Social Touch Reduces Pain Perception—An fMRI Study of Cortical Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
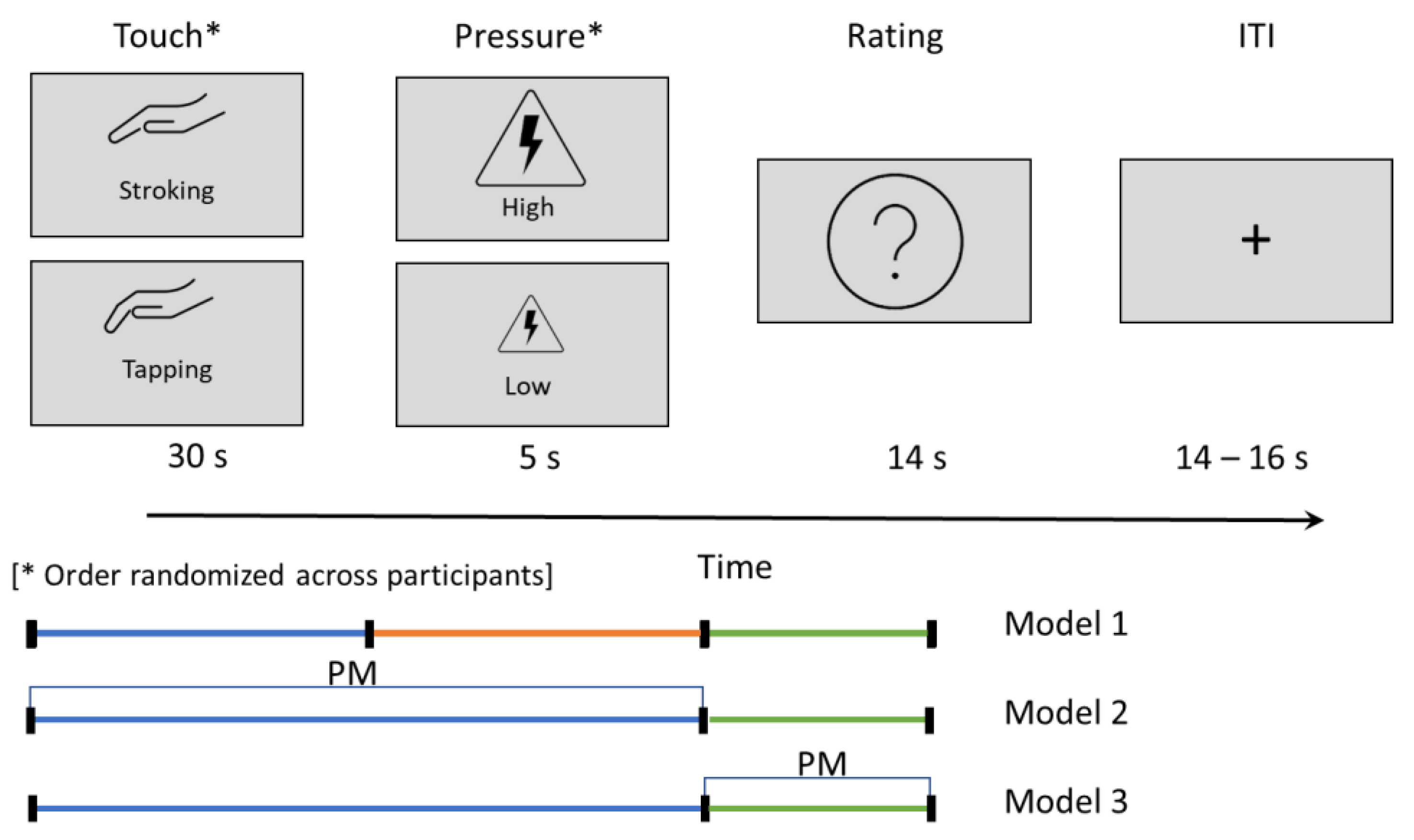
2.3. Touch–Pain Interaction Task
2.4. Brain Imaging
2.5. Statistical Analysis
3. Results
3.1. Demographics
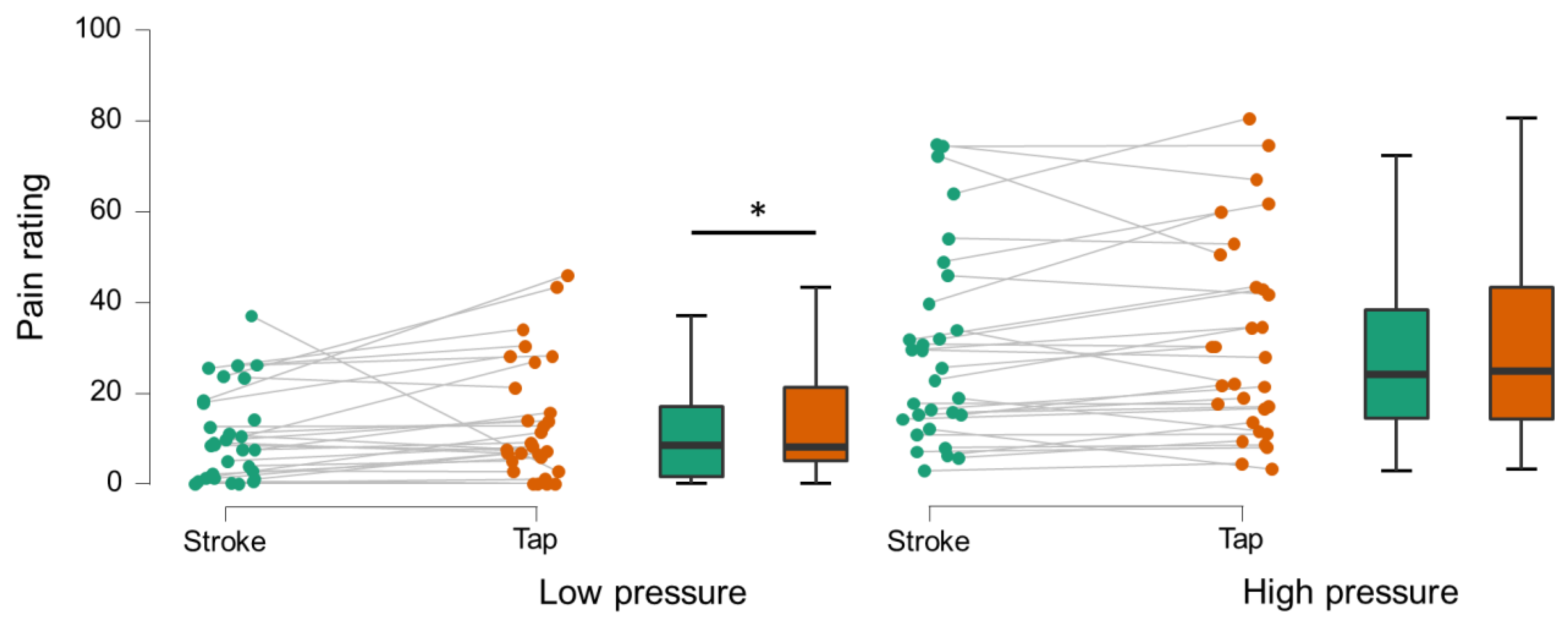
3.2. Behavior
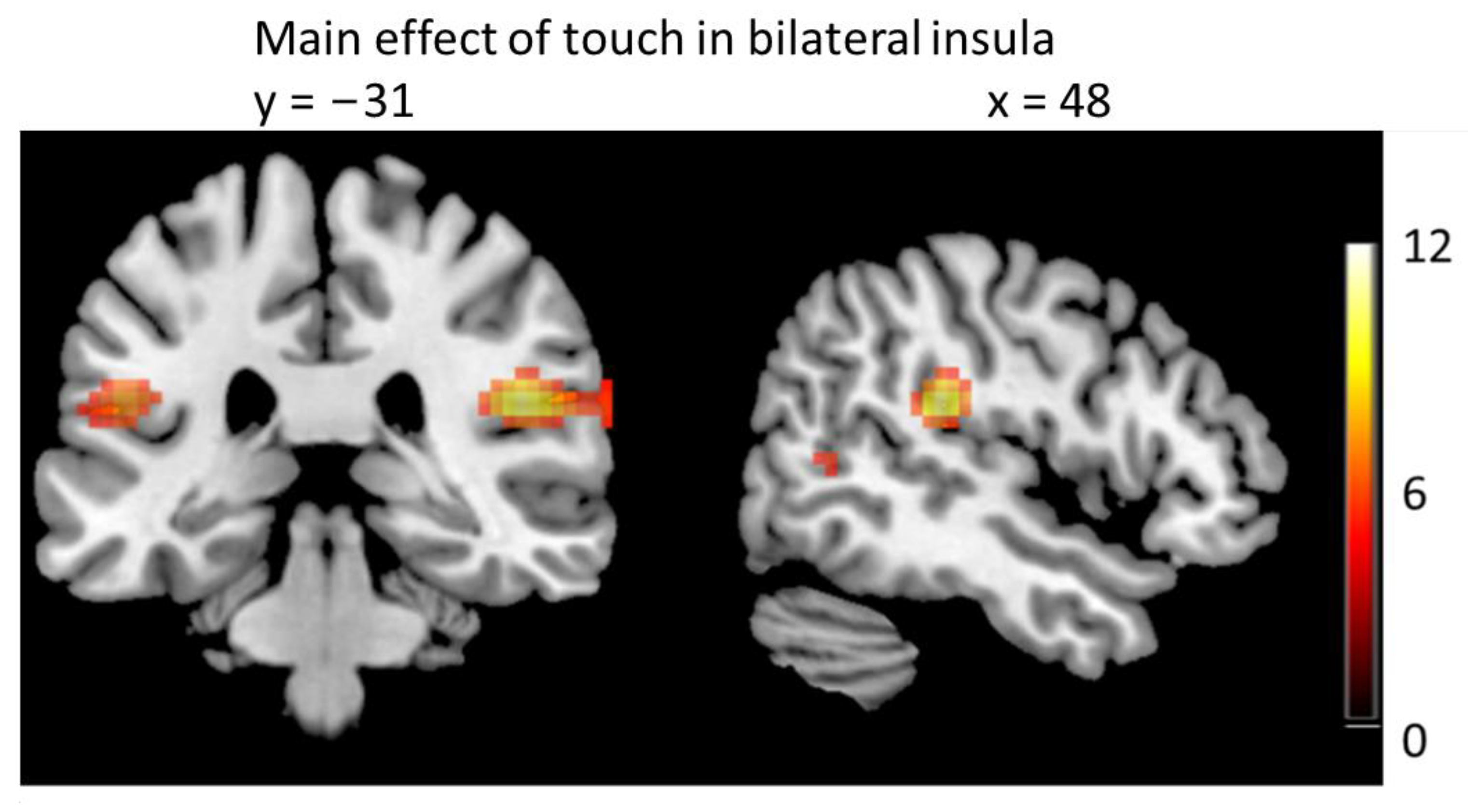
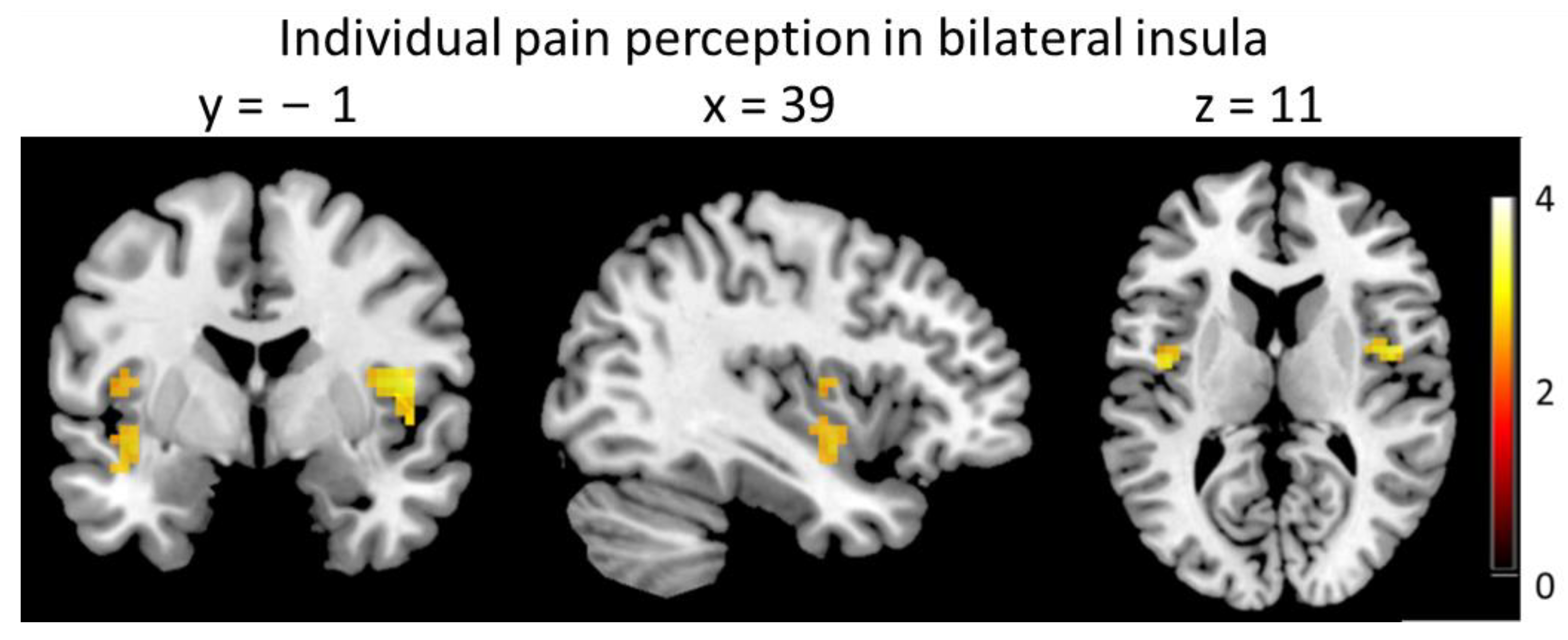
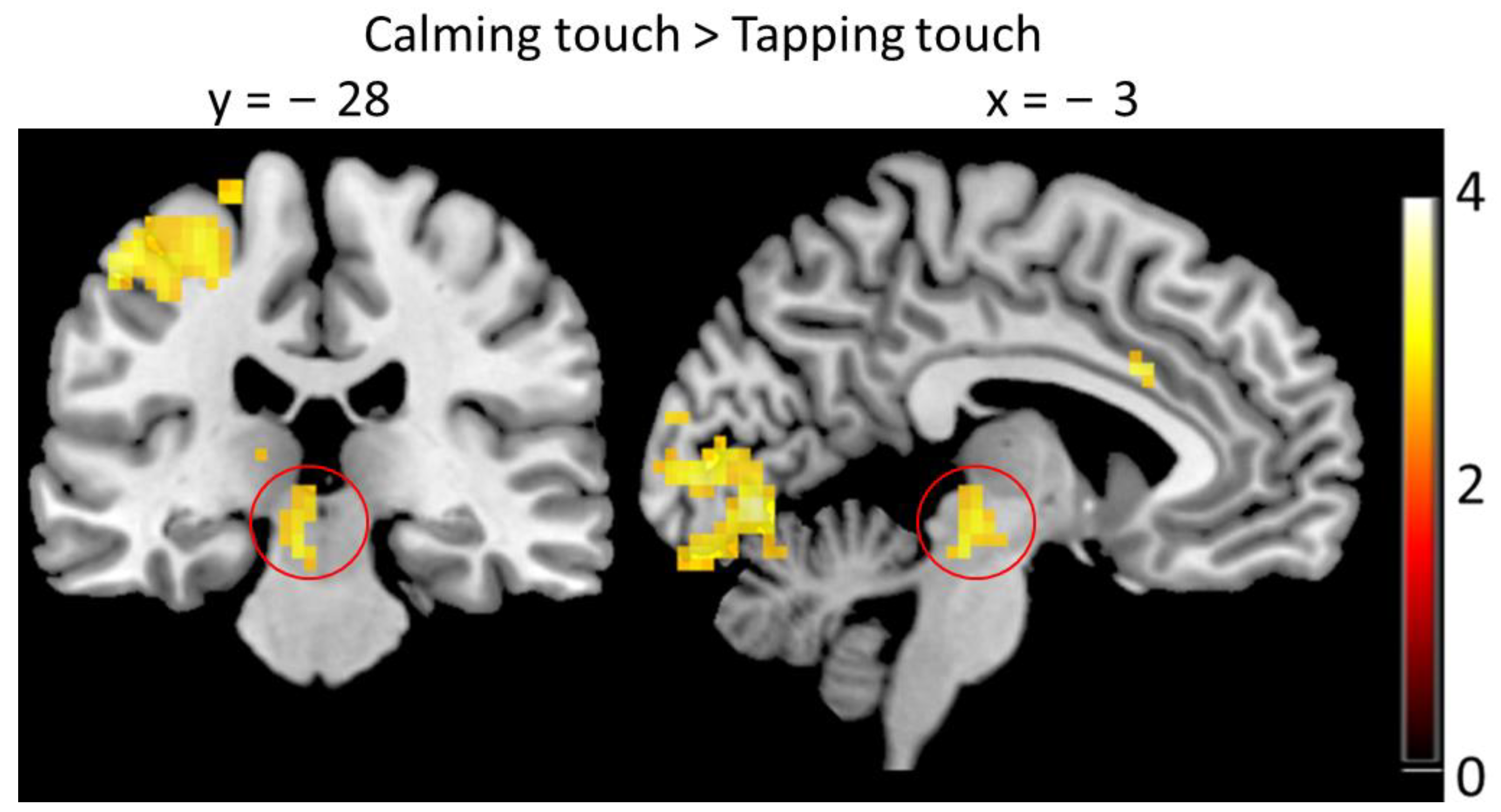
3.3. Brain Imaging
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melzack, R.; Wall, P.D. Pain mechanisms: A new theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Vallbo, A.B.; Olausson, H.; Wessberg, J. Unmyelinated afferents constitute a second system coding tactile stimuli of the human hairy skin. J. Neurophysiol. 1999, 81, 2753–2763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loken, L.S.; Wessberg, J.; Morrison, I.; McGlone, F.; Olausson, H. Coding of pleasant touch by unmyelinated afferents in humans. Nat. Neurosci. 2009, 12, 547–548. [Google Scholar] [CrossRef]
- Krahe, C.; Drabek, M.M.; Paloyelis, Y.; Fotopoulou, A. Affective touch and attachment style modulate pain: A laser-evoked potentials study. Philos. Trans. R Soc. Lond. B Biol. Sci. 2016, 371, 20160009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liljencrantz, J.; Strigo, I.; Ellingsen, D.M.; Kramer, H.H.; Lundblad, L.C.; Nagi, S.S.; Leknes, S.; Olausson, H. Slow brushing reduces heat pain in humans. Eur. J. Pain 2017, 21, 1173–1185. [Google Scholar] [CrossRef]
- Taneja, P.; Baad-Hansen, L.; Shaikh, S.; Svensson, P.; Olausson, H. Robotic Stroking on the Face and Forearm: Touch Satiety and Effects on Mechanical Pain. Front. Pain Res. 2021, 2, 90. [Google Scholar] [CrossRef]
- Fidanza, F.; Polimeni, E.; Pierangeli, V.; Martini, M. A better touch: C-tactile fibers related activity is associated to pain reduction during temporal summation of second pain. J. Pain 2021, 22, 567–576. [Google Scholar] [CrossRef]
- Gursul, D.; Goksan, S.; Hartley, C.; Mellado, G.S.; Moultrie, F.; Hoskin, A.; Adams, E.; Hathway, G.; Walker, S.; McGlone, F.; et al. Stroking modulates noxious-evoked brain activity in human infants. Curr. Biol. 2018, 28, R1380–R1381. [Google Scholar] [CrossRef] [Green Version]
- Habig, K.; Schanzer, A.; Schirner, W.; Lautenschlager, G.; Dassinger, B.; Olausson, H.; Birklein, F.; Gizewski, E.R.; Kramer, H.H. Low threshold unmyelinated mechanoafferents can modulate pain. BMC Neurol. 2017, 17, 184. [Google Scholar] [CrossRef] [Green Version]
- López-Solà, M.; Geuter, S.; Koban, L.; Coan, J.A.; Wager, T.D. Brain mechanisms of social touch-induced analgesia in females. Pain 2019, 160, 2072–2085. [Google Scholar] [CrossRef]
- Che, X.; Luo, X.; Chen, Y.; Li, B.; Li, X.; Li, X.; Qiao, L. Social touch modulates pain-evoked increases in facial temperature. Curr. Psychol. 2021, 1–10. [Google Scholar] [CrossRef]
- Hauser, S.C.; Nagi, S.S.; McIntyre, S.; Israr, A.; Olausson, H.; Gerling, G.J. From human-to-human touch to peripheral nerve responses. In Proceedings of the 2019 IEEE World Haptics Conference (WHC), Tokyo, Japan, 9–12 July 2019; pp. 592–597. [Google Scholar]
- McIntyre, S.; Hauser, S.C.; Kusztor, A.; Boehme, R.; Moungou, A.; Isager, P.M.; Homman, L.; Novembre, G.; Nagi, S.S.; Israr, A.; et al. The language of social touch is intuitive and quantifiable. Psychol. Sci. 2022, 33, 1477–1494. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, S.; Moungou, A.; Boehme, R.; Isager, P.M.; Lau, F.; Israr, A.; Lumpkin, E.A.; Abnousi, F.; Olausson, H. Affective touch communication in close adult relationships. In Proceedings of the 2019 IEEE World Haptics Conference (WHC), Tokyo, Japan, 9–12 July 2019; pp. 175–180. [Google Scholar]
- McGlone, F.; Wessberg, J.; Olausson, H. Discriminative and affective touch: Sensing and feeling. Neuron 2014, 82, 737–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, I. ALE meta-analysis reveals dissociable networks for affective and discriminative aspects of touch. In Human Brain Mapping; Wiley-Blackwell: Malden, MA, USA, 2016; Volume 37, pp. 1308–1320. [Google Scholar]
- Craig, A.D. How do you feel—Now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009, 10, 59–70. [Google Scholar] [CrossRef]
- Craig, A.D. How do you feel? Interoception: The sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002, 3, 655–666. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Nomi, J.S.; Hébert-Seropian, B.; Ghaziri, J.; Boucher, O. Structure and function of the human insula. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 2017, 34, 300. [Google Scholar] [CrossRef]
- Boehme, R.; Hauser, S.; Gerling, G.J.; Heilig, M.; Olausson, H. Distinction of self-produced touch and social touch at cortical and spinal cord levels. Proc. Natl. Acad. Sci. USA 2019, 116, 2290–2299. [Google Scholar] [CrossRef] [Green Version]
- Dunbar, R.I. The social role of touch in humans and primates: Behavioural function and neurobiological mechanisms. Neurosci. Biobehav. Rev. 2010, 34, 260–268. [Google Scholar] [CrossRef]
- Holt-Lunstad, J.; Birmingham, W.A.; Light, K.C. Influence of a “warm touch” support enhancement intervention among married couples on ambulatory blood pressure, oxytocin, alpha amylase, and cortisol. Psychosom. Med. 2008, 70, 976–985. [Google Scholar] [CrossRef] [Green Version]
- Scheele, D.; Kendrick, K.M.; Khouri, C.; Kretzer, E.; Schlapfer, T.E.; Stoffel-Wagner, B.; Gunturkun, O.; Maier, W.; Hurlemann, R. An oxytocin-induced facilitation of neural and emotional responses to social touch correlates inversely with autism traits. Neuropsychopharmacology 2014, 39, 2078–2085. [Google Scholar] [CrossRef]
- Uvnas-Moberg, K.; Handlin, L.; Petersson, M. Self-soothing behaviors with particular reference to oxytocin release induced by non-noxious sensory stimulation. Front. Psychol. 2014, 5, 1529. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Miao, W.; Ji, E.; Huang, S.; Jin, S.; Zhu, X.; Liu, M.-Z.; Sun, Y.-G.; Xu, F.; Yu, X. Social touch-like tactile stimulation activates a tachykinin 1-oxytocin pathway to promote social interactions. Neuron 2022, 110, 1051–1067. [Google Scholar] [CrossRef] [PubMed]
- Linnman, C.; Moulton, E.A.; Barmettler, G.; Becerra, L.; Borsook, D. Neuroimaging of the periaqueductal gray: State of the field. Neuroimage 2012, 60, 505–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-Y.; You, T.; Lee, C.-H.; Im, G.H.; Seo, H.; Woo, C.-W.; Kim, S.-G. Role of anterior cingulate cortex inputs to periaqueductal gray for pain avoidance. Curr. Biol. 2022, 32, 2834–2847.e5. [Google Scholar] [CrossRef] [PubMed]
- Pankow, A.; Katthagen, T.; Diner, S.; Deserno, L.; Boehme, R.; Kathmann, N.; Gleich, T.; Gaebler, M.; Walter, H.; Heinz, A. Aberrant salience is related to dysfunctional self-referential processing in psychosis. Schizophr. Bull. 2015, 42, 67–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uddin, L.Q.; Iacoboni, M.; Lange, C.; Keenan, J.P. The self and social cognition: The role of cortical midline structures and mirror neurons. Trends Cogn. Sci. 2007, 11, 153–157. [Google Scholar] [CrossRef]
- Perini, I.; Olausson, H.; Morrison, I. Seeking pleasant touch: Neural correlates of behavioral preferences for skin stroking. Front. Behav. Neurosci. 2015, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Boehme, R.; Karlsson, M.F.; Heilig, M.; Olausson, H.; Capusan, A.J. Sharpened self-other distinction in attention deficit hyperactivity disorder. NeuroImage Clin. 2020, 27, 102317. [Google Scholar] [CrossRef]
- Boehme, R.; van Ettinger-Veenstra, H.; Olausson, H.; Gerdle, B.; Nagi, S.S. Anhedonia to Gentle Touch in Fibromyalgia: Normal Sensory Processing but Abnormal Evaluation. Brain Sci. 2020, 10, 306. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Unified segmentation. Neuroimage 2005, 26, 839–851. [Google Scholar] [CrossRef]
- Corbin, N.; Todd, N.; Friston, K.J.; Callaghan, M.F. Accurate modeling of temporal correlations in rapidly sampled fMRI time series. Hum. Brain Mapp. 2018, 39, 3884–3897. [Google Scholar] [CrossRef] [Green Version]
- Olszowy, W.; Aston, J.; Rua, C.; Williams, G.B. Accurate autocorrelation modeling substantially improves fMRI reliability. Nat. Commun. 2019, 10, 1220. [Google Scholar] [CrossRef] [Green Version]
- Larsson, M.B.; Tillisch, K.; Craig, A.; Engström, M.; Labus, J.; Naliboff, B.; Lundberg, P.; Ström, M.; Mayer, E.A.; Walter, S.A. Brain responses to visceral stimuli reflect visceral sensitivity thresholds in patients with irritable bowel syndrome. Gastroenterology 2012, 142, 463–472.e3. [Google Scholar] [CrossRef] [Green Version]
- Olausson, H.; Wessberg, J.; Morrison, I.; McGlone, F.; Vallbo, A. The neurophysiology of unmyelinated tactile afferents. Neurosci. Biobehav. Rev. 2010, 34, 185–191. [Google Scholar] [CrossRef]
- Ferraro, S.; Klugah-Brown, B.; Tench, C.R.; Yao, S.; Nigri, A.; Demichelis, G.; Pinardi, C.; Bruzzone, M.G.; Becker, B. Dysregulated anterior insula reactivity as robust functional biomarker for chronic pain—Meta-analytic evidence from neuroimaging studies. Hum. Brain Mapp. 2022, 43, 998–1010. [Google Scholar] [CrossRef]
- Wager, T.D.; Atlas, L.Y.; Lindquist, M.A.; Roy, M.; Woo, C.-W.; Kross, E. An fMRI-based neurologic signature of physical pain. N. Engl. J. Med. 2013, 368, 1388–1397. [Google Scholar] [CrossRef] [Green Version]
- Gee, J.R.; Chang, J.; Dublin, A.B.; Vijayan, N. The association of brainstem lesions with migraine-like headache: An imaging study of multiple sclerosis. Headache J. Head Face Pain 2005, 45, 670–677. [Google Scholar] [CrossRef]
- Wager, T.D.; Scott, D.J.; Zubieta, J.-K. Placebo effects on human μ-opioid activity during pain. Proc. Natl. Acad. Sci. USA 2007, 104, 11056–11061. [Google Scholar] [CrossRef] [Green Version]
- Eippert, F.; Bingel, U.; Schoell, E.D.; Yacubian, J.; Klinger, R.; Lorenz, J.; Büchel, C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 2009, 63, 533–543. [Google Scholar] [CrossRef] [Green Version]
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef]
- Tracey, I.; Ploghaus, A.; Gati, J.S.; Clare, S.; Smith, S.; Menon, R.S.; Matthews, P.M. Imaging attentional modulation of pain in the periaqueductal gray in humans. J. Neurosci. 2002, 22, 2748–2752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beauregard, M.; Courtemanche, J.; Paquette, V.; St-Pierre, É.L. The neural basis of unconditional love. Psychiatry Res. Neuroimaging 2009, 172, 93–98. [Google Scholar] [CrossRef]
- Noriuchi, M.; Kikuchi, Y.; Senoo, A. The functional neuroanatomy of maternal love: Mother’s response to infant’s attachment behaviors. Biol. Psychiatry 2008, 63, 415–423. [Google Scholar] [CrossRef]
- Blood, A.J.; Zatorre, R.J. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc. Natl. Acad. Sci. USA 2001, 98, 11818–11823. [Google Scholar] [CrossRef] [Green Version]
- Zald, D.H.; Pardo, J.V. The neural correlates of aversive auditory stimulation. Neuroimage 2002, 16, 746–753. [Google Scholar] [CrossRef] [Green Version]
- Kambrun, C.; Roca-Lapirot, O.; Salio, C.; Landry, M.; Moqrich, A.; Le Feuvre, Y. TAFA4 Reverses Mechanical Allodynia through Activation of GABAergic Transmission and Microglial Process Retraction. Cell Rep. 2018, 22, 2886–2897. [Google Scholar] [CrossRef] [Green Version]

| Age | 23.5 (IQR 9.75) |
|---|---|
| Sex | 13 male, 19 female |
| State-Trait Anxiety Inventory | 29 (IQR 8) 35.2 ± 8.5 |
| Beck’s Depression Inventory | 3 (IQR 5) |
| McGill Pain Questionnaire | 0 (IQR 8) |
| Area | X | Y | Z | Clustersize | T | p (FWE-corr) |
|---|---|---|---|---|---|---|
| Anterior lobe | 15 | −40 | −25 | 128 | 7.27 | <0.001 |
| Culmen | 9 | −49 | −16 | 6.70 | <0.001 | |
| Declive | 6 | −64 | −19 | 6.68 | <0.001 | |
| Posterior Lobe | 15 | −61 | −19 | 5.38 | 0.03 | |
| Inferior Semi-Lunar Lobule | 15 | −73 | −37 | 13 | 6.04 | <0.001 |
| Area | Hemisphere | Cluster-size | p (FWE-corr) | T | X | Y | Z |
|---|---|---|---|---|---|---|---|
| Insula | R | 2912 | <0.001 | 19.36 | 42 | −1 | 11 |
| 17.39 | 51 | −28 | 20 | ||||
| 12.81 | 36 | 14 | 5 | ||||
| 11.87 | 33 | 23 | 2 | ||||
| 11.84 | 39 | −19 | 17 | ||||
| 11.60 | 42 | 11 | −4 | ||||
| 11.51 | 42 | 5 | −7 | ||||
| Postcentral Gyrus | <0.001 | 17.70 | 54 | −25 | 23 | ||
| 14.26 | 54 | −16 | 17 | ||||
| 13.79 | 54 | −19 | 38 | ||||
| 9.95 | 45 | −28 | 53 | ||||
| 9.88 | 42 | −31 | 56 | ||||
| Precentral Gyrus | <0.001 | 14.28 | 54 | 8 | 8 | ||
| Inferior Frontal Gyrus | <0.001 | 13.30 | 39 | 17 | −7 | ||
| 13.19 | 57 | 11 | 23 | ||||
| 12.91 | 60 | 8 | 29 | ||||
| Inferior Parietal Lobule | L | 1357 | <0.001 | 16.38 | −63 | −25 | 23 |
| 16.19 | −60 | −22 | 29 | ||||
| Insula | <0.001 | 13.98 | −39 | −1 | −7 | ||
| 13.95 | −42 | −4 | 2 | ||||
| 13.63 | −45 | −7 | 8 | ||||
| 13.36 | −33 | 14 | 2 | ||||
| 12.89 | −39 | −4 | 14 | ||||
| 9.54 | −30 | 23 | 8 | ||||
| Postcentral Gyrus | L | <0.001 | 13.27 | −63 | −22 | 38 | |
| Superior Temporal Gyrus | <0.001 | 12.29 | −51 | −4 | 5 | ||
| 8.53 | −54 | 5 | −1 | ||||
| Precentral Gyrus | <0.001 | 9.69 | −54 | 2 | 8 | ||
| Inferior Frontal Gyrus | <0.001 | 9.35 | −57 | 8 | 23 | ||
| 9.23 | −57 | 5 | 17 | ||||
| Supramarginal Gyrus | 0.01 | 5.33 | −66 | −43 | 29 | ||
| Lentiform Nucleus | R | 110 | <0.001 | 12.18 | 12 | 8 | −4 |
| Parahippocampal Gyrus | <0.001 | 7.41 | 18 | −1 | −10 | ||
| Lateral Globus Pallidus | L | 64 | <0.001 | 8.61 | −9 | 5 | −4 |
| Inferior Frontal Gyrus | R | 87 | <0.001 | 7.88 | 45 | 41 | 5 |
| Middle Frontal Gyrus | <0.001 | 5.62 | 45 | 44 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savallampi, M.; Maallo, A.M.S.; Shaikh, S.; McGlone, F.; Bariguian-Revel, F.J.; Olausson, H.; Boehme, R. Social Touch Reduces Pain Perception—An fMRI Study of Cortical Mechanisms. Brain Sci. 2023, 13, 393. https://doi.org/10.3390/brainsci13030393
Savallampi M, Maallo AMS, Shaikh S, McGlone F, Bariguian-Revel FJ, Olausson H, Boehme R. Social Touch Reduces Pain Perception—An fMRI Study of Cortical Mechanisms. Brain Sciences. 2023; 13(3):393. https://doi.org/10.3390/brainsci13030393
Chicago/Turabian StyleSavallampi, Mattias, Anne M. S. Maallo, Sumaiya Shaikh, Francis McGlone, Frédérique J. Bariguian-Revel, Håkan Olausson, and Rebecca Boehme. 2023. "Social Touch Reduces Pain Perception—An fMRI Study of Cortical Mechanisms" Brain Sciences 13, no. 3: 393. https://doi.org/10.3390/brainsci13030393
APA StyleSavallampi, M., Maallo, A. M. S., Shaikh, S., McGlone, F., Bariguian-Revel, F. J., Olausson, H., & Boehme, R. (2023). Social Touch Reduces Pain Perception—An fMRI Study of Cortical Mechanisms. Brain Sciences, 13(3), 393. https://doi.org/10.3390/brainsci13030393






