Supramarginal Gyrus and Angular Gyrus Subcortical Connections: A Microanatomical and Tractographic Study for Neurosurgeons
Abstract
:1. Introduction
2. Materials and Methods
3. Results
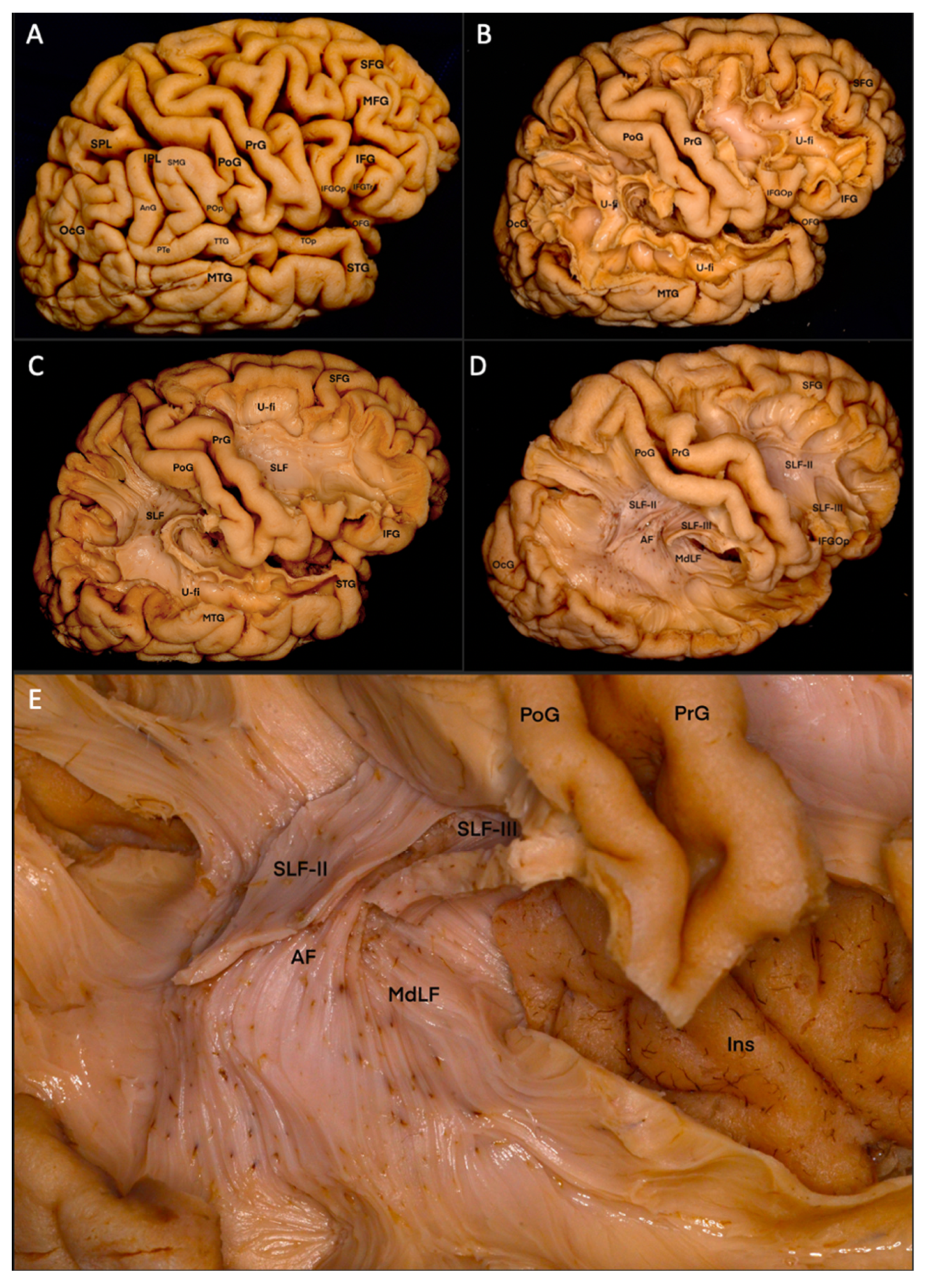
3.1. Fiber Dissection Procedure: Lateral to Medial Dissection
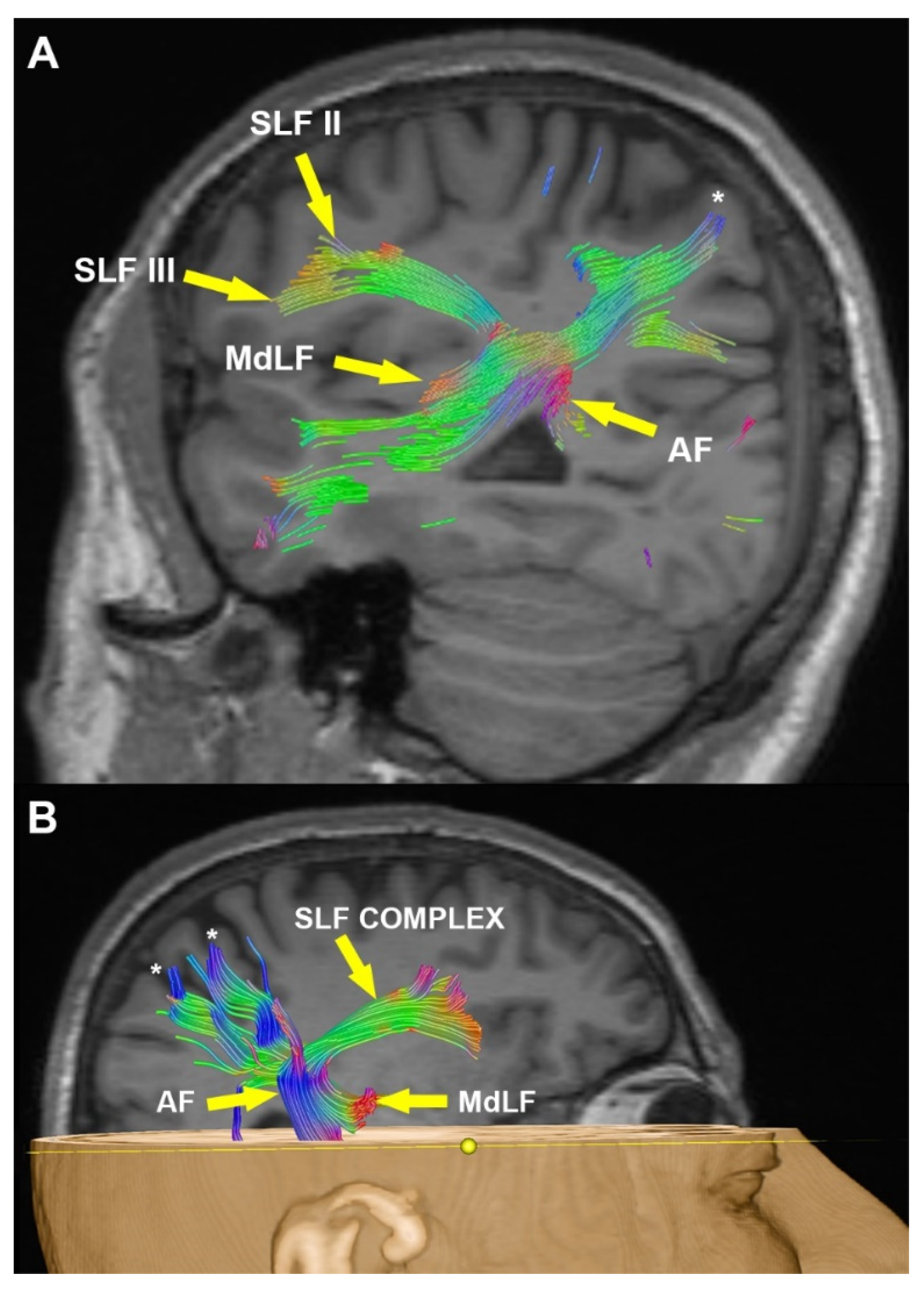
3.2. Radiological Examination
4. Discussion
4.1. Topographic Anatomy
4.2. Functional Anatomy
4.3. Surgical Perspective
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SLF | Superior longitudinal fasciculus |
| SLF I | Superior longitudinal fasciculus I |
| SLF II | Superior longitudinal fasciculus II |
| SLF III | Superior longitudinal fasciculus III |
| AF | Arcuat fasciculus |
| MdLF | Middle longitudinal fasciculus |
| SMG | Supramarginal gyrus |
| AnG | Angular gyrus |
| MRI | Magnetic resonance images |
| DTI | Diffusion tensor imaging |
| FT | Fiber tractography |
References
- Porto de Oliveira, J.V.M.; Raquelo-Menegassio, A.F.; Maldonado, I.L. What’s your name again? A review of the superior longitudinal and arcuate fasciculus evolving nomenclature. Clin. Anat. 2021, 34, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Klingler, J. Erleichterung der Makrokopischen Präparation des Gehirns Durch Den Gefrierprozess; Orell Füssli: Zurich, Switzerland, 1935. [Google Scholar]
- Catani, M.; Mesulam, M. The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex 2008, 44, 953–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalyvas, A.; Koutsarnakis, C.; Komaitis, S.; Karavasilis, E.; Christidi, F.; Skandalakis, G.P.; Liouta, E.; Papakonstantinou, O.; Kelekis, N.; Duffau, H. Mapping the human middle longitudinal fasciculus through a focused anatomo-imaging study: Shifting the paradigm of its segmentation and connectivity pattern. Brain Struct. Funct. 2020, 225, 85–119. [Google Scholar] [CrossRef] [PubMed]
- Faried, A.; Bachani, A.M.; Sendjaja, A.N.; Hung, Y.W.; Arifin, M.Z. Characteristics of moderate and severe traumatic brain injury of motorcycle crashes in Bandung, Indonesia. World Neurosurg. 2017, 100, 195–200. [Google Scholar] [CrossRef]
- Chang, E.F.; Raygor, K.P.; Berger, M.S. Contemporary model of language organization: An overview for neurosurgeons. J. Neurosurg. 2015, 122, 250–261. [Google Scholar] [CrossRef] [Green Version]
- Reitan, R.M. Validity of the Trail Making Test as an indicator of organic brain damage. Percept. Mot. Ski. 1958, 8, 271–276. [Google Scholar] [CrossRef]
- Jackendoff, R. A parallel architecture perspective on language processing. Brain Res. 2007, 1146, 2–22. [Google Scholar] [CrossRef]
- Rhoton, A.L., Jr. The cerebrum. Anatomy. Neurosurgery 2007, 61, 37–118. [Google Scholar] [CrossRef]
- Sarubbo, S.; De Benedictis, A.; Merler, S.; Mandonnet, E.; Barbareschi, M.; Dallabona, M.; Chioffi, F.; Duffau, H. Structural and functional integration between dorsal and ventral language streams as revealed by blunt dissection and direct electrical stimulation. Hum. Brain Mapp. 2016, 37, 3858–3872. [Google Scholar] [CrossRef]
- Price, A.R.; Bonner, M.F.; Peelle, J.E.; Grossman, M. Converging evidence for the neuroanatomic basis of combinatorial semantics in the angular gyrus. J. Neurosci. 2015, 35, 3276–3284. [Google Scholar] [CrossRef] [Green Version]
- Sato, W.; Kochiyama, T.; Yoshikawa, S.; Naito, E.; Matsumura, M. Enhanced neural activity in response to dynamic facial expressions of emotion: An fMRI study. Cogn. Brain Res. 2004, 20, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.A.; Ramos, L.; Segreti, A.; Brent, D.A.; Phillips, M.L. Right superior temporal gyrus volume in adolescents with a history of suicide attempt. Br. J. Psychiatry 2015, 206, 339–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakar, F.; Çeltikçi, P.; Doğruel, Y.; Egemen, E.; Güngör, A. The connectivity-based parcellation of the angular gyrus: Fiber dissection and MR tractography study. Brain Struct. Funct. 2023, 228, 121–130. [Google Scholar] [CrossRef]
- Klingler, J.; Gloor, P. The connections of the amygdala and of the anterior temporal cortex in the human brain. J. Comp. Neurol. 1960, 115, 333–369. [Google Scholar] [CrossRef] [PubMed]
- Türe, U.; Yaşargil, M.G.; Friedman, A.H.; Al-Mefty, O. Fiber dissection technique: Lateral aspect of the brain. Neurosurgery 2000, 47, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Peltier, J.; Travers, N.; Destrieux, C.; Velut, S. Optic radiations: A microsurgical anatomical study. J. Neurosurg. 2006, 105, 294–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmahmann, J.; Pandya, D. Fiber Pathways of the Brain; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Catani, M.; Jones, D.K.; Ffytche, D.H. Perisylvian language networks of the human brain. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2005, 57, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Makris, N.; Kennedy, D.; McInerney, S.; Sorensen, A.G.; Wang, R.; Caviness, V.S.; Pandya, D.N. Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo, DT-MRI study. Cereb. Cortex 2005, 15, 854–869. [Google Scholar] [CrossRef]
- Bernal, B.; Altman, N. The connectivity of the superior longitudinal fasciculus: A tractography DTI study. Magn. Reson. Imaging 2010, 28, 217–225. [Google Scholar] [CrossRef]
- Varghese, R.; Winstein, C.J. Relationship between motor capacity of the contralesional and ipsilesional hand depends on the side of stroke in chronic stroke survivors with mild-to-moderate impairment. Front. Neurol. 2020, 10, 1340. [Google Scholar] [CrossRef] [Green Version]
- Güngör, A.; Baydin, S.; Middlebrooks, E.H.; Tanriover, N.; Isler, C.; Rhoton, A.L. The white matter tracts of the cerebrum in ventricular surgery and hydrocephalus. J. Neurosurg. 2017, 126, 945–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seltzer, B.; Pandya, D. Further observations on parieto-temporal connections in the rhesus monkey. Exp. Brain Res. 1984, 55, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Makris, N.; Papadimitriou, G.M.; Kaiser, J.R.; Sorg, S.; Kennedy, D.N.; Pandya, D.N. Delineation of the middle longitudinal fascicle in humans: A quantitative, in vivo, DT-MRI study. Cereb. Cortex 2009, 19, 777–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conner, A.K.; Briggs, R.G.; Rahimi, M.; Sali, G.; Baker, C.M.; Burks, J.D.; Glenn, C.A.; Battiste, J.D.; Sughrue, M.E. A connectomic atlas of the human cerebrum—Chapter 12: Tractographic description of the middle longitudinal fasciculus. Oper. Neurosurg. 2018, 15, S429. [Google Scholar] [CrossRef]
- Makris, N.; Zhu, A.; Papadimitriou, G.; Mouradian, P.; Ng, I.; Scaccianoce, E.; Baselli, G.; Baglio, F.; Shenton, M.; Rathi, Y. Mapping temporo-parietal and temporo-occipital cortico-cortical connections of the human middle longitudinal fascicle in subject-specific, probabilistic, and stereotaxic Talairach spaces. Brain Imaging Behav. 2017, 11, 1258–1277. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, I.L.; De Champfleur, N.M.; Velut, S.; Destrieux, C.; Zemmoura, I.; Duffau, H. Evidence of am iddle l ongitudinal f asciculus in the human brain from fiber dissection. J. Anat. 2013, 223, 38–45. [Google Scholar] [CrossRef]
- Wang, Y.; Fernández-Miranda, J.C.; Verstynen, T.; Pathak, S.; Schneider, W.; Yeh, F.-C. Rethinking the role of the middle longitudinal fascicle in language and auditory pathways. Cereb. Cortex 2013, 23, 2347–2356. [Google Scholar] [CrossRef] [Green Version]
- D’Andrea, G.; Familiari, P.; Di Lauro, A.; Angelini, A.; Sessa, G. Safe resection of gliomas of the dominant angular gyrus availing of preoperative FMRI and intraoperative DTI: Preliminary series and surgical technique. World Neurosurg. 2016, 87, 627–639. [Google Scholar] [CrossRef]
- Wada, S.; Honma, M.; Masaoka, Y.; Yoshida, M.; Koiwa, N.; Sugiyama, H.; Iizuka, N.; Kubota, S.; Kokudai, Y.; Yoshikawa, A. Volume of the right supramarginal gyrus is associated with a maintenance of emotion recognition ability. PLoS ONE 2021, 16, e0254623. [Google Scholar] [CrossRef]
- Bello, L.; Gambini, A.; Castellano, A.; Carrabba, G.; Acerbi, F.; Fava, E.; Giussani, C.; Cadioli, M.; Blasi, V.; Casarotti, A. Motor and language DTI Fiber Tracking combined with intraoperative subcortical mapping for surgical removal of gliomas. Neuroimage 2008, 39, 369–382. [Google Scholar] [CrossRef]
- Duffau, H.; Lopes, M.; Arthuis, F.; Bitar, A.; Sichez, J.; Van Effenterre, R.; Capelle, L. Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: A comparative study between two series without (1985–1996) and with (1996–2003) functional mapping in the same institution. J. Neurol. Neurosurg. Psychiatry 2005, 76, 845–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leclercq, D.; Duffau, H.; Delmaire, C.; Capelle, L.; Gatignol, P.; Ducros, M.; Chiras, J.; Lehéricy, S. Comparison of diffusion tensor imaging tractography of language tracts and intraoperative subcortical stimulations. J. Neurosurg. 2010, 112, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Spena, G.; Nava, A.; Cassini, F.; Pepoli, A.; Bruno, M.; D’Agata, F.; Cauda, F.; Sacco, K.; Duca, S.; Barletta, L. Preoperative and intraoperative brain mapping for the resection of eloquent-area tumors. A prospective analysis of methodology, correlation, and usefulness based on clinical outcomes. Acta Neurochir. 2010, 152, 1835–1846. [Google Scholar] [CrossRef]
- Nimsky, C.; Ganslandt, O.; Fahlbusch, R. Implementation of fiber tract navigation. Neurosurgery 2007, 61, ONS-292–ONS-304. [Google Scholar] [CrossRef] [PubMed]
- De Benedictis, A.; Duffau, H. Brain hodotopy: From esoteric concept to practical surgical applications. Neurosurgery 2011, 68, 1703–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffau, H. The anatomo-functional connectivity of language revisited: New insights provided by electrostimulation and tractography. Neuropsychologia 2008, 46, 927–934. [Google Scholar] [CrossRef]
- Drane, D.L.; Roraback-Carson, J.; Hebb, A.O.; Hersonskey, T.; Lucas, T.; Ojemann, G.A.; Lettich, E.; Silbergeld, D.L.; Miller, J.W.; Ojemann, J.G. Cortical stimulation mapping and Wada results demonstrate a normal variant of right hemisphere language organization. Epilepsia 2012, 53, 1790–1798. [Google Scholar] [CrossRef] [Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şahin, M.H.; Akyüz, M.E.; Karadağ, M.K.; Yalçın, A. Supramarginal Gyrus and Angular Gyrus Subcortical Connections: A Microanatomical and Tractographic Study for Neurosurgeons. Brain Sci. 2023, 13, 430. https://doi.org/10.3390/brainsci13030430
Şahin MH, Akyüz ME, Karadağ MK, Yalçın A. Supramarginal Gyrus and Angular Gyrus Subcortical Connections: A Microanatomical and Tractographic Study for Neurosurgeons. Brain Sciences. 2023; 13(3):430. https://doi.org/10.3390/brainsci13030430
Chicago/Turabian StyleŞahin, Mehmet Hakan, Mehmet Emin Akyüz, Mehmet Kürşat Karadağ, and Ahmet Yalçın. 2023. "Supramarginal Gyrus and Angular Gyrus Subcortical Connections: A Microanatomical and Tractographic Study for Neurosurgeons" Brain Sciences 13, no. 3: 430. https://doi.org/10.3390/brainsci13030430
APA StyleŞahin, M. H., Akyüz, M. E., Karadağ, M. K., & Yalçın, A. (2023). Supramarginal Gyrus and Angular Gyrus Subcortical Connections: A Microanatomical and Tractographic Study for Neurosurgeons. Brain Sciences, 13(3), 430. https://doi.org/10.3390/brainsci13030430







