EEG Features in Autism Spectrum Disorder: A Retrospective Analysis in a Cohort of Preschool Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical and Instrumental Investigations in ASD Subjects
2.2. Polysomnographic Recordings and Analysis
2.3. Inclusion and Exclusion Criteria
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maj, M.M.; Massimo, B. DSM-5®: Manuale Diagnostico e Statistico dei Disturbi Mentali, 5th ed.; R. Cortina: Milano, Italy, 2014; in print. [Google Scholar]
- Jeste, S.S. The neurology of autism spectrum disorders. Curr. Opin. Neurol. 2011, 24, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/ncbddd/autism/data.html (accessed on 23 December 2022).
- Narzisi, A.; Posada, M.; Barbieri, F.; Chericoni, N.; Ciuffolini, D.; Pinzino, M.; Romano, R.; Scattoni, M.L.; Tancredi, R.; Calderoni, S.; et al. Prevalence of Autism Spectrum Disorder in a large Italian catchment area: A school-based population study within the ASDEU project. Epidemiol. Psychiatr. Sci. 2018, 29, e5. [Google Scholar] [CrossRef] [PubMed]
- Muhle, R.A.; Reed, H.E.; Stratigos, K.A.; Veenstra-VanderWeele, J. The Emerging Clinical Neuroscience of Autism Spectrum Disorder: A Review. JAMA Psychiatry 2018, 75, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Christian, P.S. Autism Genetics—An Overview: Autism Genetics. Prenat. Diagn. 2017, 37, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Donovan, A.P.A.; Basson, M.A. The neuroanatomy of autism—A developmental perspective. J. Anat. 2016, 230, 4–15. [Google Scholar] [CrossRef]
- Yasuhara, A. Correlation between EEG abnormalities and symptoms of autism spectrum disorder (ASD). Brain Dev. 2010, 32, 791–798. [Google Scholar] [CrossRef]
- Gurau, O.; Bosl, W.J.; Newton, C.R. How Useful Is Electroencephalography in the Diagnosis of Autism Spectrum Disorders and the Deline-ation of Subtypes: A Systematic Review. Front. Psychiatry 2017, 8, 121. [Google Scholar] [CrossRef]
- Capal, J.K.; Carosella, C.; Corbin, E.; Horn, P.S.; Caine, R.; Manning-Courtney, P. EEG endophenotypes in autism spectrum disorder. Epilepsy Behav. 2018, 88, 341–348. [Google Scholar] [CrossRef]
- Boutros, N.N.; Lajiness-O’Neill, R.; Zillgitt, A.; Richard, A.E.; Bowyer, S.M. EEG changes associated with autistic spectrum disorders. Neuropsychiatr. Electrophysiol. 2015, 1, 577. [Google Scholar] [CrossRef]
- Nicotera, A.G.; Hagerman, R.J.; Catania, M.V.; Buono, S.; Di Nuovo, S.; Liprino, E.M.; Stracuzzi, E.; Giusto, S.; Di Vita, G.; Musumeci, S.A. EEG Abnormalities as a Neurophysiological Biomarker of Severity in Autism Spectrum Disorder: A Pilot Cohort Study. J. Autism Dev. Disord. 2019, 49, 2337–2347. [Google Scholar] [CrossRef]
- Parmeggiani, A.; Barcia, G.; Posar, A.; Raimondi, E.; Santucci, M.; Scaduto, M.C. Epilepsy and EEG paroxysmal abnormalities in autism spectrum disorders. Brain Dev. 2010, 32, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, J.A.; Molloy, C.A.; Manning-Courtney, P. Electroencephalogram Abnormalities in Children with Autism Spectrum Disorders. J. Neurosci. Nurs. 2005, 37, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Canitano, R. Epilepsy in Autism Spectrum Disorders. Eur. Child Adolesc. Psychiatry 2007, 16, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Hrdlicka, M.; Komarek, V.; Propper, L.; Kulisek, R.; Zumrova, A.; Faladova, L.; Havlovicova, M.; Sedlacek, Z.; Blatny, M.; Urbanek, T. Not EEG Abnormalities but Epilepsy Is Associated with Autistic Regression and Mental Function-ing in Childhood Autism. Eur. Child Adolesc. Psychiatry 2004, 13, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Colombi, C.; Tancredi, R.; Persico, A.; Faggioli, R. Manuale Ados 2; Hogrefe: Firenze, Italy, 2013. [Google Scholar]
- Rutter, M.; Le Couteur, A.; Lord, C. ADI-R Autism Diagnostic Interview—Revised. In Curatore Edizione Italiana; Faggioli, R., Saccani, M., Persico, A.M., Tancredi, R., Parrini, B., Igliozzi, R., Eds.; Giunti Psychometrics: Firenze, Italy, 2005. [Google Scholar]
- Sannio Fancello, G.; Cianchetti, C. WPPSI-3: Contributo Alla Taratura Italiana; Giunti OS: Firenze, Italy, 2008. [Google Scholar]
- SINPIA. Available online: http://sinpia.eu/wp-content/uploads/atom/allegato/148.pdf (accessed on 23 December 2022).
- Mukherjee, S.B. Identification, Evaluation, and Management of Children with Autism Spectrum Disorder: American Academy of Pediatrics 2020 Clinical Guidelines. Indian Pediatr. 2020, 57, 959–962. [Google Scholar] [CrossRef]
- Dauchez, T.; Camelot, G.; Levy, C.; Rajerison, T.; Briot, K.; Pizano, A.; Geoffray, M.-M.; Landrieu, L.; Bouvard, M.; Amestoy, A. Diagnostic Process for Autism Spectrum Disorder: A Meta-Analysis of Worldwide Clinical Practice Guidelines for the Initial Somatic Assessment. Children 2022, 9, 1886. [Google Scholar] [CrossRef]
- Kane, N.; Acharya, J.; Beniczky, S.; Caboclo, L.; Finnigan, S.; Kaplan, P.W.; Shibasaki, H.; Pressler, R.; van Putten, M.J. A revised glossary of terms most commonly used by clinical electroencephalographers and updated proposal for the report format of the EEG findings. Revision 2017. Clin. Neurophysiol. Prac. 2017, 2, 170–185. [Google Scholar] [CrossRef]
- Capdevila, O.S.; Dayyat, E.; Gozal, L.; Gozal, D. Prevalence of epileptiform activity in healthy children during sleep. Sleep Med. 2008, 9, 303–309. [Google Scholar] [CrossRef]
- Akshoomoff, N.; Farid, N.; Courchesne, E.; Haas, R. Abnormalities on the Neurological Examination and EEG in Young Children with Pervasive Developmental Disorders. J. Autism Dev. Disord. 2006, 37, 887–893. [Google Scholar] [CrossRef]
- Baird, G.; Robinson, R.O.; Boyd, S.; Charman, T. Sleep Electroencephalograms in Young Children with Autism with and without Regression. Dev. Med. Child Neurol. 2006, 48, 604–608. [Google Scholar] [CrossRef]
- Spence, S.J.; Schneider, M.T. The Role of Epilepsy and Epileptiform EEGs in Autism Spectrum Disorders. Pediatr. Res. 2009, 65, 599–606. [Google Scholar] [CrossRef] [PubMed]
- El Achkar, C.M.; Sarah, J.S. Clinical Characteristics of Children and Young Adults with Co-Occurring Autism Spectrum Disorder and Epilepsy. Epilepsy Behav. 2015, 47, 183–190. [Google Scholar] [PubMed]
- Precenzano, F.; Parisi, L.; Lanzara, V.; Vetri, L.; Operto, F.F.; Pastorino, G.M.G.; Ruberto, M.; Messina, G.; Risoleo, M.C.; Santoro, C.; et al. Electroencephalographic Abnormalities in Autism Spectrum Disorder: Characteristics and Therapeutic Implications. Medicina 2020, 56, 419. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.L.; Carvalho, D.Z.; St Louis, E.K.; Bazil, C. Sleep and Epilepsy: A Focused Review of Pathophysiology, Clinical Syndromes, Co-Morbidities, and Therapy. Neurotherapeutics 2021, 18, 170–180. [Google Scholar]
- Herman, S.; Walczak, T.; Bazil, C. Distribution of partial seizures during the sleep-wake cycle: Differences by seizure onset site. Neurology 2001, 56, 1453–1459. [Google Scholar] [CrossRef]
- Donnelly, N.A.; Bartsch, U.; Moulding, H.A.; Eaton, C.; Marston, H.; Hall, J.H.; Hall, J.; Owen, M.J.; Bree, M.B.V.D.; Jones, M.W. Sleep EEG in young people with 22q11.2 deletion syndrome: A cross-sectional study of slow-waves, spindles and correlations with memory and neurodevelopmental symptoms. Elife 2022, 11, e75482. [Google Scholar] [CrossRef]
- Hamburg, S.; Bush, D.; Strydom, A.; Startin, C.M. Comparison of resting-state EEG between adults with Down syndrome and typically developing controls. J. Neurodev. Disord. 2021, 13, 48. [Google Scholar] [CrossRef]
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism Spectrum Disorder. Nat. Reviews. Dis. Prim. 2020, 6, 5. [Google Scholar]
- Alfei, E.; Raviglione, F.; Franceschetti, S.; D’Arrigo, S.; Milani, D.; Selicorni, A.; Riva, D.; Zuffardi, O.; Pantaleoni, C.; Binelli, S. Seizures and EEG features in 74 patients with genetic-dysmorphic syndromes. Am. J. Med. Genet. Part A 2014, 164, 3154–3161. [Google Scholar] [CrossRef]
- Liu, X.; Sun, X.; Sun, C.; Zou, M.; Chen, Y.; Huang, J.; Wu, L.; Chen, W.-X. Prevalence of epilepsy in autism spectrum disorders: A systematic review and meta-analysis. Autism 2021, 26, 33–50. [Google Scholar] [CrossRef]
- Begeer, S.; Mandell, D.; Wijnker-Holmes, B.; Venderbosch, S.; Rem, D.; Stekelenburg, F.; Koot, H.M. Sex Differences in the Timing of Identification Among Children and Adults with Autism Spectrum Disorders. J. Autism Dev. Disord. 2012, 43, 1151–1156. [Google Scholar] [CrossRef]
- Shattuck, P.T.; Durkin, M.; Maenner, M.; Newschaffer, C.; Mandell, D.S.; Wiggins, L.; Lee, L.-C.; Rice, C.; Giarelli, E.; Kirby, R.; et al. Timing of Identification among Children with an Autism Spectrum Disorder: Findings from a Population-Based Surveillance Study. J. Am. Acad. Child Adolesc. Psychiatry 2009, 48, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Siklos, S.; Kerns, K.A. Assessing the diagnostic experiences of a small sample of parents of children with autism spectrum disorders. Res. Dev. Disabil. 2007, 28, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Bargiela, S.; Steward, R.; Mandy, W. The Experiences of Late-diagnosed Women with Autism Spectrum Conditions: An Investigation of the Female Autism Phenotype. J. Autism Dev. Disord. 2016, 46, 3281–3294. [Google Scholar] [CrossRef] [PubMed]
- Cridland, E.K.; Jones, S.C.; Caputi, P.; Magee, C.A. Being a Girl in a Boys’ World: Investigating the Experiences of Girls with Autism Spectrum Disorders During Adolescence. J. Autism Dev. Disord. 2013, 44, 1261–1274. [Google Scholar] [CrossRef]
- Trubanova, A.; Donlon, K.; Kreiser, N.L.; Ollendick, T.H.; White, S.W. Underidentification of Autism Spectrum Disorder in Females: A Case Series Illustrating the Unique Presentation of this Disorder in Young Women. Scand. J. Child Adolesc. Psychiatry Psychol. 2013, 2, 66–76. [Google Scholar] [CrossRef]
- Fombonne, E. Epidemiology of Pervasive Developmental Disorders. Pediatr. Res. 2009, 65, 591–598. [Google Scholar] [CrossRef]
- Kirkovski, M.; Enticott, P.G.; Fitzgerald, P.B. A Review of the Role of Female Gender in Autism Spectrum Disorders. J. Autism Dev. Disord. 2013, 43, 2584–2603. [Google Scholar] [CrossRef]
- Clarke, E.; Hull, L.; Loomes, R.; McCormick, C.E.; Sheinkopf, S.J.; Mandy, W. Assessing gender differences in autism spectrum disorder using the Gendered Autism Behavioral Scale (GABS): An exploratory study. Res. Autism Spectr. Disord. 2021, 88, 101844. [Google Scholar] [CrossRef]
- Volkmar, F.R.; Szatmari, P.; Sparrow, S.S. Sex differences in pervasive developmental disorders. J. Autism Dev. Disord. 1993, 23, 579–591. [Google Scholar] [CrossRef]
- Kreiser, N.L.; Susan, W.W. ASD in Females: Are We Overstating the Gender Difference in Diagnosis? Clin. Child Fam. Psychol. Rev. 2014, 17, 67–84. [Google Scholar] [PubMed]
- Lai, M.-C.; Baron-Cohen, S.; Buxbaum, J.D. Understanding autism in the light of sex/gender. Mol. Autism 2015, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Halladay, A.K.; Bishop, S.; Constantino, J.N.; Daniels, A.M.; Koenig, K.; Palmer, K.; Messinger, D.; Pelphrey, K.; Sanders, S.J.; Singer, A.T.; et al. Sex and gender differences in autism spectrum disorder: Summarizing evidence gaps and identifying emerging areas of priority. Mol. Autism 2015, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Hiller, R.M.; Meiser-Stedman, R.; Fearon, P.; Lobo, S.; McKinnon, A.; Fraser, A.; Halligan, S.L. Research Review: Changes in the prevalence and symptom severity of child post-traumatic stress disorder in the year following trauma—A meta-analytic study. J. Child Psychol. Psychiatry 2016, 57, 884–898. [Google Scholar] [CrossRef]
- Messinger, D.S.; Young, G.S.; Webb, S.J.; Ozonoff, S.; Bryson, S.E.; Carter, A.; Carver, L.; Charman, T.; Chawarska, K.; Curtin, S.; et al. Early sex differences are not autism-specific: A Baby Siblings Research Consortium (BSRC) study. Mol. Autism 2015, 6, 32. [Google Scholar] [CrossRef]
- Hartley, S.L.; Darryn, M.S. Sex Differences in Autism Spectrum Disorder: An Examination of Developmental Functioning, Autistic Symptoms, and Coexisting Behavior Problems in Toddlers. J. Autism Dev. Disord. 2009, 39, 1715–1722. [Google Scholar] [PubMed]
- Chawarska, K.; Paul, R.; Klin, A.; Hannigen, S.; Dichtel, L.E.; Volkmar, F. Parental Recognition of Developmental Problems in Toddlers with Autism Spectrum Disorders. J. Autism Dev. Disord. 2006, 37, 62–72. [Google Scholar] [CrossRef]
- Knickmeyer, R.C.; Wheelwright, S.; Baron-Cohen, S.B. Sex-typical Play: Masculinization/Defeminization in Girls with an Autism Spectrum Condition. J. Autism Dev. Disord. 2007, 38, 1028–1035. [Google Scholar] [CrossRef]
- Lord, C.; Schopler, E.; Revicki, D. Sex Differences in Autism. Int. J. Rehabil. Res. 1989, 12, 113–114. [Google Scholar] [CrossRef]
- Chez, M.G.; Chang, M.; Krasne, V.; Coughlan, C.; Kominsky, M.; Schwartz, A. Frequency of epileptiform EEG abnormalities in a sequential screening of autistic patients with no known clinical epilepsy from 1996 to 2005. Epilepsy Behav. 2006, 8, 267–271. [Google Scholar] [CrossRef]
- Lord, C.; Brugha, T.S.; Charman, T.; Cusack, J.; Dumas, G.; Frazier, T.; Jones, E.J.; Jones, R.M.; Pickles, A.; State, M.W.; et al. Autism Spectrum Disorder. Nat. Rev. Dis. Prim. 2020, 6, 5. [Google Scholar] [PubMed]
- Romero-González, M.; Navas-Sánchez, P.; Marín-Gámez, E.; Barbancho-Fernández, M.A.; Fernández-Sánchez, V.E.; Lara-Muñoz, J.P.; Guzmán-Parra, J. EEG abnormalities and clinical phenotypes in pre-school children with autism spectrum disorder. Epilepsy Behav. 2022, 129, 108619. [Google Scholar] [CrossRef] [PubMed]
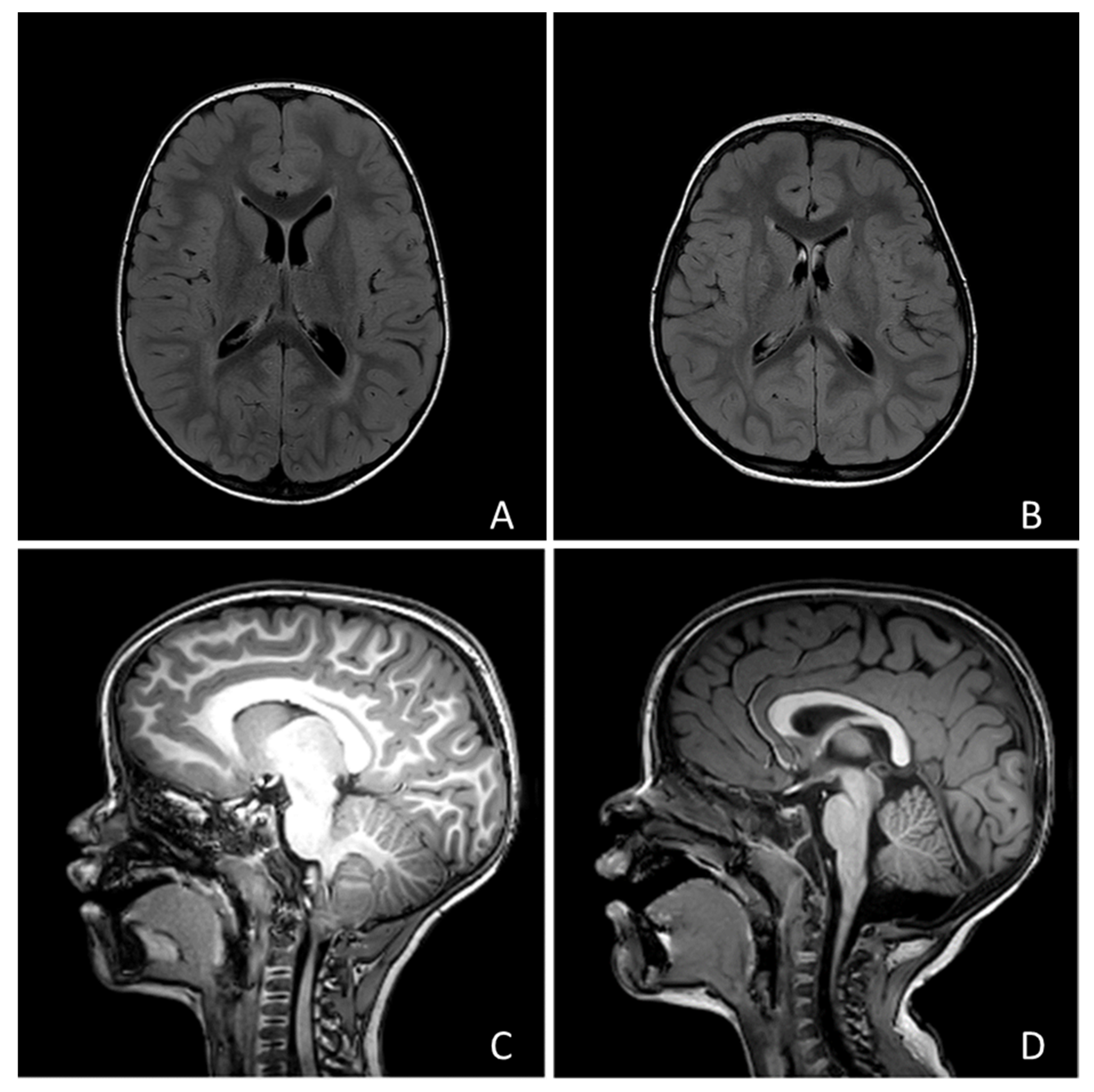


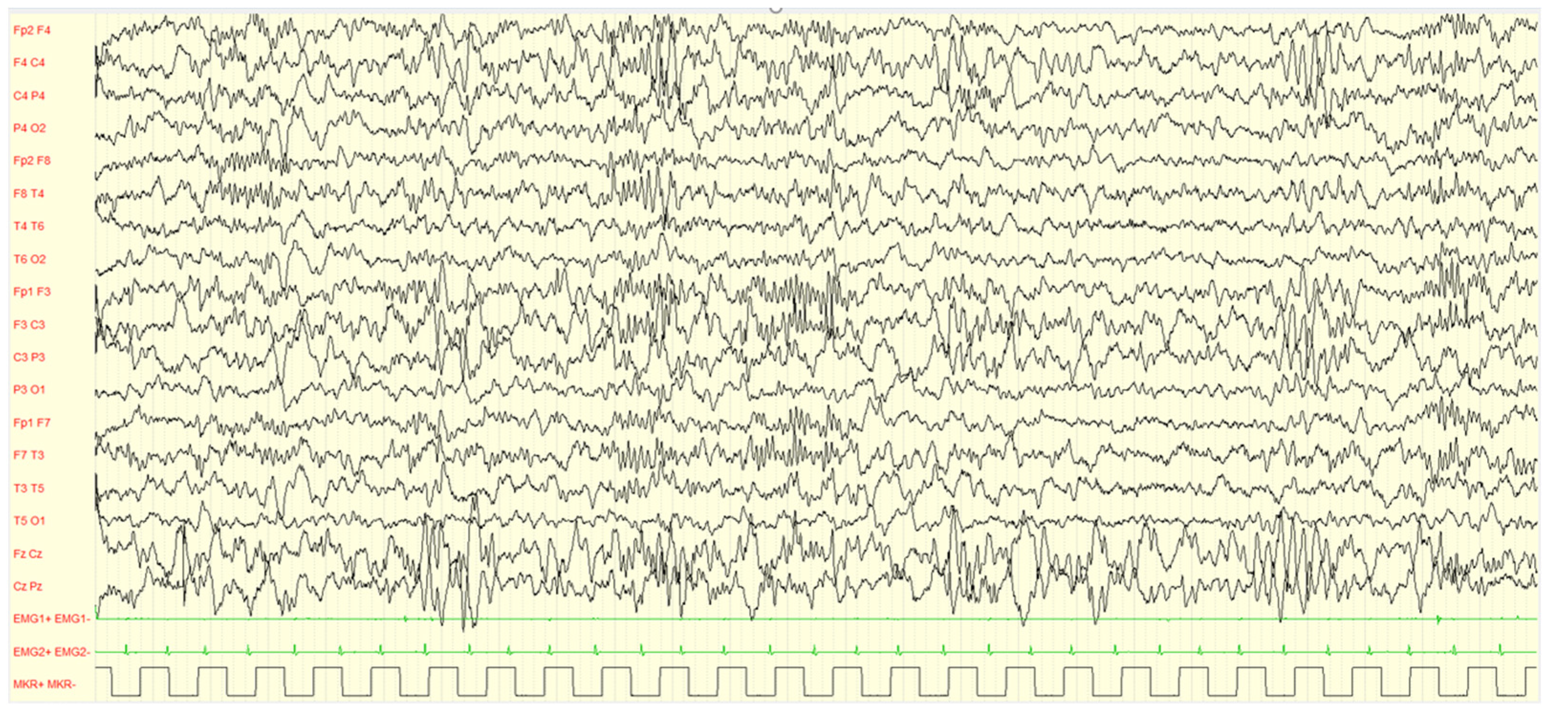

| TOTAL SUBJECTS | 292 |
| M | 248 (84.9%) |
| F | 44 (15.1%) |
| MEAN AGE AT RECORDING (MONTHS) | 34.6 |
| DEVELOPMENTAL DELAY | 190 (69.5%) |
| FEMALES with DEVELOPMENTAL DELAY | 37 (84.0%) |
| MALES with DEVELOPMENTAL DELAY | 153 (61.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santarone, M.E.; Zambrano, S.; Zanotta, N.; Mani, E.; Minghetti, S.; Pozzi, M.; Villa, L.; Molteni, M.; Zucca, C. EEG Features in Autism Spectrum Disorder: A Retrospective Analysis in a Cohort of Preschool Children. Brain Sci. 2023, 13, 345. https://doi.org/10.3390/brainsci13020345
Santarone ME, Zambrano S, Zanotta N, Mani E, Minghetti S, Pozzi M, Villa L, Molteni M, Zucca C. EEG Features in Autism Spectrum Disorder: A Retrospective Analysis in a Cohort of Preschool Children. Brain Sciences. 2023; 13(2):345. https://doi.org/10.3390/brainsci13020345
Chicago/Turabian StyleSantarone, Marta Elena, Stefania Zambrano, Nicoletta Zanotta, Elisa Mani, Sara Minghetti, Marco Pozzi, Laura Villa, Massimo Molteni, and Claudio Zucca. 2023. "EEG Features in Autism Spectrum Disorder: A Retrospective Analysis in a Cohort of Preschool Children" Brain Sciences 13, no. 2: 345. https://doi.org/10.3390/brainsci13020345
APA StyleSantarone, M. E., Zambrano, S., Zanotta, N., Mani, E., Minghetti, S., Pozzi, M., Villa, L., Molteni, M., & Zucca, C. (2023). EEG Features in Autism Spectrum Disorder: A Retrospective Analysis in a Cohort of Preschool Children. Brain Sciences, 13(2), 345. https://doi.org/10.3390/brainsci13020345





