Nguyen, T.; Lee, D.K.; Lynch, K.R.; Cheng, R.; Heng, H.H.; George, S.R.; O’Dowd, B.F. Identification and cloning of three novel human G protein-coupled receptor genes GPR52, PsiGPR53 and GPR55: GPR55 is extensively expressed in human brain. Brain Res. Mol. Brain Res. 1999, 64, 193–198. [Google Scholar] [CrossRef]
- Shi, Q.X.; Yang, L.K.; Shi, W.L.; Wang, L.; Zhou, S.-M.; Guan, S.-Y.; Zhao, M.-G.; Yang, Q. The novel cannabinoid receptor GPR55 mediates anxiolytic-like effects in the medial orbital cortex of mice with acute stress. Mol. Brain 2017, 10, 38. [Google Scholar] [CrossRef]
- Ryberg, E.; Larsson, N.; Sjögren, S.; Hjorth, S.; Hermansson, N.O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P.J. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007, 152, 1092–1101. [Google Scholar] [CrossRef]
- Overton, H.A.; Babbs, A.J.; Doel, S.M.; Fyfe, M.C.; Gardner, L.S.; Griffin, G.; Jackson, H.C.; Procter, M.J.; Rasamison, C.M.; Tang-Christensen, M.; et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006, 3, 167–175. [Google Scholar] [CrossRef]
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular targets of the phytocannabinoids: A complex picture. Prog. Chem. Org. Nat. Prod. 2017, 103, 103–131. [Google Scholar]
- Pérez-Olives, C.; Rivas-Santisteban, R.; Lillo, J.; Navarro, G.; Franco, R. Recent Advances in the Potential of Cannabinoids for Neuroprotection in Alzheimer’s, Parkinson’s, and Huntington’s Diseases. Adv. Exp. Med. Biol. 2021, 1264, 81–92. [Google Scholar] [CrossRef]
- Callén, L.; Moreno, E.; Barroso-Chinea, P.; Moreno-Delgado, D.; Cortés, A.; Mallol, J.; Casadó, V.; Lanciego, J.L.; Franco, R.; Lluis, C.; et al. Cannabinoid receptors CB1 and CB2 form functional heteromers in brain. J. Biol. Chem. 2012, 287, 20851–20865. [Google Scholar] [CrossRef]
- Glass, M.; Felder, C.C. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: Evidence for a Gs linkage to the CB1 receptor. J. Neurosci. 1997, 17, 5327–5333. [Google Scholar] [CrossRef]
- Przybyla, J.A.; Watts, V.J. Ligand-induced regulation and localization of cannabinoid CB1 and dopamine D2L receptor heterodimers. J. Pharmacol. Exp. Ther. 2010, 332, 710–719. [Google Scholar] [CrossRef]
- Pazos, M.R.; Mohammed, N.; Lafuente, H.; Santos, M.; Martínez-Pinilla, E.; Moreno, E.; Valdizan, E.; Romero, J.; Pazos, A.; Franco, R.; et al. Mechanisms of cannabidiol neuroprotection in hypoxic-ischemic newborn pigs: Role of 5HT(1A) and CB2 receptors. Neuropharmacology 2013, 71, 282–291. [Google Scholar] [CrossRef]
- Vinals, X.; Moreno, E.; Lanfumey, L.; Cordomí, A.; Pastor, A.; De La Torre, R.; Gasperini, P.; Navarro, G.; Howell, L.A.; Pardo, L.; et al. Cognitive impairment induced by Delta9-tetrahydrocannabinol occurs through heteromers between cannabinoid CB1 and serotonin 5-HT2A receptors. PLoS Biol. 2015, 13, e1002194. [Google Scholar] [CrossRef]
- Orgado, J.M.; Fernández-Ruiz, J.; Romero, J. The endocannabinoid system in neuropathological states. Int. Rev. Psychiatry 2009, 21, 172–180. [Google Scholar] [CrossRef]
- Martınez-Pinilla, E.; Reyes-Resina, I.; Oñatibia-Astibia, A.; Zamarbide, M.; Ricobaraza, A.; Navarro, G.; Moreno, E.; Dopeso-Reyes, I.; Sierra, S.; Rico, A.; et al. CB1 and GPR55 receptors are co-expressed and form heteromers in rat and monkey striatum. Exp. Neurol. 2014, 261, 44–52. [Google Scholar] [CrossRef]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R.; et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [CrossRef]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamashita, A.; Waku, K. 2-Arachidonoylglycerol: A possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995, 215, 89–97. [Google Scholar] [CrossRef]
- Mechoulam, R.; Hanus, L.O.; Pertwee, R.; Howlett, A.C. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat. Rev. Neurosci. 2014, 15, 757–764. [Google Scholar] [CrossRef]
- Hanus, L.; Abu-Lafi, S.; Fride, E.; Breuer, A.; Vogel, Z.; Shalev, D.E.; Kustanovich, I.; Mechoulam, R. 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc. Natl. Acad. Sci. USA 2001, 98, 3662–3665. [Google Scholar] [CrossRef]
- Huang, S.M.; Bisogno, T.; Trevisani, M.; Al-Hayani, A.; De Petrocellis, L.; Fezza, F.; Tognetto, M.; Petros, T.J.; Krey, J.F.; Chu, C.J.; et al. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl. Acad. Sci. USA 2002, 99, 8400–8405. [Google Scholar] [CrossRef]
- Porter, A.C.; Sauer, J.M.; Knierman, M.D.; Becker, G.W.; Berna, M.J.; Bao, J.; Nomikos, G.G.; Carter, P.; Bymaster, F.P.; Leese, A.B.; et al. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J. Pharmacol. Exp. Ther. 2002, 301, 1020–1024. [Google Scholar] [CrossRef]
- Leggett, J.D.; Aspley, S.; Beckett, S.R.; D’Antona, A.M.; Kendall, D.A.; Kendall, D.A. Oleamide is a selective endogenous agonist of rat and human CB1 cannabinoid receptors. Br. J. Pharmacol. 2004, 141, 253–262. [Google Scholar] [CrossRef]
- Cravatt, B.F.; Giang, D.K.; Mayfield, S.P.; Boger, D.L.; Lerner, R.A.; Gilula, N.B. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 1996, 384, 83–87. [Google Scholar] [CrossRef]
- Blankman, J.L.; Simon, G.M.; Cravatt, B.F. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem. Biol. 2007, 14, 1347–1356. [Google Scholar] [CrossRef]
- Kozak, K.R.; Marnett, L.J. Oxidative metabolism of endocannabinoids. Prostaglandins Leukot. Essent. Fat. Acids (PLEFA) 2002, 66, 211–220. [Google Scholar] [CrossRef]
- van der Stelt, M.; van Kuik, J.A.; Bari, M.; van Zadelhoff, G.; Leeflang, B.R.; Veldink, G.A.; Finazzi-Agrò, A.; Vliegenthart, J.F.; Maccarrone, M. Oxygenated metabolites of anandamide and 2-arachidonoylglycerol: Conformational analysis and interaction with cannabinoid receptors, membrane transporter, and fatty acid amide hydrolase. J. Med. Chem. 2002, 45, 3709–3720. [Google Scholar] [CrossRef]
- Woodward, D.F.; Carling, R.W.; Cornell, C.L.; Fliri, H.G.; Martos, J.L.; Pettit, S.N.; Liang, Y.; Wang, J.W. The pharmacology and therapeutic relevance of endocannabinoid derived cyclo-oxygenase (COX)-2 products. Pharmacol. Ther. 2008, 120, 71–80. [Google Scholar] [CrossRef]
- Wilson, R.I.; Nicoll, R.A. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 2001, 410, 588–592, Erratum in: Nature 2001, 411, 974. [Google Scholar] [CrossRef]
- Araque, A.; Castillo, P.E.; Manzoni, O.J.; Tonini, R. Synaptic functions of endocannabinoid signaling in health and disease. Neuropharmacology 2017, 124, 13–24. [Google Scholar] [CrossRef]
- Castillo, P.E.; Younts, T.J.; Chávez, A.E.; Hashimotodani, Y. Endocannabinoid signaling and synaptic function. Neuron 2012, 76, 70–81. [Google Scholar] [CrossRef]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [CrossRef]
- Metna-Laurent, M.; Marsicano, G. Rising stars: Modulation of brain functions by astroglial type-1 cannabinoid receptors. Glia 2015, 63, 353–364. [Google Scholar] [CrossRef]
- Navarrete, M.; Díez, A.; Araque, A. Astrocytes in endocannabinoid signalling. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130599. [Google Scholar] [CrossRef]
- Chevaleyre, V.; Takahashi, K.A.; Castillo, P.E. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu. Rev. Neurosci. 2006, 29, 37–76. [Google Scholar] [CrossRef]
- Mátyás, F.; Gabriella, M.U.; Masahiko, W.; Ken, M.; Andreas, Z.; Tamás, F.F.; István, K. Identification of the sites of 2-arachidonoylglycerol synthesis and action imply retrograde endocannabinoid signaling at both GABAergic and glutamatergic synapses in the ventral tegmental area. Neuropharmacology 2008, 54, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M.L.; Shobin, E.; Pickel, V.M. Cannabinoid modulation of the dopaminergic circuitry: Implications for limbic and striatal output. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 38, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Covey, D.P.; Mateo, Y.; Sulzer, D.; Cheer, J.F.; Lovinger, D.M. Endocannabinoid modulation of dopamine neurotransmission. Neuropharmacology 2017, 124, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Szabo, B.; Siemes, S.; Wallmichrath, I. Inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. Eur. J. Neurosci. 2002, 15, 2057–2061. [Google Scholar] [CrossRef] [PubMed]
- Riegel, A.C.; Lupica, C.R. Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J. Neurosci. 2004, 24, 11070–11078. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Pistis, M. Hub and switches: Endocannabinoid signalling in midbrain dopamine neurons. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 3276–3285. [Google Scholar] [CrossRef]
- Wang, T.; Collet, J.P.; Shapiro, S.; Ware, M.A. Adverse effects of medical cannabinoids: A systematic review. CMAJ 2008, 178, 1669–1678. [Google Scholar] [CrossRef]
- Talani, G.; Licheri, V.; Biggio, F.; Locci, V.; Mostallino, M.C.; Secci, P.P.; Melis, V.; Dazzi, L.; Carta, G.; Banni, S.; et al. Enhanced Glutamatergic Synaptic Plasticity in the Hippocampal CA1 Field of Food-Restricted Rats: Involvement of CB1 Receptors. Neuropsychopharmacology 2016, 41, 1308–1318. [Google Scholar] [CrossRef]
- Melis, M.; Pistis, M.; Perra, S.; Muntoni, A.L.; Pillolla, G.; Gessa, G.L. Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J. Neurosci. 2004, 24, 53–62. [Google Scholar] [CrossRef]
- Spanagel, R. Cannabinoids and the endocannabinoid system in reward processing and addiction: From mechanisms to interventions. Dialogues Clin. Neurosci. 2020, 22, 241–250. [Google Scholar] [CrossRef]
- Robbe, D.; Kopf, M.; Remaury, A.; Bockaert, J.; Manzoni, O.J. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc. Natl. Acad. Sci. USA 2002, 99, 8384–8388. [Google Scholar] [CrossRef] [PubMed]
- Zlebnik, N.E.; Cheer, J.F. Beyond the CB1 receptor: Is cannabidiol the answer for disorders of motivation? Annu. Rev. Neurosci. 2016, 39, 1–17. [Google Scholar] [CrossRef]
- Heifets, B.D.; Castillo, P.E. Endocannabinoid Signaling and Long-Term Synaptic Plasticity. Annu. Rev. Physiol. 2009, 71, 283–306. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.J. Anandamide uptake explained? Trends Pharmacol. Sci. 2012, 33, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Poleszak, E.; Wośko, S.; Sławińska, K.; Szopa, A.; Wróbel, A.; Serefko, A. Cannabinoids in depressive disorders. Life Sci. 2018, 213, 18–24. [Google Scholar] [CrossRef]
- Hill, M.N.; Gorzalka, B.B. Is there a role for the endocannabinoid system in the etiology and treatment of melancholic depression? Behav. Pharmacol. 2005, 16, 333–352. [Google Scholar] [CrossRef] [PubMed]
- El-Alfy, A.T.; Ivey, K.; Robinson, K.; Ahmed, S.; Radwan, M.; Slade, D.; Khan, I.; ElSohly, M.; Ross, S. Antidepressant-like effect of Δ9-tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L. Pharmacol. Biochem. Behav. 2010, 95, 434–442. [Google Scholar] [CrossRef]
- Bambico, F.R.; Hattan, P.R.; Garant, J.P.; Gobbi, G. Effect of delta-9-tetrahydrocannabinol on behavioral despair and on pre- and postsynaptic serotonergic transmission. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 38, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Bambico, F.R.; Duranti, A.; Tontini, A.; Tarzia, G.; Gobbi, G. Endocannabinoids in the treatment of mood disorders: Evidence from animal models. Curr. Pharm. Des. 2009, 15, 1623–1646. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, P.; Golda, A.; McCreary, A.C.; Filip, M.; Przegaliriski, E. Activation of endocannabinoid transmission induces antidepressant-like effects in rats. Acta Physiol. Pol. 2008, 59, 217. [Google Scholar]
- Hayase, T. Differential effects of TRPV1 receptor ligands against nicotine-induced depression-like behaviors. BMC Pharmacol. 2011, 11, 6. [Google Scholar] [CrossRef]
- Umathe, S.N.; Manna, S.S.; Jain, N.S. Involvement of endocannabinoids in antidepressant and anti-compulsive effect of fluoxetine in mice. Behav. Brain Res. 2011, 223, 125–134. [Google Scholar] [CrossRef]
- Rubino, T.; Parolaro, D. Long lasting consequences of cannabis exposure in adolescence. Mol. Cell. Endocrinol. 2008, 286, S108–S113. [Google Scholar] [CrossRef] [PubMed]
- Long, J.Z.; Li, W.; Booker, L.; Burston, J.J.; Kinsey, S.G.; Schlosburg, J.E.; Pavon, F.J.; Serrano, A.M.; Selley, D.E.; Parsons, L.H.; et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat. Chem. Biol. 2009, 5, 37–44. [Google Scholar] [CrossRef]
- O’Brien, L.D.; Wills, K.L.; Segsworth, B.; Dashney, B.; Rock, E.M.; Limebeer, C.L.; Parker, L.A. Effect of chronic exposure to rimonabant and phytocannabinoids on anxiety-like behavior and saccharin palatability. Pharmacol. Biochem. Behav. 2013, 103, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Wilson, R.; Jackson, R.; Ball, M.; Shetty, H.; Broadbent, M.; Stewart, R.; McGuire, P.; Bhattacharyya, S. Cannabis use and treatment resistance in first episode psychosis: A natural language processing study. Lancet 2015, 385, S79. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.A.; Kaiser, N.; Monory, K.; Lutz, B. Reduced anxietylike behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology 2008, 54, 141–150. [Google Scholar] [CrossRef]
- Patel, S.; Hillard, C.J. Role of endocannabinoid signaling in anxiety and depression. Curr. Top. Behav. Neurosci. 2009, 1, 347–371. [Google Scholar]
- Haller, J.; Varga, B.; Ledent, C.; Freund, T.F. CB1 cannabinoid receptors mediate anxiolytic effects: Convergent genetic and pharmacological evidence with CB1-specific agents. Behav. Pharmacol. 2004, 15, 299–304. [Google Scholar] [CrossRef]
- Spanagel, R.; Bilbao, A. Approved cannabinoids for medical purposes—Comparative systematic review and meta-analysis for sleep and appetite. Neuropharmacology 2021, 196, 108680. [Google Scholar] [CrossRef]
- Degroot, A.; Nomikos, G.G. Genetic deletion and pharmacological blockade of CB1 receptors modulates anxiety in the shock-probe burying test. Eur. J. Neurosci. 2004, 20, 1059–1064. [Google Scholar] [CrossRef]
- Kathuria, S.; Gaetani, S.; Fegley, D.; Valiño, F.; Duranti, A.; Tontini, A.; Mor, M.; Tarzia, G.; La Rana, G.; Calignano, A.; et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat. Med. 2003, 9, 76–81. [Google Scholar] [CrossRef]
- Bortolato, M.; Campolongo, P.; Mangieri, R.A.; Scattoni, M.L.; Frau, R.; Trezza, V.; La Rana, G.; Russo, R.; Calignano, A.; Gessa, G.L.; et al. Anxiolytic-like properties of the anandamide transport inhibitor AM404. Neuropsychopharmacology 2006, 31, 2652–2659. [Google Scholar] [CrossRef]
- Gorzalka, B.B.; Hill, M.N. Putative role of endocannabinoid signaling in the etiology of depression and actions of antidepressants. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 1575–1585. [Google Scholar] [CrossRef]
- Shearman, L.P.; Rosko, K.M.; Fleischer, R.; Wang, J.; Xu, S.; Tong, X.S.; Rocha, B.A. Antidepressant-like and anorectic effects of the cannabinoid CB1 receptor inverse agonist AM251 in mice. Behav. Pharmacol. 2003, 14, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Tzavara, E.T.; Davis, R.J.; Perry, K.W.; Li, X.; Salhoff, C.; Bymaster, F.P.; Witkin, J.M.; Nomikos, G.G. The CB1 receptor antagonist selectively increases monoaminergic neurotransmission in the medial prefrontal cortex: Implications for therapeutic actions. Br. J. Pharmacol. 2003, 138, 544–553. [Google Scholar] [CrossRef]
- Witkin, J.M.; Tzavara, E.T.; Davis, R.J.; Li, X.; Nomikos, G.G. A therapeutic role for cannabinoid CB1 receptor antagonists in major depressive disorders. Trends Pharmacol. Sci. 2005, 26, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Griebel, G.; Stemmelin, J.; Scatton, B. Effects of the cannabinoid CB1 receptor antagonist rimonabant in models of emotional reactivity in rodents. Biol. Psychiatry 2005, 57, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Sanchis-Segura, C.; Cline, B.H.; Marsicano, G.; Lutz, B.; Spanagel, R. Reduced sensitivity to reward in CB1 knockout mice. Psychopharmacology 2004, 176, 223–232. [Google Scholar] [CrossRef]
- Haller, J.; Varga, B.; Ledent, C.; Barna, I.; Freund, T.F. Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behaviour in mice. Eur. J. Neurosci. 2004, 19, 1906–1912. [Google Scholar] [CrossRef]
- Haller, J.; Bakos, N.; Szirmay, M.; Ledent, C.; Freund, T.F. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur. J. Neurosci. 2002, 16, 1395–1398. [Google Scholar] [CrossRef]
- Mikics, E.; Vas, J.; Aliczki, M.; Halasz, J.; Haller, J. Interactions between the anxiogenic effects of CB1 gene disruption and 5-HT3 neurotransmission. Behav. Pharmacol. 2009, 20, 265–272. [Google Scholar] [CrossRef]
- Aso, E.; Ozaita, A.; Valdizán, E.M.; Ledent, C.; Pazos, Á.; Maldonado, R.; Valverde, O. BDNF impairment in the hippocampus is related to enhanced despair behavior in CB1 knockout mice. J. Neurochem. 2008, 105, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Steiner, M.A.; Wanisch, K.; Monory, K.; Marsicano, G.; Borroni, E.; Bächli, H.; Holsboer, F.; Lutz, B.; Wotjak, C.T. Impaired cannabinoid receptor type 1 signaling interferes with stress-coping behavior in mice. Pharm. J. 2008, 8, 196–208. [Google Scholar] [CrossRef]
- Patel, S.; Hillard, C.J. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: Further evidence for an anxiolytic role for endogenous cannabinoid signaling. J. Pharmacol. Exp. Ther. 2006, 318, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; Carrier, E.J.; McLaughlin, R.J.; Morrish, A.C.; Meier, S.E.; Hillard, C.J.; Gorzalka, B.B. Regional alterations in the endocannabinoid system in an animal model of depression: Effects of concurrent antidepressant treatment. J. Neurochem. 2008, 106, 2322–2336. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; Gorzalka, B.B. Enhancement of anxiety-like responsiveness to the cannabinoid CB(1) receptor agonist HU-210 following chronic stress. Eur. J. Pharmacol. 2004, 499, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Reich, C.G.; Taylor, M.E.; McCarthy, M.M. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav. Brain Res. 2009, 203, 264–269. [Google Scholar] [CrossRef]
- Hill, K.P. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: A clinical review. JAMA 2015, 313, 2474–2483. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, D.J.; Meier, S.E.; Shi, L.; Ho WS, V.; Jarrahian, A.; Hillard, C.J. Effects of acute and repeated restraint stress on endocannabinoid content in the amygdala, ventral striatum, and medial prefrontal cortex in mice. Neuropharmacology 2008, 54, 108–116. [Google Scholar] [CrossRef]
- Rossi, S.; De Chiara, V.; Musella, A.; Kusayanagi, H.; Mataluni, G.; Bernardi, G.; Usiello, A.; Centonze, D. Chronic psychoemotional stress impairs cannabinoid-receptor-mediated control of GABA transmission in the striatum. J. Neurosci. 2008, 28, 7284–7292. [Google Scholar] [CrossRef] [PubMed]
- Wamsteeker, J.I.; Kuzmiski, J.B.; Bains, J.S. Repeated stress impairs endocannabinoid signaling in the paraventricular nucleus of the hypothalamus. J. Neurosci. 2010, 30, 11188–11196. [Google Scholar] [CrossRef] [PubMed]
- Legare, C.A.; Raup-Konsavage, W.M.; Vrana, K.E. Therapeutic Potential of Cannabis, Cannabidiol, and Cannabinoid-Based Pharmaceuticals. Pharmacology 2022, 107, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, G.; Bambico, F.; Mangieri, R.; Bortolato, M.; Campolongo, P.; Solinas, M.; Cassano, T.; Morgese, M.G.; Debonnel, G.; Duranti, A.; et al. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc. Natl. Acad. Sci. USA 2005, 102, 18620–18625. [Google Scholar] [CrossRef] [PubMed]
- Naidu, P.S.; Varvel, S.A.; Ahn, K.; Cravatt, B.F.; Martin, B.R.; Lichtman, A.H. Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacology 2007, 192, 61–70. [Google Scholar] [CrossRef]
- Chadwick, V.L.; Rohleder, C.; Koethe, D.; Leweke, F.M. Cannabinoids and the endocannabinoid system in anxiety, depression, and dysregulation of emotion in humans. Curr. Opin. Psychiatry 2020, 33, 20–42. [Google Scholar] [CrossRef] [PubMed]
- Black, N.; Stockings, E.; Campbell, G.; Tran, L.T.; Zagic, D.; Hall, W.D.; Farrell, M.; Degenhardt, L. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: A systematic review and meta-analysis. Lancet Psychiatry 2019, 6, 995–1010, Erratum in: Lancet Psychiatry 2020, 7, e3. [Google Scholar] [CrossRef]
- Hungund, B.L.; Vinod, K.Y.; Kassir, S.A.; Basavarajappa, B.S.; Yalamanchili, R.; Cooper, T.B.; Mann, J.J.; Arango, V. Upregulation of CB 1 receptors and agonist-stimulated [35 S] GTP γ S binding in the prefrontal cortex of depressed suicide victims. Mol. Psychiatry 2004, 9, 184–190. [Google Scholar] [CrossRef]
- Vinod, K.Y.; Xie, S.; Psychoyos, D.; Hungund, B.L.; Cooper, T.B.; Tejani-Butt, S.M. Dysfunction in fatty acid amide hydrolase is associated with depressive-like behavior in Wistar Kyoto rats. PLoS ONE 2012, 7, e36743. [Google Scholar] [CrossRef]
- Mato, S.; Pilar-Cuéllar, F.; Valdizán, E.M.; González-Maeso, J.; Rodríguez-Puertas, R.; Meana, J.; Sallés, J.; Crespo-Facorro, B.; Pazos, Á. Selective up-regulation of cannabinoid CB1 receptor coupling to Go-proteins in suicide victims with mood disorders. Biochem. Pharmacol. 2018, 157, 258–265. [Google Scholar] [CrossRef]
- Koethe, D.; Llenos, I.C.; Dulay, J.R.; Hoyer, C.; Torrey, E.F.; Leweke, F.M.; Weis, S. Expression of CB1 cannabinoid receptor in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression. J. Neural Transm. Vienna 2007, 114, 1055–1063. [Google Scholar] [CrossRef]
- Dlugos, A.; Childs, E.; Stuhr, K.L.; Hillard, C.J.; de Wit, H. Acute stress increases circulating anandamide and other N-acylethanolamines in healthy humans. Neuropsychopharmacology 2012, 37, 2416–2427. [Google Scholar] [CrossRef] [PubMed]
- Crombie, K.M.; Leitzelar, B.N.; Brellenthin, A.G.; Hillard, C.J.; Koltyn, K.F. Loss of exercise- and stress-induced increases in circulating 2-arachidonoylglycerol concentrations in adults with chronic PTSD. Biol. Psychol. 2019, 145, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kranaster, L.; Hoyer, C.; Aksay, S.S.; Bumb, J.M.; Müller, N.; Zill, P.; Schwarz, M.J.; Moll, N.; Lutz, B.; Bindila, L.; et al. Biomarkers for antidepressant efficacy of electroconvulsive therapy: An exploratory cerebrospinal fluid study. Neuropsychobiology 2019, 77, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Mayo, L.M.; Asratian, A.; Lindé, J.; Holm, L.; Nätt, D.; Augier, G.; Stensson, N.; Vecchiarelli, H.A.; Balsevich, G.; Aukema, R.J.; et al. Protective effects of elevated anandamide on stress and fear-related behaviors: Translational evidence from humans and mice. Mol. Psychiatry 2020, 25, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Bifulco, M.; Maina, G.; Tortorella, A.; Gazzerro, P.; Proto, M.C.; Di Filippo, C.; Monteleone, F.; Canestrelli, B.; Buonerba, G. Investigation of CNR1 and FAAH endocannabinoid gene polymorphisms in bipolar disorder and major depression. Pharmacol. Res. 2010, 61, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; Gorzalka, B.B. Impairments in endocannabinoid signaling and depressive illness. JAMA 2009, 301, 1165–1166. [Google Scholar] [CrossRef]
- Hill, M.N.; Gorzalka, B.B. The endocannabinoid system and the treatment of mood and anxiety disorders. CNS Neurol. Disord. Drug Targets 2009, 8, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.; Kristensen, P.K.; Bartels, E.M.; Bliddal, H.; Astrup, A. Efficacy and safety of the weight-loss drug rimonabant: A meta-analysis of randomised trials. Lancet 2007, 370, 1706–1713. [Google Scholar] [CrossRef]
- Nissen, S.E.; Nicholls, S.J.; Wolski, K.; Rodés-Cabau, J.; Cannon, C.P.; Deanfield, J.E.; Després, J.-P.; Kastelein, J.J.P.; Steinhubl, S.R.; Kapadia, S.; et al. Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: The STRADIVARIUS randomized controlled trial. JAMA 2008, 299, 1547–1560. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, G.; Chase, D.; Pegg, E.; Downey, D.; Toth, Z.G.; Stones, K.; Platt, H.; Mekli, K.; Payton, A.; Elliott, R.; et al. CNR1 gene is associated with high neuroticism and low agreeableness and interacts with recent negative life events to predict current depressive symptoms. Neuropsychopharmacology 2009, 34, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- Domschke, K.; Dannlowski, U.; Ohrmann, P.; Lawford, B.; Bauer, J.; Kugel, H.; Heindel, W.; Young, R.; Morris, P.; Arolt, V.; et al. Cannabinoid receptor 1 (CNR1) gene: Impact on antidepressant treatment response and emotion processing in major depression. Eur. Neuropsychopharmacol. 2008, 18, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Rana, S.; Coulter, C. Simultaneous identification of 2-carboxy-tetrahydrocannabinol, tetrahydrocannabinol, cannabinol and cannabidiol in oral fluid. J. Chromatogr. B 2007, 852, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Baler, R.D.; Compton, W.M.; Weiss, S.R. Adverse health effects of marijuana use. N. Engl. J. Med. 2014, 370, 2219–2227. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Swanson, J.M.; Evins, A.E.; DeLisi, L.E.; Meier, M.H.; Gonzalez, R.; Bloomfield, M.A.; Curran, H.V.; Baler, R. Effects of Cannabis Use on Human Behavior, Including Cognition, Motivation, and Psychosis: A Review. JAMA Psychiatry 2016, 73, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D.J.E.; Sasiadek, J.D.; Coles, A.S.; George, T.P. Cannabis and mental illness: A review. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Solowij, N.; Stephens, R.S.; Roffman, R.A.; Babor, T.; Kadden, R.; Miller, M.; Christiansen, K.; McRee, B.; Vendetti, J. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA 2002, 287, 1123–1131. [Google Scholar] [CrossRef]
- Crean, R.D.; Crane, N.A.; Mason, B.J. An evidence based review of acute and longterm effects of cannabis use on executive cognitive functions. J. Addict. Med. 2011, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Bossong, M.G.; Jansma, J.M.; van Hell, H.H.; Jager, G.; Oudman, E.; Saliasi, E.; Kahn, R.S.; Ramsey, N.F. Effects of δ9-tetrahydrocannabinol on human working memory function. Biol. Psychiatry 2012, 71, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Meier, M.H.; Caspi, A.; Ambler, A.; Harrington, H.; Houts, R.; Keefe, R.S.; McDonald, K.; Ward, A.; Poulton, R.; Moffitt, T.E. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc. Natl. Acad. Sci. USA 2012, 109, E2657–E2664. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Chandra, S.; Radwan, M.; Majumdar, C.G.; Church, J.C. A Comprehensive Review of Cannabis Potency in the United States in the Last Decade. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Hill, M.N.; Cheer, J.F.; Wotjak, C.T.; Holmes, A. The endocannabinoid system as a target for novel anxiolytic drugs. Neurosci. Biobehav. Rev. 2017, 76 Pt A, 56–66. [Google Scholar] [CrossRef]
- Zehra, A.; Burns, J.; Liu, C.K.; Manza, P.; Wiers, C.E.; Volkow, N.D.; Wang, G.J. Cannabis Addiction and the Brain: A Review. J. Neuroimmune Pharmacol. 2018, 13, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Compton, W.M.; Blanco, C.; Jones, C.M. Time since first cannabis use and 12-month prevalence of cannabis use disorder among youth and emerging adults in the United States. Addiction 2019, 114, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.C.K.; Becker, D.; Butterworth, P.; Hines, L.; Coffey, C.; Hall, W.; Patton, G. Young-adult compared to adolescent onset of regular cannabis use: A 20-year prospective cohort study of later consequences. Drug Alcohol. Rev. 2021, 40, 627–636. [Google Scholar] [CrossRef]
- Karila, L.; Roux, P.; Rolland, B.; Benyamina, A.; Reynaud, M.; Aubin, H.J.; Lançon, C. Acute and long-term effects of cannabis use: A review. Curr. Pharm. Des. 2014, 20, 4112–4118. [Google Scholar] [CrossRef]
- Katz, G.; Lobel, T.; Tetelbaum, A.; Raskin, S. Cannabis Withdrawal—A New Diagnostic Category in DSM-5. Isr. J. Psychiatry Relat. Sci. 2014, 51, 270–275. [Google Scholar]
- Copersino, M.L.; Boyd, S.J.; Tashkin, D.P.; Huestis, M.A.; Heishman, S.J.; Dermand, J.C.; Simmons, M.S.; Gorelick, D.A. Cannabis withdrawal among non-treatment-seeking adult cannabis users. Am. J. Addict. 2006, 15, 8–14. [Google Scholar] [CrossRef]
- Cornelius, J.R.; Chung, T.; Martin, C.; Wood, D.S.; Clark, D.B. Cannabis withdrawal is common among treatment-seeking adolescents with cannabis dependence and major depression, and is associated with rapid relapse to dependence. Addict. Behav. 2008, 33, 1500–1505. [Google Scholar] [CrossRef]
- Levin, F.R.; Brooks, D.J.; Bisaga, A.; Raby, W.; Rubin, E.; Aharonovich, E.; Nunes, E.V. Severity of dependence and motivation for treatment: Comparison of marijuana- and cocaine-dependent treatment seekers. J. Addict. Dis. 2006, 25, 33–41. [Google Scholar] [CrossRef]
- Bahji, A.; Stephenson, C.; Tyo, R.; Hawken, E.R.; Seitz, D.P. Prevalence of Cannabis Withdrawal Symptoms Among People With Regular or Dependent Use of Cannabinoids A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e202370. [Google Scholar] [CrossRef] [PubMed]
- Fergusson, D.M.; Boden, J.M.; Horwood, L.J. Cannabis use and other illicit drug use: Testing the cannabis gateway hypothesis. Addiction 2006, 101, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Madras, B.K. Are THC Levels in Oral Fluids and Blood Plasma Comparable after Oral Ingestion of Edibles Containing Cannabis or THC? Clin. Chem. 2017, 63, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.; Vemuri, K.; Pu, M.; Qu, L.; Han, G.W.; Wu, Y.; Zhao, S.; Shui, W.; Li, S.; Korde, A.; et al. Crystal structure of the human cannabinoid CB1. Cell 2016, 167, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Yin, J.; Chapman, K.; Grzemska, M.; Clark, L.; Wang, J.; Rosenbaum, D.M. High-resolution crystal structure of the human CB1 cannabinoid receptor. Nature 2016, 540, 602–606. [Google Scholar] [CrossRef]
- Katsidoni, V.; Kastellakis, A.; Panagis, G. Biphasic effects of Δ9-tetrahydrocannabinol on brain stimulation reward and motor activity. Int. J. Neuropsychopharmacol. 2013, 16, 2273–2284. [Google Scholar] [CrossRef]
- Vann, R.E.; Gamage, T.F.; Warner, J.A.; Marshall, E.M.; Taylor, N.L.; Martin, B.R.; Wiley, J.L. Divergent effects of cannabidiol on the discriminative stimulus and place conditioning effects of Δ9-tetrahydrocannabinol. Drug Alcohol Depend. 2008, 94, 191–198. [Google Scholar] [CrossRef]
- Schoeler, T.; Theobald, D.; Pingault, J.-B.; Farrington, D.P.; Coid, J.W.; Bhattacharyya, S. Developmental sensitivity to cannabis use patterns and risk for major depressive disorder in mid-life: Findings from 40 years of follow-up. Psychol. Med. 2018, 48, 2169–2176. [Google Scholar] [CrossRef]
- Feingold, D.; Weiser, M.; Rehm, J.; Lev-Ran, S. The association between cannabis use and mood disorders: A longitudinal study. J. Affect. Disord. 2015, 172, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Feingold, D.; Weiser, M.; Rehm, J.; Lev-Ran, S. The association between cannabis use and anxiety disorders: Results from a population-based representative sample. Eur. Neuropsychopharmacol. 2016, 26, 493–505. [Google Scholar] [CrossRef]
- Danielsson, A.-K.; Lundin, A.; Agardh, E.; Allebeck, P.; Forsell, Y. Cannabis use, depression and anxiety: A 3-year prospective population-based study. J. Affect. Disord. 2016, 193, 103–108. [Google Scholar] [CrossRef]
- Cougle, J.R.; Hakes, J.K.; Macatee, R.J.; Chavarria, J.; Zvolensky, M.J. Quality of life and risk of psychiatric disorders among regular users of alcohol, nicotine, and cannabis: An analysis of the national epidemiological survey on alcohol and related conditions (nesarc). J. Psychiatr. Res. 2015, 66, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, M.L.; Nordentoft, M.; Hjorthøj, C. Associations between substance use disorders and suicide or suicide attempts in people with mental illness: A danish nation-wide, prospective, register-based study of patients diagnosed with schizophrenia, bipolar disorder, unipolar depression or personality disorder. Addiction 2017, 112, 1250–1259. [Google Scholar]
- Van Dam, N.T.; Bedi, G.; Earleywine, M. Characteristics of clinically anxious versus non-anxious regular, heavy marijuana users. Addict. Behav. 2012, 37, 1217–1223. [Google Scholar] [CrossRef]
- Sagar, K.A.; Dahlgren, M.K.; Racine, M.T.; Dreman, M.W.; Olson, D.P.; Gruber, S.A. Joint effects: A pilot investigation of the impact of bipolar disorder and marijuana use on cognitive function and mood. PLoS ONE 2016, 11, e0157060. [Google Scholar] [CrossRef] [PubMed]
- Hjorthøj, C.; Østergaard, M.L.D.; Benros, M.E.; Toftdahl, N.G.; Erlangsen, A.; Andersen, J.T.; Nordentoft, M. Association between alcohol and substance use disorders and allcause and cause-specific mortality in schizophrenia, bipolar disorder, and unipolar depression: A nationwide, prospective, register-based study. Lancet Psychiatry 2015, 2, 801–808. [Google Scholar] [CrossRef]
- Duperrouzel, J.; Hawes, S.W.; Lopez-Quintero, C.; Pacheco-Colón, I.; Comer, J.; Gonzalez, R. The association between adolescent cannabis use and anxiety: A parallel process analysis. Addict. Behav. 2018, 78, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Copeland, J.; Rooke, S.; Swift, W. Changes in cannabis use among young people: Impact on mental health. Curr. Opin. Psychiatry 2013, 26, 325–329. [Google Scholar] [CrossRef]
- Degenhardt, L.; Coffey, C.; Romaniuk, H.; Swift, W.; Carlin, J.B.; Hall, W.D.; Patton, G.C. The persistence of the association between adolescent cannabis use and common mental disorders into young adulthood. Addiction 2013, 108, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Hayatbakhsh, M.R.; Najman, J.M.; Jamrozik, K.; Mamun, A.A.; Alati, R.; Bor, W. Cannabis and anxiety and depression in young adults: A large prospective study. J. Am. Acad. Child Adolesc. Psychiatry 2007, 46, 408–417. [Google Scholar] [CrossRef]
- Earleywine, M.; Bolles, J.R. Marijuana, expectancies, and post-traumatic stress symptoms: A preliminary investigation. J. Psychoact. Drugs 2014, 46, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Manhapra, A.; Stefanovics, E.; Rosenheck, R. Treatment outcomes for veterans with PTSD and substance use: Impact of specific substances and achievement of abstinence. Drug Alcohol Depend. 2015, 156, 70–77. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research; The National Academies Press: Washington, DC, USA, 2017. [Google Scholar]
- Campeny, E.; López-Pelayo, H.; Nutt, D.; Blithikioti, C.; Oliveras, C.; Nuño, L.; Maldonado, R.; Florez, G.; Arias, F.; Fernández-Artamendi, S.; et al. The blind men and the elephant: Systematic review of systematic reviews of cannabis use related health harms. Eur. Neuropsychopharmacol. 2020, 33, 1–35. [Google Scholar] [CrossRef]
- Ney, L.J.; Matthews, A.; Bruno, R.; Felmingham, K.L. Cannabinoid interventions for PTSD: Where to next? Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 93, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Rock, E.M.; Parker, L.A. Constituents of Cannabis Sativa. In Cannabinoids and Neuropsychiatric Disorders. Advances in Experimental Medicine and Biology; Murillo-Rodriguez, E., Pandi-Perumal, S.R., Monti, J.M., Eds.; Springer: Cham, Switzerland, 2021; Volume 1264. [Google Scholar]
- Ahmed, M.; Boileau, I.; Le Foll, B.; Carvalho, A.F.; Kloiber, S. The endocannabinoid system in social anxiety disorder: From pathophysiology to novel therapeutics. Braz. J. Psychiatry 2022, 44, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.G.; Hallak, J.E.; Crippa, J.A.S. Neuropharmacological effects of the main phytocannabinoids: A narrative review. In Cannabinoids and Neuropsychiatric Disorders; Springer: Berlin/Heidelberg, Germany, 2021; pp. 29–45. [Google Scholar]
- Stanciu, C.N.; Brunette, M.F.; Teja, N.; Budney, A.J. Evidence for Use of Cannabinoids in Mood Disorders, Anxiety Disorders, and PTSD: A Systematic Review. Psychiatr. Serv. 2021, 72, 429–436. [Google Scholar] [CrossRef]
- Orsolini, L.; Chiappini, S.; Volpe, U.; Berardis, D.; Latini, R.; Papanti, G.D.; Corkery, A.J.M. Use of Medicinal Cannabis and Synthetic Cannabinoids in Post-Traumatic Stress Disorder (PTSD): A Systematic Review. Medicina 2019, 55, 525. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Morrison, P.D.; Fusar-Poli, P.; Martin-Santos, R.; Borgwardt, S.; Winton-Brown, T.; Nosarti, C.; O’Carroll, C.M.; Seal, M.; Allen, P. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology 2010, 35, 764–774. [Google Scholar] [CrossRef]
- Bossong, M.G.; Jansma, J.M.; van Hell, H.H.; Jager, G.; Kahn, R.S.; Ramsey, N.F. Default mode network in the effects of Δ9-Tetrahydrocannabinol (THC) on human executive function. PLoS ONE 2013, 8, e70074. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, P.; Allen, P.; Bhattacharyya, S.; Crippa, J.A.; Mechelli, A.; Borgwardt, S.; Martin-Santos, R.; Seal, M.L.; O’Carrol, C.; Atakan, Z.; et al. Modulation of effective connectivity during emotional processing by Δ9-tetrahydrocannabinol and cannabidiol. Int. J. Neuropsychopharmacol. 2010, 13, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Karniol, I.G.; Shirakawa, I.; Kasinski, N.; Pfeferman, A.; Carlini, E.A. Cannabidiol interferes with the effects of delta 9-tetrahydrocannabinol in man. Eur. J. Pharmacol. 1974, 28, 172–177. [Google Scholar] [CrossRef]
- Martin-Santos, R.; a Crippa, J.; Batalla, A.; Bhattacharyya, S.; Atakan, Z.; Borgwardt, S.; Allen, P.; Seal, M.; Langohr, K.; McGuire, K.P.; et al. Acute effects of a single, oral dose of d9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr. Pharm. Des. 2012, 18, 4966–4979. [Google Scholar] [CrossRef]
- Turna, J.; Patterson, B.; Van Ameringen, M. Is cannabis treatment for anxiety, mood, and related disorders ready for prime time? Depress. Anxiety 2017, 34, 1006–1017. [Google Scholar] [CrossRef]
- Gallego-Landin, I.; García-Baos, A.; Castro-Zavala, A.; Valverde, O. Reviewing the Role of the Endocannabinoid System in the Pathophysiology of Depression. Front. Pharmacol. 2021, 12, 762738. [Google Scholar] [CrossRef]
- Nelson, K.; Walsh, D.; Deeter, P.; Sheehan, F. A phase II study of delta-9-tetrahydrocannabinol for appetite stimulation in cancer-associated anorexia. J. Palliat Care 1994, 10, 14–18. [Google Scholar]
- Chang, A.E.; Shiling, D.J.; Stillman, R.C.; Goldberg, N.H.; Seipp, C.A.; Barofsky, I.; Simon, R.M.; Rosenberg, S.A. Delta-9-tetrahydrocannabinol as an antiemetic in cancer patients receiving high-dose methotrexate. A prospective, randomized evaluation. Ann. Intern. Med. 1979, 91, 819–824. [Google Scholar] [CrossRef]
- Frytak, S.; Moertel, C.G.; O’Fallon, J.R.; Rubin, J.; Creagan, E.T.; O’Connell, M.J.; Schutt, A.J.; Schwartau, N.W. Delta-9-tetrahydrocannabinol as an antiemetic for patients receiving cancer chemotherapy. A comparison with prochlorperazine and a placebo. Ann. Intern. Med. 1979, 91, 825–830. [Google Scholar] [CrossRef]
- Costa, B.; Comelli, F. Pain. In Handbook of Cannabis; Pertwee, R.G., Ed.; Oxford University Press: Oxford, UK, 2014; pp. 473–486. [Google Scholar]
- Ware, M.A.; Wang, T.; Shapiro, S.; Robinson, A.; Ducruet, T.; Huynh, T.; Gamsa, A.; Bennett, G.J.; Collet, J.-P. Smoked cannabis for chronic neuropathic pain: A randomized controlled trial. CMAJ 2010, 182, E694–E701. [Google Scholar] [CrossRef]
- Wilsey, B.; Marcotte, T.; Deutsch, R.; Gouaux, B.; Sakai, S.; Donaghe, H. Low-dose vaporized cannabis significantly improves neuropathic pain. J. Pain 2013, 14, 136–148. [Google Scholar] [CrossRef]
- Wilsey, B.; Marcotte, T.D.; Deutsch, R.; Zhao, H.; Prasad, H.; Phan, A. An Exploratory Human Laboratory Experiment Evaluating Vaporized Cannabis in the Treatment of Neuropathic Pain From Spinal Cord Injury and Disease. J. Pain 2016, 17, 982–1000. [Google Scholar] [CrossRef]
- Betthauser, K.; Pilz, J.; Vollmer, L.E. Use and effects of cannabinoids in military veterans with posttraumatic stress disorder. Am. J. Health Syst. Pharm. 2015, 72, 1279–1284. [Google Scholar] [CrossRef]
- Jetly, R.; Heber, A.; Fraser, G.; Boisvert, D. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: A preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology 2015, 51, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Rock, E.M.; Parker, L.A. Cannabinoids As Potential Treatment for Chemotherapy-Induced Nausea and Vomiting. Front. Pharmacol. 2016, 7, 221. [Google Scholar] [CrossRef] [PubMed]
- Herman, T.S.; Jones, S.E.; Dean, J.; Leigh, S.; Dorr, R.; E Moon, T.; Salmon, E.S. Nabilone: A potent antiemetic cannabinol with minimal euphoria. Biomedicine 1977, 27, 331–334. [Google Scholar] [PubMed]
- Beal, J.E.; Olson, R.; Laubenstein, L.; Morales, J.O.; Bellman, P.; Yangco, B.; Lefkowitz, L.; Plasse, T.F.; Shepard, K.V.; John, S. Dronabinol as a Treatment for Anorexia Associated with Weight Loss in Patients with AIDS. J. Pain Symptom Manag. 1995, 10, 89–97. [Google Scholar] [CrossRef]
- Badowski, M.E.; Yanful, P.K. Dronabinol oral solution in the management of anorexia and weight loss in AIDS and cancer. Ther. Clin. Risk Manag. 2018, 14, 643–651. [Google Scholar] [CrossRef]
- Parker, L.A.; Mechoulam, R.; Schlievert, C.; Abbott, L.; Fudge, M.L.; Burton, P. Effects of cannabinoids on lithium-induced conditioned rejection reactions in a rat model of nausea. Psychopharmacology 2003, 166, 156–162. [Google Scholar] [CrossRef]
- Cluny, N.L.; Naylor, R.J.; Whittle, B.A.; Javid, F.A. The effects of cannabidiol and tetrahydrocannabinol on motion-induced emesis in Suncus murinus. Basic Clin. Pharmacol. Toxicol. 2008, 103, 150–156. [Google Scholar] [CrossRef]
- Parker, L.A.; Kwiatkowska, M.; Burton, P.; Mechoulam, R. Effect of cannabinoids on lithium-induced vomiting in the Suncus murinus (house musk shrew). Psychopharmacology 2004, 171, 156–161. [Google Scholar] [CrossRef]
- van Vliet, S.A.; Vanwersch, R.A.; Jongsma, M.J.; Olivier, B.; Philippens, I.H. Therapeutic effects of delta9-THC and modafinil in a marmoset Parkinson model. Eur. Neuropsychopharmacol. 2008, 18, 383–389. [Google Scholar] [CrossRef]
- Raman, C.; McAllister, S.D.; Rizvi, G. Amyotrophic lateral sclerosis: Delayed disease progression in mice by treatment with a cannabinoid. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2004, 5, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Crippa, J.A.; Guimarães, F.S.; Campos, A.C.; Zuardi, A.W. Translational investigation of the therapeutic potential of cannabidiol (CBD): Toward a new age. Front. Immunol. 2018, 9, 2009. [Google Scholar] [CrossRef] [PubMed]
- Rock, E.M.; Bolognini, D.; Limebeer, C.L.; Cascio, M.G.; Anavi-Goffer, S.; Fletcher, P.J.; Mechoulam, R.; Pertwee, R.G.; Parker, L.A. Cannabidiol, a non-psychotropic component of cannabis, attenuates vomiting and nausea-like behaviour via indirect agonism of 5-HT(1A) somatodendritic autoreceptors in the dorsal raphe nucleus. Br. J. Pharmacol. 2012, 165, 2620–2634. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I. Medical use of cannabinoids. Drugs 2018, 78, 1665–1703. [Google Scholar] [CrossRef]
- Jacobs, D.S.; Kohut, S.J.; Jiang, S.; Nikas, S.P.; Makriyannis, A.; Bergman, J. Acute and chronic effects of cannabidiol on Δ9-tetrahydrocannabinol (Δ9-THC)-induced disruption in stop signal task performance. Exp. Clin. Psychopharmacol. 2016, 24, 320–330. [Google Scholar] [CrossRef]
- Zuardi, A.W.; Cosme, R.A.; Graeff, F.G.; Guimaraes, F.S. Effects of ipsapirone and cannabidiol on human experimental anxiety. J. Psychopharmacol. 1993, 7 (Suppl. S1), 82–88. [Google Scholar] [CrossRef]
- Linares, I.M.; Zuardi, A.W.; Pereira, L.C.; Queiroz, R.H.; Mechoulam, R.; Guimarães, F.S.; Crippa, J.A. Cannabidiol presents an inverted U-shaped dose-response curve in a simulated public speaking test. Braz. J. Psychiatry 2019, 41, 9–14. [Google Scholar] [CrossRef]
- Nader, D.A.; Sanchez, Z.M. Effects of regular cannabis use on neurocognition, brain structure, and function: A systematic review of findings in adults. Am. J. Drug Alcohol Abuse 2018, 44, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Chagas, M.H.; Zuardi, A.W.; Tumas, V.; Pena-Pereira, M.A.; Sobreira, E.T.; Bergamaschi, M.M.; Dos Santos, A.C.; Teixeira, A.L.; Hallak, J.E.C.; Crippa, J.A.S. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: An exploratory double-blind trial. J. Psychopharmacol. 2014, 28, 1088–1098. [Google Scholar] [CrossRef]
- Bergamaschi, M.M.; Queiroz, R.H.; Chagas, M.H.; De Oliveira, D.C.G.; De Martinis, B.; Kapczinski, F.; de Quevedo, J.L.; Roesler, R.; Schroder, N.; Nardi, A.E.; et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology 2011, 36, 1219–1226. [Google Scholar] [CrossRef]
- Crippa, J.A.; Derenusson, G.N.; Ferrari, T.B.; Wichert-Ana, L.; Duran, F.L.; Martin-Santos, R.; Simões, M.V.; Bhattacharyya, S.; Fusar-Poli, P.; Atakan, Z.; et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: A preliminary report. J. Psychopharmacol. 2011, 25, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Rock, E.M.; Limebeer, C.L.; Parker, L.A. Effect of combined doses of Δ(9)-tetrahydrocannabinol (THC) and cannabidiolic acid (CBDA) on acute and anticipatory nausea using rat (Sprague- Dawley) models of conditioned gaping. Psychopharmacology 2015, 232, 4445–4454. [Google Scholar] [CrossRef] [PubMed]
- Rock, E.M.; Limebeer, C.L.; Parker, L.A. Effect of cannabidiolic acid and Δ9-tetrahydrocannabinol on carrageenan-induced hyperalgesia and edema in a rodent model of inflammatory pain. Psychopharmacology 2018, 235, 3259–3271. [Google Scholar] [CrossRef] [PubMed]
- Rock, E.M.; Limebeer, C.L.; Petrie, G.N.; Williams, L.A.; Mechoulam, R.; Parker, L.A. Effect of prior foot shock stress and Δ9-tetrahydrocannabinol, cannabidiolic acid, and cannabidiol on anxiety-like responding in the light-dark emergence test in rats. Psychopharmacology 2017, 234, 2207–2217. [Google Scholar] [CrossRef] [PubMed]
- Skrabek, R.Q.; Galimova, L.; Ethans, K.; Perry, D. Nabilone for the treatment of pain in fibromyalgia. J. Pain 2008, 9, 164–173. [Google Scholar] [CrossRef]
- Maida, V.; Ennis, M.; Irani, S.; Corbo, M.; Dolzhykov, M. Adjunctive nabilone in cancer pain and symptom management: A prospective observational study using propensity scoring. J. Support Oncol. 2008, 6, 119–124. [Google Scholar]
- Weber, J.; Schley, M.; Casutt, M.; Gerber, H.; Schuepfer, G.; Rukwied, R.; Schleinzer, W.; Ueberall, M.; Konrad, C. Tetrahydrocannabinol (Delta 9-THC) treatment in chronic central neuropathic pain and fibromyalgia patients: Results of a multicenter survey. Anesthesiol. Res. Pract. 2009, 2009, 827290. [Google Scholar] [CrossRef] [PubMed]
- Schley, M.; Legler, A.; Skopp, G.; Schmelz, M.; Konrad, C.; Rukwied, R. Delta-9-THC based monotherapy in fibromyalgia patients on experimentally induced pain, axon reflex flare, and pain relief. Curr. Med. Res. Opin. 2006, 22, 1269–1276. [Google Scholar] [CrossRef]
- Phan, K.L.; Angstadt, M.; Golden, J.; Onyewuenyi, I.; Popovska, A.; de Wit, H. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. J. Neurosci. 2008, 28, 2313–2319. [Google Scholar] [CrossRef]
- Colizzi, M.; Ruggeri, M.; Bhattacharyya, S. Unraveling the Intoxicating and Therapeutic Effects of Cannabis Ingredients on Psychosis and Cognition. Front. Psychol. 2020, 11, 833. [Google Scholar] [CrossRef]
- Rabinak, C.A.; Angstadt, M.; Sripada, C.S.; Abelson, J.L.; Liberzon, I.; Milad, M.R.; Phan, K.L. Cannabinoid facilitation of fear extinction memory recall in humans. Neuropharmacology 2013, 64, 396–402. [Google Scholar] [CrossRef]
- Rabinak, C.A.; Phan, K.L. Cannabinoid modulation of fear extinction brain circuits: A novel target to advance anxiety treatment. Curr. Pharm. 2014, 20, 2212–2217. [Google Scholar] [CrossRef] [PubMed]
- Fabre, L.F.; Mclendon, D.A.V.I.D. The efficacy and safety of nabilone (a synthetic cannabinoid) in the treatment of anxiety. J. Clin. Pharmacol. 1981, 21, 377S–382S. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Brook, J.S.; Finch, S.J.; Brook, D.W. Trajectories of cannabis use beginning in adolescence associated with symptoms of posttraumatic stress disorder in the midthirties. Subst. Abuse 2018, 39, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Withey, S.L.; Kangas, B.D.; Charles, S.; Gumbert, A.B.; Eisold, J.E.; George, S.R.; Bergman, J.; Madras, B.K. Effects of daily Δ9-Tetrahydrocannabinol (THC) alone or combined with cannabidiol (CBD) on cognition-based behavior and activity in adolescent nonhuman primates. Drug Alcohol Depend. 2021, 221, 108629. [Google Scholar] [CrossRef]
- Hasbi, A.; Madras, B.K.; Bergman, J.; Kohut, S.; Lin, Z.; Withey, S.L.; George, S.R. Δ-9-Tetrahydrocannabinol Increases Dopamine D1-D2 Receptor Heteromer and Elicits Phenotypic Reprogramming in Adult Primate Striatal Neurons. IScience 2020, 23, 100794. [Google Scholar] [CrossRef]
- Withey, S.L.; Bergman, J.; Huestis, M.A.; George, S.R.; Madras, B.K. THC and CBD blood and brain concentrations following daily administration to adolescent primates. Drug Alcohol Depend. 2020, 213, 108129. [Google Scholar] [CrossRef]
- Englund, A.; Freeman, T.P.; Murray, R.M.; McGuire, P. Can we make cannabis safer? Lancet Psychiatry 2017, 4, 643–648. [Google Scholar] [CrossRef]
- Schubart, C.D.; Sommer, I.E.; van Gastel, W.A.; Goetgebuer, R.L.; Kahn, R.S.; Boks, M.P. Cannabis with high cannabidiol content is associated with fewer psychotic experiences. Schizophr. Res. 2011, 130, 216–221. [Google Scholar] [CrossRef]
- Colizzi, M.; Bhattacharyya, S. Does cannabis composition matter? Differential effects of delta-9-tetrahydrocannabinol and cannabidiol on human cognition. Curr. Addict. Rep. 2017, 4, 62–74. [Google Scholar] [CrossRef]
- Sarne, Y. Beneficial and deleterious effects of cannabinoids in the brain: The case of ultra-low dose of THC. Am. J. Drugs Alcohol Abuse 2019, 45, 551–562. [Google Scholar] [CrossRef] [PubMed]

| Species | Context and Cannabinoid Used | Summary of Results | References |
|---|---|---|---|
| Rodents (rat, mice) | CB1 antagonist SR141716 Depression- or anxiety-like behavior | Depressive-like phenotype | [80,81] |
| Rodents (rat, mice) | CB1 receptor antagonists, Rimonabant and AM251 | anxiogenic effects | [80,81,82] |
| Rodents (rat, mice) | Rimonabant Depression- or anxiety-like behavior | No effect | [80,83,84,85] |
| Rodents (rat, mice) | Rimonabant Depression- or anxiety-like behavior | Anxiolytic effect | [80] |
| Rodents (rat, mice) | CB1 agonists HU210 or oleamide Depression- or anxiety-like behavior | Anxiolytic and/or antidepression-like behavior | [10,68] |
| Rodents (rat, mice) | CB1 agonist THC Depression- or anxiety-like behavior | Anxiolytic and/or antidepression-like behavior | [10,69,70,80] |
| Rodents (rat, mice) | Synthetic CB1 agonists WIN55,212-2; CP55,940 Depression- or anxiety-like behavior | Anxiolytic and/or antidepression-like behavior | [10,71,72,80] |
| Rodents (rat, mice) | Higher doses of CB1 agonists Depression- or anxiety-like behavior | Pro-depressive and anxiogenic | [69,75,76,77,78,80] |
| Rodents (rat, mice) | The endogenous AEA Depression- or anxiety-like behavior | Anxiolytic and/or antidepression-like behavior | [73,74,80] |
| C57BL/6 mice | CBD Chronic stress | Treatment precludes anxiety-like behavior | reviewed in [80] |
| C57BL/6 mice | WIN55,212-2 Stress-induced anxiety | Reversal of social stress-induced anxiety-like phenotype | reviewed in [80] |
| C57BL/6 mice | MAGL inhibitor Chronic stress | Enhanced adult neurogenesis involved in anxiolytic like effects | reviewed in [80] |
| CB1 KO mice | Role of CB1 receptor in social anxiety and memory | Acute CB1 antagonist AM251 increased anxiety-like behavior in CB1 KOs and WT | reviewed in [80] |
| CB1 KO mice | Role of CB1 receptor in anxiety | Anxiogenic phenotype only under high light conditions | reviewed in [80] |
| CB1 KO mice | Role of CB1 receptor in stress coping behaviors | Increased passive stress coping behavior | reviewed in [80] |
| CB1 KO mice | Behavioral characterization of CB1-KO mice | Increased anxiety-like behavior. Reversed by cannabinoid antagonist | reviewed in [80] |
| DAGL-α KO mice | Role of DAGL-α, and 2-AG in anxiety-like behavior | Enhanced anxiety, stress and fear responses in DAGL-α KO | reviewed in [80] |
| Cannabinoid-Based Drug | Dosage (Where Indicated) | Outcomes for Anxiety/Depression | Related Condition | Systematic Reviews (References) |
|---|---|---|---|---|
| Cannabis | NA | ↓Anxiety | HIV | Huang et al., 2016 Ref. [5] |
| Cannabis | NA | ↓Anxiety ↓Depression ↑Quality of life | Antidepressant, anxiolytic and quality of life | Black et al., 2019 Chadwick et al., 2020 Refs. [108,109] |
| Cannabis sativa | NA | ↓Anxiety ↓Depression | Antidepressant | Black et al., 2019 Ref. [109] |
| Nabilone | NA | ↓Anxiety | Fibromyalgia | Huang et al., 2016 Ref. [5] |
| Nabilone | NA | ↓Anxiety and Stress | Cancer | Huang et al., 2016 Ref. [5] |
| Nabilone | 28 days of 1 mg | ↓Anxiety | Psychoneurotic anxiety disorder | Stanciu et al., 2021 Ref. [171] |
| Nabilone | once 2 mg, then weekly 0.5–5 mg for five weeks | No improvement in anxiety. | Anxiety neurosis or generalized anxiety disorder | Stanciu et al., 2021 Ref. [171] |
| Dronabinol [THC] | NA | ↓Anxiety | Chronic neuropathic pain | Huang et al., 2016 Ref. [5] |
| Dronabinol (oral) | 16.6 mg (7.5–25 mg) | ↓Anxiety ↓Depression ↑Quality of life | Analgesic and impact on mental health outcomes | Black et al., 2019 Ref. [109] |
| Dronabinol (oral) | 5–10 mg | ↓Depression ↑Quality of life | Antidepressant and quality of life | Black et al., 2019 Chadwick et al., 2020 Refs. [108,109] |
| Dronabinol (oral) | 10 mg (2.5–10 mg) | ↓Anxiety ↑Quality of life | Antidepressant and quality of life | Black et al., 2019 Chadwick et al., 2020 Refs. [108,109] |
| THC | 7 days of 0.3 mg/kg | No improvement in depressive symptoms. | Unipolar and bipolar major depression | Stanciu et al., 2021 Ref. [171] |
| CBD | 4 weeks of 300 mg | ↓Anxiety | Social anxiety disorder | Stanciu et al., 2021 Ref. [171] |
| CBD | Single dose of 600 mg | ↓Anxiety | Social anxiety disorder | Stanciu et al., 2021 Ref. [171] |
| CBD | Single dose of 400 mg | ↓Anxiety | Social anxiety disorder | Stanciu et al., 2021 Ref. [171] |
| Sativex [THC+CBD] | NA | ↓Anxiety ↑Quality of life | Diabetic-neuropathy | Huang et al., 2016 Ref. [5] |
| CBD + THC | NA | Some but not all studies: ↓Anxiety ↓Depression ↓Stress ↑Quality of life | PTSD | Orsolini et al., 2019 Ref. [172] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasbi, A.; Madras, B.K.; George, S.R. Endocannabinoid System and Exogenous Cannabinoids in Depression and Anxiety: A Review. Brain Sci. 2023, 13, 325. https://doi.org/10.3390/brainsci13020325
Hasbi A, Madras BK, George SR. Endocannabinoid System and Exogenous Cannabinoids in Depression and Anxiety: A Review. Brain Sciences. 2023; 13(2):325. https://doi.org/10.3390/brainsci13020325
Chicago/Turabian StyleHasbi, Ahmed, Bertha K. Madras, and Susan R. George. 2023. "Endocannabinoid System and Exogenous Cannabinoids in Depression and Anxiety: A Review" Brain Sciences 13, no. 2: 325. https://doi.org/10.3390/brainsci13020325
APA StyleHasbi, A., Madras, B. K., & George, S. R. (2023). Endocannabinoid System and Exogenous Cannabinoids in Depression and Anxiety: A Review. Brain Sciences, 13(2), 325. https://doi.org/10.3390/brainsci13020325





