Anticipatory Postural Adjustments and Compensatory Postural Responses to Multidirectional Perturbations—Effects of Medication and Subthalamic Nucleus Deep Brain Stimulation in Parkinson’s Disease
Abstract
:1. Introduction
2. Methods
2.1. Subjects
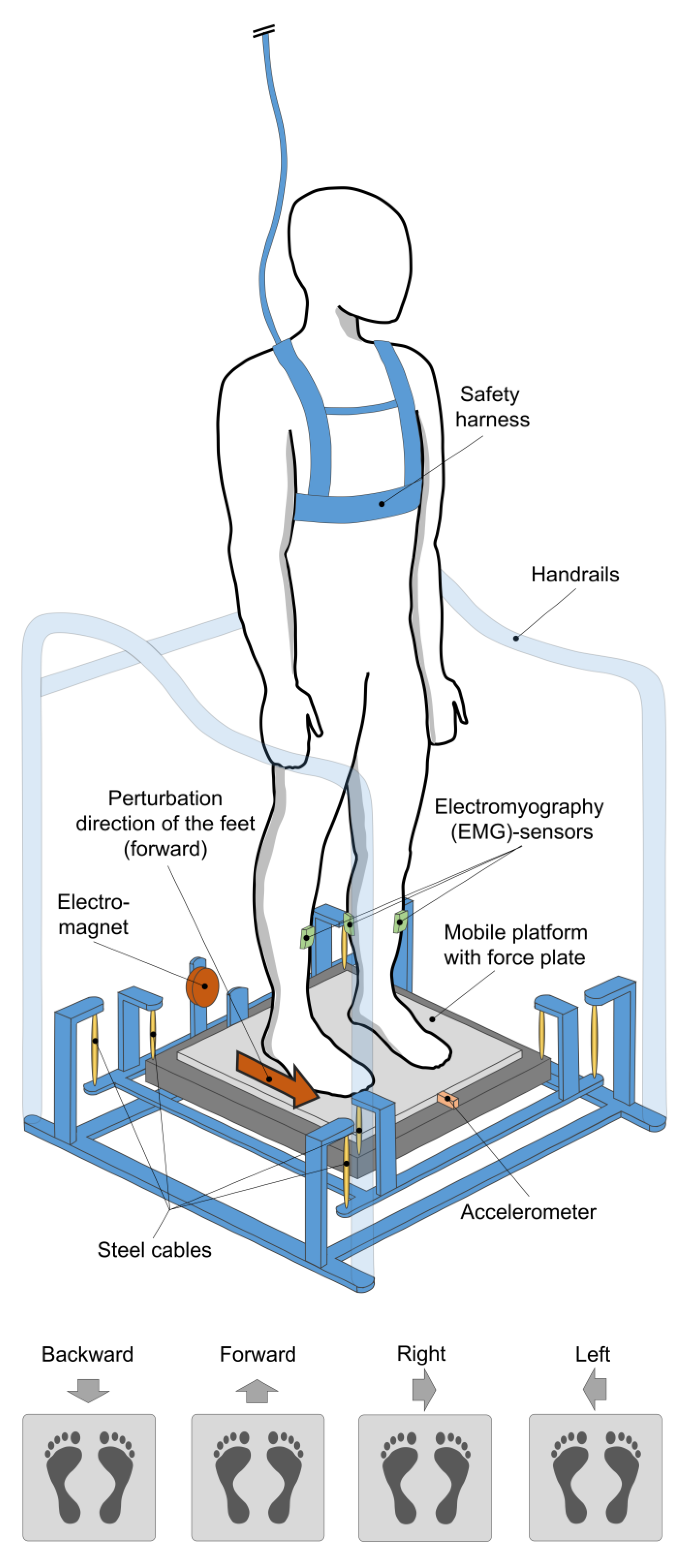
2.2. Equipment
2.3. Testing Procedure and Data Acquisition
2.4. Data Processing
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Data
3.2. EMG
3.2.1. Muscle Activity
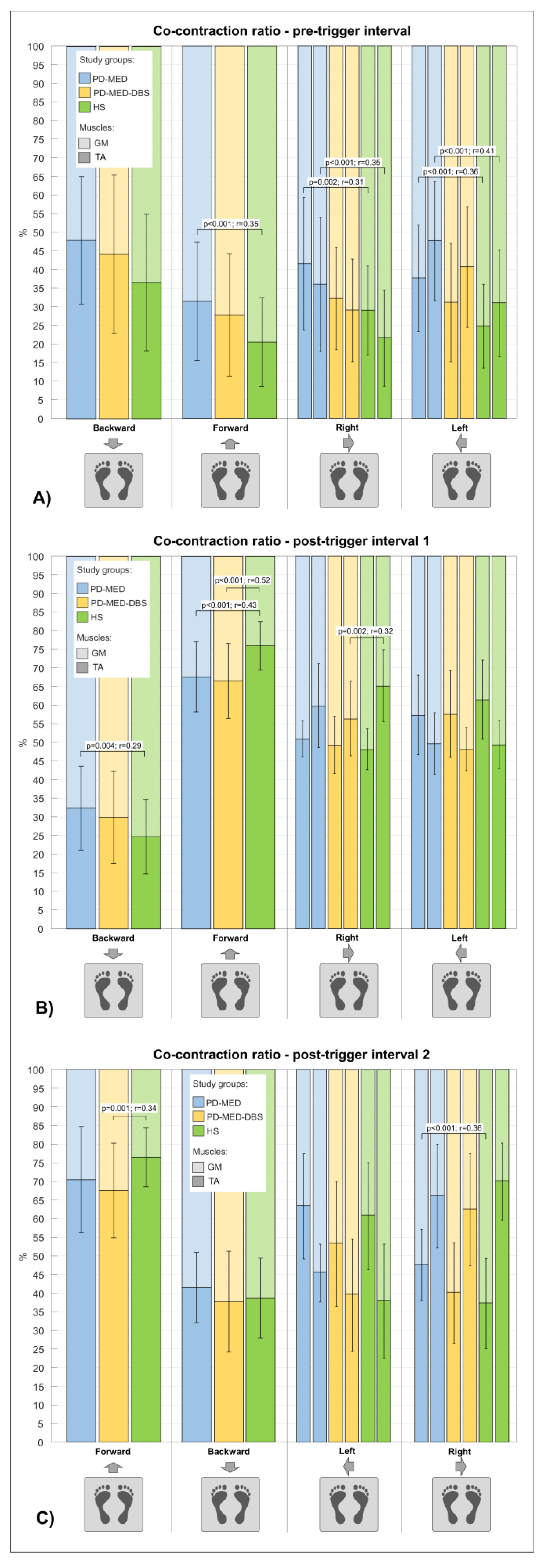
3.2.2. Co-Contraction Ratio
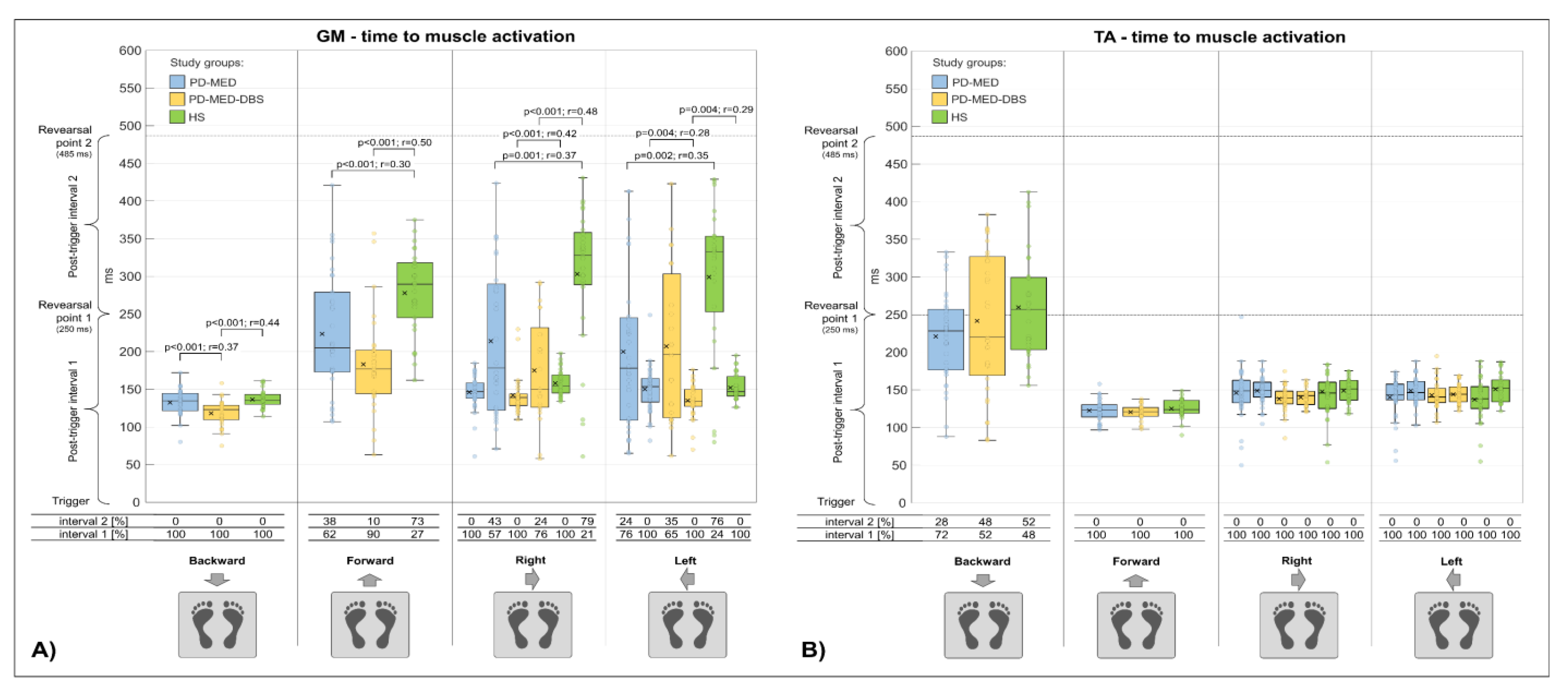
3.2.3. Time to Muscle Activation
3.3. COP Range
4. Discussion
4.1. Anticipatory Postural Adjustments
4.2. Compensatory Postural Responses
4.2.1. Timing of Compensatory Postural Responses
4.2.2. Magnitude of Compensatory Postural Responses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weerasak, M.; Aju, M.; Hiroyuki, H.; Seidel, D. A systematic review of the worldwide prevalence and incidence of Parkinson’s disease. J. Med. Assoc. Thail. 2011, 94, 19–55. [Google Scholar]
- Tysnes, O.-B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z.; Pan, J.; Tang, S.; Duan, D.; Yu, D.; Nong, H.; Wang, Z. Global Trends in the Incidence, Prevalence, and Years Lived with Disability of Parkinson’s Disease in 204 Countries/Territories from 1990 to 2019. Front. Public Health 2021, 9, 776847. [Google Scholar] [CrossRef]
- Berardelli, A. Neurophysiology of basal ganglia diseases. In Parkinson’s Disease and Related Disorders, Part I; Elsevier: Amsterdam, The Netherlands, 2007; pp. 67–75. ISBN 9780444519009. [Google Scholar]
- Shohamy, D.; Myers, C.E.; Onlaor, S.; Gluck, M.A. Role of the basal ganglia in category learning: How do patients with Parkinson’s disease learn? Behav. Neurosci. 2004, 118, 676–686. [Google Scholar] [CrossRef] [Green Version]
- Agid, Y. Parkinson’s disease: Pathophysiology. Lancet 1991, 337, 1321–1324. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Prim. 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Blandini, F.; Nappi, G.; Tassorelli, C.; Martignoni, E. Functional changes of the basal ganglia circuitry in Parkinson’s disease. Prog. Neurobiol. 2000, 62, 63–88. [Google Scholar] [CrossRef] [PubMed]
- Sveinbjornsdottir, S. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016, 139, 318–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, K.K.; Zhang, Y.; Lim, K.L.; Tanaka, Y.; Huang, H.; Gao, J.; Ross, C.A.; Dawson, V.L.; Dawson, T.M. Parkin ubiquitinates the alpha-synuclein-interacting protein, synphilin-1: Implications for Lewy-body formation in Parkinson disease. Nat. Med. 2001, 7, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H. Science, medicine, and the future: Parkinson’s disease. BMJ 1999, 318, 311–314. [Google Scholar] [CrossRef]
- Horak, F.B.; Dimitrova, D.; Nutt, J.G. Direction-specific postural instability in subjects with Parkinson’s disease. Exp. Neurol. 2005, 193, 504–521. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Bai, Y.; Zou, L.; Zhang, X.; Wang, H.; Gao, D.; Qin, G.; Ma, R.; Zhang, K.; Meng, F.; et al. Balance response to levodopa predicts balance improvement after bilateral subthalamic nucleus deep brain stimulation in Parkinson’s disease. NPJ Parkinsons. Dis. 2021, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Beretta, V.S.; Vitório, R.; Santos, P.C.R.D.; Orcioli-Silva, D.; Gobbi, L.T.B. Postural control after unexpected external perturbation: Effects of Parkinson’s disease subtype. Hum. Mov. Sci. 2019, 64, 12–18. [Google Scholar] [CrossRef]
- Benatru, I.; Vaugoyeau, M.; Azulay, J.-P. Postural disorders in Parkinson’s disease. Neurophysiol. Clin. 2008, 38, 459–465. [Google Scholar] [CrossRef]
- Siragy, T.; Nantel, J. Quantifying Dynamic Balance in Young, Elderly and Parkinson’s Individuals: A Systematic Review. Front. Aging Neurosci. 2018, 10, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.D.; Allen, N.E.; Canning, C.G.; Fung, V.S.C. Postural instability in patients with Parkinson’s disease. Epidemiology, pathophysiology and management. CNS Drugs 2013, 27, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Shagam, J.Y. Unlocking the Secrets of Parkinson Disease. Radiol. Technol. 2008, 79, 227–239. [Google Scholar]
- Lau, B.; Meier, N.; Serra, G.; Czernecki, V.; Schuepbach, M.; Navarro, S.; Cornu, P.; Grabli, D.; Agid, Y.; Vidailhet, M.; et al. Axial symptoms predict mortality in patients with Parkinson disease and subthalamic stimulation. Neurology 2019, 92, e2559–e2570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Am Grimbergen, Y.; Munneke, M.; Bloem, B.R. Falls in Parkinson’s disease. Curr. Opin. Neurol. 2004, 17, 405–415. [Google Scholar] [CrossRef]
- Stylianou, A.P.; McVey, M.A.; Lyons, K.E.; Pahwa, R.; Luchies, C.W. Postural sway in patients with mild to moderate Parkinson’s disease. Int. J. Neurosci. 2011, 121, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Palakurthi, B.; Burugupally, S.P. Postural Instability in Parkinson’s Disease: A Review. Brain Sci. 2019, 9, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorsey, E.R.; Elbaz, A.; Nichols, E.; Abbasi, N.; Abd-Allah, F.; Abdelalim, A.; Adsuar, J.C.; Ansha, M.G.; Brayne, C.; Choi, J.-Y.J.; et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbieri, F.A.; Polastri, P.F.; Baptista, A.M.; Lirani-Silva, E.; Simieli, L.; Orcioli-Silva, D.; Beretta, V.S.; Gobbi, L.T. Effects of disease severity and medication state on postural control asymmetry during challenging postural tasks in individuals with Parkinson’s disease. Hum. Mov. Sci. 2016, 46, 96–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Din, S.; Godfrey, A.; Coleman, S.; Galna, B.; Lord, S.; Rochester, L. Time-dependent changes in postural control in early Parkinson’s disease: What are we missing? Med. Biol. Eng. Comput. 2016, 54, 401–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, W.R.W.; Wieler, M. Treatment of Parkinson’s disease. Can. J. Neurol. Sci. 2003, 30, S27–S33. [Google Scholar] [CrossRef] [Green Version]
- Ganga, G.; Alty, J.E.; Clissold, B.G.; McColl, C.D.; Reardon, K.A.; Schiff, M.; Kempster, P.A. Longitudinal study of levodopa in Parkinson’s disease: Effects of the advanced disease phase. Mov. Disord. 2013, 28, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Thanvi, B.R.; Lo, T.C.N. Long term motor complications of levodopa: Clinical features, mechanisms, and management strategies. Postgrad. Med. J. 2004, 80, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groiss, S.J.; Wojtecki, L.; Südmeyer, M.; Schnitzler, A. Deep brain stimulation in Parkinson’s disease. Ther. Adv. Neurol. Disord. 2009, 2, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Castrioto, A.; Lozano, A.M.; Poon, Y.-Y.; Lang, A.E.; Fallis, M.; Moro, E. Ten-year outcome of subthalamic stimulation in Parkinson disease: A blinded evaluation. Arch. Neurol. 2011, 68, 1550–1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goetz, C.G.; Poewe, W.; Rascol, O.; Sampaio, C. Evidence-based medical review update: Pharmacological and surgical treatments of Parkinson’s disease: 2001 to 2004. Mov. Disord. 2005, 20, 523–539. [Google Scholar] [CrossRef]
- Bonnet, A.-M.; Loria, Y.; Saint-Hilaire, M.-H.; Lhermitte, F.; Agid, Y. Does long-term aggravation of Parkinson\textquoterights disease result from nondopaminergic lesions? Neurology 1987, 37, 1539. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lloret, S.; Barrantes, F.J. Deficits in cholinergic neurotransmission and their clinical correlates in Parkinson’s disease. NPJ Parkinson’s Dis. 2016, 2, 16001. [Google Scholar] [CrossRef] [Green Version]
- Di Giulio, I.; St George, R.J.; Kalliolia, E.; Peters, A.L.; Limousin, P.; Day, B.L. Maintaining balance against force perturbations: Impaired mechanisms unresponsive to levodopa in Parkinson’s disease. J. Neurophysiol. 2016, 116, 493–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colnat-Coulbois, S. Bilateral subthalamic nucleus stimulation improves balance control in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2005, 76, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Rinne, J.O.; Ma, S.Y.; Lee, M.S.; Collan, Y.; Röyttä, M. Loss of cholinergic neurons in the pedunculopontine nucleus in Parkinson’s disease is related to disability of the patients. Park. Relat. Disord. 2008, 14, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Beckley, D.J.; van Dijk, J.G.; Zwinderman, A.H.; Remler, M.P.; Roos, R.A. Influence of dopaminergic medication on automatic postural responses and balance impairment in Parkinson’s disease. Mov. Disord. 1996, 11, 509–521. [Google Scholar] [CrossRef]
- Rissanen, S.M.; Ruonala, V.; Pekkonen, E.; Kankaanpää, M.; Airaksinen, O.; Karjalainen, P.A. Signal features of surface electromyography in advanced Parkinson’s disease during different settings of deep brain stimulation. Clin. Neurophysiol. 2015, 126, 2290–2298. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.; Gill, S.; Varma, T.; Jenkinson, C.; Quinn, N.; Mitchell, R.; Scott, R.; Ives, N.; Rick, C.; Daniels, J.; et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial): A randomised, open-label trial. Lancet Neurol. 2010, 9, 581–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Follett, K.A.; Weaver, F.M.; Stern, M.; Hur, K.; Harris, C.L.; Luo, P.; Marks, W.J., Jr.; Rothlind, J.; Sagher, O.; Moy, C.; et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N. Engl. J. Med. 2010, 362, 2077–2091. [Google Scholar] [CrossRef] [Green Version]
- Yamada, K.; Goto, S.; Hamasaki, T.; Kuratsu, J.-I. Effect of bilateral subthalamic nucleus stimulation on levodopa-unresponsive axial symptoms in Parkinson’s disease. Acta Neurochir. 2008, 150, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Agnesi, F.; Johnson, M.D.; Vitek, J.L. Deep brain stimulation: How does it work? Handb. Clin. Neurol. 2013, 116, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Beurrier, C.; Bioulac, B.; Audin, J.; Hammond, C. High-Frequency Stimulation Produces a Transient Blockade of Voltage-Gated Currents in Subthalamic Neurons. J. Neurophysiol. 2001, 85, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Llinas, R.R.; Leznik, E.; Urbano, F.J. Temporal binding via cortical coincidence detection of specific and nonspecific thalamocortical inputs: A voltage-dependent dye-imaging study in mouse brain slices. Proc. Natl. Acad. Sci. USA 2002, 99, 449–454. [Google Scholar] [CrossRef] [Green Version]
- Dostrovsky, J.O.; Levy, R.; Wu, J.P.; Hutchison, W.D.; Tasker, R.R.; Lozano, A.M. Microstimulation-Induced Inhibition of Neuronal Firing in Human Globus Pallidus. J. Neurophysiol. 2000, 84, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Perlmutter, J.S.; Mink, J.W. Deep brain stimulation. Annu. Rev. Neurosci. 2006, 29, 229–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benabid, A.L. Deep brain stimulation for Parkinson’s disease. Curr. Opin. Neurobiol. 2003, 13, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.; D’Alessandro, G.; Bioulac, B.; Hammond, C. High-frequency stimulation in Parkinson’s disease: More or less? Trends Neurosci. 2005, 28, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Pötter-Nerger, M.; Volkmann, J. Deep brain stimulation for gait and postural symptoms in Parkinson’s disease. Mov. Disord. 2013, 28, 1609–1615. [Google Scholar] [CrossRef]
- Johnsen, E.L. Gait and postural instability in Parkinson’s disease treated with deep brain stimulation of the subthalamic nucleus. Dan. Med. Bull. 2011, 58, B4334. [Google Scholar]
- Rizzone, M.G.; Fasano, A.; Daniele, A.; Zibetti, M.; Merola, A.; Rizzi, L.; Piano, C.; Piccininni, C.; Romito, L.M.; Lopiano, L.; et al. Long-term outcome of subthalamic nucleus DBS in Parkinson’s disease: From the advanced phase towards the late stage of the disease? Park. Relat. Disord. 2014, 20, 376–381. [Google Scholar] [CrossRef]
- Zibetti, M.; Merola, A.; Rizzi, L.; Ricchi, V.; Angrisano, S.; Azzaro, C.; Artusi, C.A.; Arduino, N.; Marchisio, A.; Lanotte, M.; et al. Beyond nine years of continuous subthalamic nucleus deep brain stimulation in Parkinson’s disease. Mov. Disord. 2011, 26, 2327–2334. [Google Scholar] [CrossRef]
- Fasano, A.; Romito, L.M.; Daniele, A.; Piano, C.; Zinno, M.; Bentivoglio, A.R.; Albanese, A. Motor and cognitive outcome in patients with Parkinson’s disease 8 years after subthalamic implants. Brain 2010, 133, 2664–2676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bejjani, B.P.; Gervais, D.; Arnulf, I.; Papadopoulos, S.; Demeret, S.; Bonnet, A.M.; Cornu, P.; Damier, P.; Agid, Y. Axial parkinsonian symptoms can be improved: The role of levodopa and bilateral subthalamic stimulation. J. Neurol. Neurosurg. Psychiatry 2000, 68, 595–600. [Google Scholar] [CrossRef]
- Krack, P.; Batir, A.; van Blercom, N.; Chabardes, S.; Fraix, V.; Ardouin, C.; Koudsie, A.; Limousin, P.D.; Benazzouz, A.; LeBas, J.F.; et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N. Engl. J. Med. 2003, 349, 1925–1934. [Google Scholar] [CrossRef] [Green Version]
- De La Casa-Fages, B.; Alonso-Frech, F.; Grandas, F. Effect of subthalamic nucleus deep brain stimulation on balance in Parkinson’s disease: A static posturographic analysis. Gait Posture 2017, 52, 374–380. [Google Scholar] [CrossRef]
- Rocchi, L.; Carlson-Kuhta, P.; Chiari, L.; Burchiel, K.J.; Hogarth, P.; Horak, F.B. Effects of deep brain stimulation in the subthalamic nucleus or globus pallidus internus on step initiation in Parkinson disease: Laboratory investigation. J. Neurosurg. 2012, 117, 1141–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belmonte-Hernández, A.; Daras, P.; Theodoridis, T.; González, M.B.; Hernández-Peñaloza, G.; Solachidis, V.; Ramos, J.J.; Álvarez, F.; Vretos, N.; Carrasco, L. A novel framework for physical therapy rehabilitation monitoring and assessment in Parkinson disease patients using depth information. In Proceedings of the PETRA’19: Proceedings of the 12th ACM International Conference on PErvasive Technologies Related to Assistive Environments, Rhodes, Greece, 5–7 June 2019; Makedon, F., Ed.; ACM Press: New York, NY, USA, 2019; pp. 535–539, ISBN 9781450362320. [Google Scholar]
- Bloem, B.R.; Beckley, D.J.; van Hilten, B.J.; Roos, R.A. Clinimetrics of Postural Instability in Parkinson’s Disease: A Collection of Articles on Psychological Theories, Ethical Development and Human Understanding; Allen & Unwin: London, UK, 1998; ISBN 0041500504. [Google Scholar]
- Ganesan, M.; Pal, P.K.; Gupta, A.; Sathyaprabha, T.N. Dynamic posturography in evaluation of balance in patients of Parkinson’s disease with normal pull test: Concept of a diagonal pull test. Park. Relat. Disord. 2010, 16, 595–599. [Google Scholar] [CrossRef]
- Munhoz, R.P.; Teive, H.A. Pull test performance and correlation with falls risk in Parkinson’s disease. Arq. Neuropsiquiatr. 2014, 72, 587–591. [Google Scholar] [CrossRef] [Green Version]
- Bloem, B.R.; Grimbergen, Y.A.M.; Cramer, M.; Willemsen, M.; Zwinderman, A.H. Prospective assessment of falls in Parkinson’s disease. J. Neurol. 2001, 248, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Schlenstedt, C.; Mancini, M.; Horak, F.; Peterson, D. Anticipatory Postural Adjustment During Self-Initiated, Cued, and Compensatory Stepping in Healthy Older Adults and Patients with Parkinson Disease. Arch. Phys. Med. Rehabil. 2017, 98, 1316–1324.e1. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.J.; Kanekar, N.; Aruin, A.S. The role of anticipatory postural adjustments in compensatory control of posture: 1. Electromyographic analysis. J. Electromyogr. Kinesiol. 2010, 20, 388–397. [Google Scholar] [CrossRef]
- Dimitrova, D.; Horak, F.B.; Nutt, J.G. Postural muscle responses to multidirectional translations in patients with Parkinson’s disease. J. Neurophysiol. 2004, 91, 489–501. [Google Scholar] [CrossRef] [Green Version]
- Beretta, V.S.; Carpenter, M.G.; Barbieri, F.A.; Santos, P.C.R.; Orcioli-Silva, D.; Pereira, M.P.; Gobbi, L.T.B. Does the impaired postural control in Parkinson’s disease affect the habituation to non-sequential external perturbation trials? Clin. Biomech. 2021, 85, 105363. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, M.G.; Allum, J.H.J.; Honegger, F.; Adkin, A.L.; Bloem, B.R. Postural abnormalities to multidirectional stance perturbations in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1245–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visser, J.E.; Oude Nijhuis, L.B.; Janssen, L.; Bastiaanse, C.M.; Borm, G.F.; Duysens, J.; Bloem, B.R. Dynamic posturography in Parkinson’s disease: Diagnostic utility of the “first trial effect”. Neuroscience 2010, 168, 387–394. [Google Scholar] [CrossRef]
- Oude Nijhuis, L.B.; Allum, J.H.J.; Nanhoe-Mahabier, W.; Bloem, B.R. Influence of perturbation velocity on balance control in Parkinson’s disease. PLoS ONE 2014, 9, e86650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiraoka, K.; Matsuo, Y.; Abe, K. Soleus H-reflex inhibition during gait initiation in Parkinson’s disease. Mov. Disord. 2005, 20, 858–864. [Google Scholar] [CrossRef]
- Bloem, B.R.; Beckley, D.J.; Remler, M.P.; Roos, R.A.; van Dijk, J. Postural reflexes in Parkinson’s disease during ‘resist’ and ‘yield’ tasks. J. Neurol. Sci. 1995, 129, 109–119. [Google Scholar] [CrossRef] [PubMed]
- May, D.S.; van Dillen, L.R.; Earhart, G.M.; Rawson, K.S.; Perlmutter, J.S.; Duncan, R.P. Effects of Subthalamic Nucleus Deep Brain Stimulation and Levodopa on Balance in People with Parkinson’s Disease: A Cross Sectional Study. Brain Sci. 2020, 10, 693. [Google Scholar] [CrossRef] [PubMed]
- Hall, L.M.; Brauer, S.G.; Horak, F.; Hodges, P.W. The effect of Parkinson’s disease and levodopa on adaptation of anticipatory postural adjustments. Neuroscience 2013, 250, 483–492. [Google Scholar] [CrossRef] [Green Version]
- St George, R.J.; Carlson-Kuhta, P.; King, L.A.; Burchiel, K.J.; Horak, F.B. Compensatory stepping in Parkinson’s disease is still a problem after deep brain stimulation randomized to STN or GPi. J. Neurophysiol. 2015, 114, 1417–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, M.; Nilsson, M.H.; Rehncrona, S.; Tjernström, F.; Magnusson, M.; Johansson, R.; Fransson, P.-A. Strategic alterations of posture are delayed in Parkinson’s disease patients during deep brain stimulation. Sci. Rep. 2021, 11, 23550. [Google Scholar] [CrossRef] [PubMed]
- Fransson, P.-A.; Nilsson, M.H.; Rehncrona, S.; Tjernström, F.; Magnusson, M.; Johansson, R.; Patel, M. Deep brain stimulation in the subthalamic nuclei alters postural alignment and adaptation in Parkinson’s disease. PLoS ONE 2021, 16, e0259862. [Google Scholar] [CrossRef]
- Chastan, N.; Westby, G.W.M.; Yelnik, J.; Bardinet, E.; Do, M.C.; Agid, Y.; Welter, M.L. Effects of nigral stimulation on locomotion and postural stability in patients with Parkinson’s disease. Brain 2009, 132, 172–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- St George, R.J.; Carlson-Kuhta, P.; Burchiel, K.J.; Hogarth, P.; Frank, N.; Horak, F.B. The effects of subthalamic and pallidal deep brain stimulation on postural responses in patients with Parkinson disease. J. Neurosurg. 2012, 116, 1347–1356. [Google Scholar] [CrossRef] [Green Version]
- Maurer, C.; Mergner, T.; Xie, J.; Faist, M.; Pollak, P.; Lucking, C.H. Effect of chronic bilateral subthalamic nucleus (STN) stimulation on postural control in Parkinson’s disease. Brain 2003, 126, 1146–1163. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, S.; Yu, Y.; Wang, Y.; Cheng, Y.; Yang, H.; Tong, X. Effect of Subthalamic Nucleus Deep Brain Stimulation (STN-DBS) on balance performance in Parkinson’s disease. PLoS ONE 2020, 15, e0238936. [Google Scholar] [CrossRef]
- Beuter, A.; Modolo, J. Delayed and lasting effects of deep brain stimulation on locomotion in Parkinson’s disease. Chaos 2009, 19, 26114. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Carpes, F.P.; Milani, T.L.; Germano, A.M.C. Different visual manipulations have similar effects on quasi-static and dynamic balance responses of young and older people. PeerJ 2021, 9, e11221. [Google Scholar] [CrossRef] [PubMed]
- Hülsdünker, T.; Mierau, A.; Neeb, C.; Kleinöder, H.; Strüder, H.K. Cortical processes associated with continuous balance control as revealed by EEG spectral power. Neurosci. Lett. 2015, 592, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Germano, A.M.C.; Milani, T.L. Aspects of Dynamic Balance Responses: Inter- and Intra-Day Reliability. PLoS ONE 2015, 10, e0136551. [Google Scholar] [CrossRef]
- Germano, A.M.C.; Schmidt, D.; Milani, T.L. Effects of hypothermically reduced plantar skin inputs on anticipatory and compensatory balance responses. BMC Neurosci. 2016, 17, 41. [Google Scholar] [CrossRef] [Green Version]
- Luca, C.J. de. The Use of Surface Electromyography in Biomechanics. J. Appl. Biomech. 1997, 13, 135–163. [Google Scholar] [CrossRef] [Green Version]
- Hermens, H.J. European Recommendations for Surface ElectroMyoGraphy: Results of the SENIAM Project; Roessingh Research and Development: Enschede, The Netherlands, 1999; ISBN 9075452152. [Google Scholar]
- Jacobs, J.V.; Horak, F.B. Abnormal proprioceptive-motor integration contributes to hypometric postural responses of subjects with Parkinson’s disease. Neuroscience 2006, 141, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Taube, W.; Schubert, M.; Gruber, M.; Beck, S.; Faist, M.; Gollhofer, A. Direct corticospinal pathways contribute to neuromuscular control of perturbed stance. J. Appl. Physiol. 2006, 101, 420–429. [Google Scholar] [CrossRef] [Green Version]
- Bloem, B.R.; van Dijk, J.G.; Beckley, D.J.; Roos, R.; Remler, M.P.; Bruyn, G.W. Altered postural reflexes in Parkinson’s disease: A reverse hypothesis. Med. Hypotheses 1992, 39, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Scholz, E.; Diener, H.C.; Noth, J.; Friedemann, H.; Dichgans, J.; Bacher, M. Medium and long latency EMG responses in leg muscles: Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1987, 50, 66–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halaki, M.; Gi, K. Normalization of EMG Signals: To Normalize or Not to Normalize and What to Normalize to? In Computational Intelligence in Electromyography Analysis—A Perspective on Current Applications and Future Challenges; Naik, G.R., Ed.; InTech: London, UK, 2012; ISBN 978-953-51-0805-4. [Google Scholar]
- Chalard, A.; Belle, M.; Montané, E.; Marque, P.; Amarantini, D.; Gasq, D. Impact of the EMG normalization method on muscle activation and the antagonist-agonist co-contraction index during active elbow extension: Practical implications for post-stroke subjects. J. Electromyogr. Kinesiol. 2020, 51, 102403. [Google Scholar] [CrossRef]
- De Freitas, P.B.; Knight, C.A.; Barela, J.A. Postural reactions following forward platform perturbation in young, middle-age, and old adults. J. Electromyogr. Kinesiol. 2010, 20, 693–700. [Google Scholar] [CrossRef]
- Li, G.; Shourijeh, M.S.; Di Ao; Patten, C.; Fregly, B.J. How Well Do Commonly Used Co-contraction Indices Approximate Lower Limb Joint Stiffness Trends During Gait for Individuals Post-stroke? Front. Bioeng. Biotechnol. 2020, 8, 588908. [Google Scholar] [CrossRef]
- Mancini, M.; Rocchi, L.; Horak, F.B.; Chiari, L. Effects of Parkinson’s disease and levodopa on functional limits of stability. Clin. Biomech. 2008, 23, 450–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schieppati, M.; Hugon, M.; Grasso, M.; Nardone, A.; Galante, M. The limits of equilibrium in young and elderly normal subjects and in parkinsonians. Electroencephalogr. Clin. Neurophysiol./Evoked Potentials Sect. 1994, 93, 286–298. [Google Scholar] [CrossRef]
- Menz, H.B. Two feet, or one person?: Problems associated with statistical analysis of paired data in foot and ankle medicine. Foot 2004, 14, 2–5. [Google Scholar] [CrossRef]
- Bleuse, S.; Delval, A.; Blatt, J.L.; Derambure, P.; Destée, A.; Defebvre, L. Effect of bilateral subthalamic nucleus deep brain stimulation on postural adjustments during arm movement. Clin. Neurophysiol. 2011, 122, 2032–2035. [Google Scholar] [CrossRef] [PubMed]
- Bayot, M.; Delval, A.; Moreau, C.; Defebvre, L.; Hansen, C.; Maetzler, W.; Schlenstedt, C. Initial center of pressure position prior to anticipatory postural adjustments during gait initiation in people with Parkinson’s disease with freezing of gait. Park. Relat. Disord. 2021, 84, 8–14. [Google Scholar] [CrossRef]
- Song, J.; Sigward, S.; Fisher, B.; Salem, G.J. Altered Dynamic Postural Control during Step Turning in Persons with Early-Stage Parkinson’s Disease. Parkinsons. Dis. 2012, 2012, 386962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Néstor, G.-J. Parkinson’s Disease. Neurobiol. Dis. 2007, 51–67. [Google Scholar] [CrossRef]
- Błaszczyk, J.W.; Orawiec, R. Assessment of postural control in patients with Parkinson’s disease: Sway ratio analysis. Basic Mech. Underlying Balance Control. Under Static Dyn. Cond. 2011, 30, 396–404. [Google Scholar] [CrossRef]
- Schlenstedt, C.; Gavriliuc, O.; Boße, K.; Wolke, R.; Granert, O.; Deuschl, G.; Margraf, N.G. The Effect of Medication and Deep Brain Stimulation on Posture in Parkinson’s Disease. Front. Neurol. 2019, 10, 1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz-Schaeffer, W.J.; Margraf, N.G.; Munser, S.; Wrede, A.; Buhmann, C.; Deuschl, G.; Oehlwein, C. Effect of neurostimulation on camptocormia in Parkinson’s disease depends on symptom duration. Mov. Disord. 2015, 30, 368–372. [Google Scholar] [CrossRef]
- Carpenter, M.G.; Frank, J.S.; Silcher, C.P.; Peysar, G.W. The influence of postural threat on the control of upright stance. Exp. Brain Res. 2001, 138, 210–218. [Google Scholar] [CrossRef]
- Raethjen, J.; Raethjen, P.; Schmalbach, B.; Wasner, G. Dynamic posturography and posturographic training for Parkinson’s disease in a routine clinical setting. Gait Posture 2020, 82, 281–286. [Google Scholar] [CrossRef]
- Beretta, V.S.; Gobbi, L.T.B.; Lirani-Silva, E.; Simieli, L.; Orcioli-Silva, D.; Barbieri, F.A. Challenging Postural Tasks Increase Asymmetry in Patients with Parkinson’s Disease. PLoS ONE 2015, 10, e0137722. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Kang, Y.-J.; Horak, F.B. What Is Wrong with Balance in Parkinson’s Disease? J. Mov. Disord. 2015, 8, 109–114. [Google Scholar] [CrossRef]
- Müller, M.L.T.M.; Redfern, M.S. Correlation between EMG and COP onset latency in response to a horizontal platform translation. J. Biomech. 2004, 37, 1573–1581. [Google Scholar] [CrossRef]
- Levin, O.; Mizrahi, J.; Adam, D.; Verbitsky, O.; Isakov, E. On the correlation between force plate data and EMG in various standing conditions. In Proceedings of the 5th Annual Conference of the Functional Electrical Stimulation Society, Aalborg, Denmark, June 2000; Volume 2000. [Google Scholar]
- St George, R.J.; Carlson-Kuhta, P.; Nutt, J.G.; Hogarth, P.; Burchiel, K.J.; Horak, F.B. The effect of deep brain stimulation randomized by site on balance in Parkinson’s disease. Mov. Disord. 2014, 29, 949–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Needle, A.R.; Kaminski, T.W.; Baumeister, J.; Higginson, J.S.; Farquhar, W.B.; Swanik, C.B. The Relationship Between Joint Stiffness and Muscle Activity in Unstable Ankles and Copers. J. Sport Rehabil. 2017, 26, 15–25. [Google Scholar] [CrossRef]
- Crenna, P.; Carpinella, I.; Rabuffetti, M.; Rizzone, M.; Lopiano, L.; Lanotte, M.; Ferrarin, M. Impact of subthalamic nucleus stimulation on the initiation of gait in Parkinson’s disease. Exp. Brain Res. 2006, 172, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Wylie, S.A.; van den Wildenberg, W.; Ridderinkhof, K.R.; Claassen, D.O.; Wooten, G.F.; Manning, C.A. Differential susceptibility to motor impulsivity among functional subtypes of Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2012, 83, 1149–1154. [Google Scholar] [CrossRef] [Green Version]
- Miller-Patterson, C.; Buesa, R.; McLaughlin, N.; Jones, R.; Akbar, U.; Friedman, J.H. Motor asymmetry over time in Parkinson’s disease. J. Neurol. Sci. 2018, 393, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Pahapill, P.A.; Lozano, A.M. The pedunculopontine nucleus and Parkinson’s disease. Brain 2000, 123, 1767–1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zweig, R.M.; Jankel, W.R.; Hedreen, J.C.; Mayeux, R.; Price, D.L. The pedunculopontine nucleus in Parkinson’s disease. Ann. Neurol. 1989, 26, 41–46. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Müller, M.L.T.M.; Koeppe, R.A.; Studenski, S.A.; Kilbourn, M.A.; Frey, K.A.; Albin, R.L. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology 2009, 73, 1670–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohnen, N.I.; Frey, K.A.; Studenski, S.; Kotagal, V.; Koeppe, R.A.; Scott, P.J.H.; Albin, R.L.; Müller, M.L.T.M. Gait speed in Parkinson disease correlates with cholinergic degeneration. Neurology 2013, 81, 1611–1616. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, E.C.; Graybiel, A.M.; Duyckaerts, C.; Javoy-Agid, F. Neuronal loss in the pedunculopontine tegmental nucleus in Parkinson disease and in progressive supranuclear palsy. Proc. Natl. Acad. Sci. USA 1987, 84, 5976–5980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karachi, C.; Grabli, D.; Bernard, F.A.; Tandé, D.; Wattiez, N.; Belaid, H.; Bardinet, E.; Prigent, A.; Nothacker, H.-P.; Hunot, S.; et al. Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J. Clin. Invest. 2010, 120, 2745–2754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocchi, L. Effects of deep brain stimulation and levodopa on postural sway in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2002, 73, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.B.; Frank, J.; Nutt, J. Effects of dopamine on postural control in parkinsonian subjects: Scaling, set, and tone. J. Neurophysiol. 1996, 75, 2380–2396. [Google Scholar] [CrossRef] [PubMed]
- Gradinaru, V.; Mogri, M.; Thompson, K.R.; Henderson, J.M.; Deisseroth, K. Optical Deconstruction of Parkinsonian Neural Circuitry. Science 2009, 324, 354–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamani, C.; Saint-Cyr, J.A.; Fraser, J.; Kaplitt, M.; Lozano, A.M. The subthalamic nucleus in the context of movement disorders. Brain 2004, 127, 4–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano, A.M.; Snyder, B.J. Deep brain stimulation for parkinsonian gait disorders. J. Neurol. 2008, 255, 30–31. [Google Scholar] [CrossRef] [PubMed]
- Weaver, F.M.; Follett, K.A.; Stern, M.; Luo, P.; Harris, C.L.; Hur, K.; Marks, W.J.; Rothlind, J.; Sagher, O.; Moy, C.; et al. Randomized trial of deep brain stimulation for Parkinson disease: Thirty-six-month outcomes. Neurology 2012, 79, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Collomb-Clerc, A.; Welter, M.-L. Effects of deep brain stimulation on balance and gait in patients with Parkinson’s disease: A systematic neurophysiological review. Neurophysiol. Clin. 2015, 45, 371–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallabhajosula, S.; Haq, I.U.; Hwynn, N.; Oyama, G.; Okun, M.; Tillman, M.D.; Hass, C.J. Low-frequency Versus High-frequency Subthalamic Nucleus Deep Brain Stimulation on Postural Control and Gait in Parkinson’s Disease: A Quantitative Study. Brain Stimul. 2015, 8, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Stolze, H.; Klebe, S.; Poepping, M.; Lorenz, D.; Herzog, J.; Hamel, W.; Schrader, B.; Raethjen, J.; Wenzelburger, R.; Mehdorn, H.M.; et al. Effects of bilateral subthalamic nucleus stimulation on parkinsonian gait. Neurology 2001, 57, 144–146. [Google Scholar] [CrossRef] [Green Version]
- Lubik, S.; Fogel, W.; Tronnier, V.; Krause, M.; König, J.; Jost, W.H. Gait analysis in patients with advanced Parkinson disease: Different or additive effects on gait induced by levodopa and chronic STN stimulation. J. Neural Transm. 2006, 113, 163–173. [Google Scholar] [CrossRef]
- Faist, M.; Xie, J.; Kurz, D.; Berger, W.; Maurer, C.; Pollak, P.; Lücking, C.H. Effect of bilateral subthalamic nucleus stimulation on gait in Parkinson’s disease. Brain 2001, 124, 1590–1600. [Google Scholar] [CrossRef]
- Houeto, J.L.; Damier, P.; Bejjani, P.B.; Staedler, C.; Bonnet, A.M.; Arnulf, I.; Pidoux, B.; Dormont, D.; Cornu, P.; Agid, Y. Subthalamic stimulation in Parkinson disease: A multidisciplinary approach. Arch. Neurol. 2000, 57, 461–465. [Google Scholar] [CrossRef] [Green Version]
- Limousin, P.; Krack, P.; Pollak, P.; Benazzouz, A.; Ardouin, C.; Hoffmann, D.; Benabid, A.L. Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N. Engl. J. Med. 1998, 339, 1105–1111. [Google Scholar] [CrossRef]
- Moro, E.; Scerrati, M.; Romito, L.M.; Roselli, R.; Tonali, P.; Albanese, A. Chronic subthalamic nucleus stimulation reduces medication requirements in Parkinson’s disease. Neurology 1999, 53, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, K.; Sunde, N.; Dupont, E. Effects of bilateral stimulation of the subthalamic nucleus in patients with severe Parkinson’s disease and motor fluctuations. Mov. Disord. 2002, 17, 693–700. [Google Scholar] [CrossRef]
- Jaggi, J.L.; Umemura, A.; Hurtig, H.I.; Siderowf, A.D.; Colcher, A.; Stern, M.B.; Baltuch, G.H. Bilateral Stimulation of the Subthalamic Nucleus in Parkinson’s Disease: Surgical Efficacy and Prediction of Outcome. Stereotact. Funct. Neurosurg. 2004, 82, 104–114. [Google Scholar] [CrossRef]
- McNeely, M.E.; Earhart, G.M. Medication and subthalamic nucleus deep brain stimulation similarly improve balance and complex gait in Parkinson disease. Park. Relat. Disord. 2013, 19, 86–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, R.; Hashimoto, T.; Tada, T.; Ikeda, S. Relation between changes in long-latency stretch reflexes and muscle stiffness in Parkinson’s disease—Comparison before and after unilateral pallidotomy. Clin. Neurophysiol. 2001, 112, 1814–1821. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Beckley, D.J.; van Dijk, J.G.; Zwinderman, A.H.; Roos, R. Are medium and long latency reflexes a screening tool for early Parkinson’s disease? J. Neurol. Sci. 1992, 113, 38–42. [Google Scholar] [CrossRef]
- Hunter, J.P.; Ashby, P.; Lang, A.E. Afferents contributing to the exaggerated long latency reflex response to electrical stimulation in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1988, 51, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Ishida, G.; Nakashima, K.; Takahashi, K. Skin nerve sympathetic activity reflex latency in Parkinson’s disease. Acta Neurol. Scand. 1990, 81, 121–124. [Google Scholar] [CrossRef]
- Khudados, E.; Cody, F.W.; O’Boyle, D.J. Proprioceptive regulation of voluntary ankle movements, demonstrated using muscle vibration, is impaired by Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1999, 67, 504–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demirci, M.; Grill, S.; McShane, L.; Hallett, M. A mismatch between kinesthetic and visual perception in Parkinson’s disease. Ann. Neurol. 1997, 41, 781–788. [Google Scholar] [CrossRef]
- Vitale, C.; Marcelli, V.; Furia, T.; Santangelo, G.; Cozzolino, A.; Longo, K.; Allocca, R.; Amboni, M.; Marciano, E.; Barone, P. Vestibular impairment and adaptive postural imbalance in parkinsonian patients with lateral trunk flexion. Mov. Disord. 2011, 26, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Heß, T.; Milani, T.L.; Meixensberger, J.; Krause, M. Postural performance and plantar cutaneous vibration perception in patients with idiopathic normal pressure hydrocephalus. Heliyon 2021, 7, e05811. [Google Scholar] [CrossRef] [PubMed]
- Allum, J.H.J.; Oude Nijhuis, L.B.; Carpenter, M.G. Differences in coding provided by proprioceptive and vestibular sensory signals may contribute to lateral instability in vestibular loss subjects. Exp. Brain Res. 2008, 184, 391–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delwaide, P.J.; Pepin, J.L.; Maertens de Noordhout, A. Short-latency autogenic inhibition in patients with Parkinsonian rigidity. Ann. Neurol. 1991, 30, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Chen, R.S.; Lu, C.S. Reciprocal inhibition in Parkinson’s disease. Acta Neurol. Scand. 1997, 95, 13–18. [Google Scholar] [CrossRef]
- Burne, J.A.; Lippold, O.C. Loss of tendon organ inhibition in Parkinson’s disease. Brain 1996, 119, 1115–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pötter, M.; Illert, M.; Wenzelburger, R.; Deuschl, G.; Volkmann, J. The effect of subthalamic nucleus stimulation on autogenic inhibition in Parkinson disease. Neurology 2004, 63, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Blenkinsop, G.M.; Pain, M.T.G.; Hiley, M.J. Balance control strategies during perturbed and unperturbed balance in standing and handstand. R. Soc. Open Sci. 2017, 4, 161018. [Google Scholar] [CrossRef] [Green Version]
- Horak, F.B.; Nashner, L.M.; Diener, H.C. Postural strategies associated with somatosensory and vestibular loss. Exp. Brain Res. 1990, 82, 167–177. [Google Scholar] [CrossRef]
- Pötter-Nerger, M.; Ilic, T.V.; Siebner, H.R.; Deuschl, G.; Volkmann, J. Subthalamic nucleus stimulation restores corticospinal facilitation in Parkinson’s disease. Mov. Disord. 2008, 23, 2210–2215. [Google Scholar] [CrossRef]
- Andrews, J.C.; Roy, F.D.; Ba, F.; Sankar, T. Intraoperative changes in the H-reflex pathway during deep brain stimulation surgery for Parkinson’s disease: A potential biomarker for optimal electrode placement. Brain Stimul. 2020, 13, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Hidding, U.; Bäumer, T.; Siebner, H.R.; Demiralay, C.; Buhmann, C.; Weyh, T.; Moll, C.; Hamel, W.; Münchau, A. MEP latency shift after implantation of deep brain stimulation systems in the subthalamic nucleus in patients with advanced Parkinson’s disease. Mov. Disord. 2006, 21, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- French, I.T.; Muthusamy, K.A. A Review of the Pedunculopontine Nucleus in Parkinson’s Disease. Front. Aging Neurosci. 2018, 10, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.-G.; Zhang, K.; Yang, A.-C.; Zhang, J.-G. Deep brain stimulation of the subthalamic and pedunculopontine nucleus in a patient with Parkinson’s disease. J. Korean Neurosurg. Soc. 2015, 57, 303–306. [Google Scholar] [CrossRef]
- Dietz, V.; Berger, W.; Horstmann, G.A. Posture in Parkinson’s disease: Impairment of reflexes and programming. Ann. Neurol. 1988, 24, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Beckley, D.J.; Bloem, B.R.; van Dijk, J.G.; Roos, R.A.; Remler, M.P. Electrophysiological correlates of postural instability in Parkinson’s disease. Electroencephalogr. Clin. Neurophysiol. 1991, 81, 263–268. [Google Scholar] [CrossRef]
- Winter, D.A.; Patla, A.E.; Prince, F.; Ishac, M.; Gielo-Perczak, K. Stiffness Control of Balance in Quiet Standing. J. Neurophysiol. 1998, 80, 1211–1221. [Google Scholar] [CrossRef] [Green Version]
- Boonstra, T.A.; van Vugt, J.P.P.; van der Kooij, H.; Bloem, B.R. Balance asymmetry in Parkinson’s disease and its contribution to freezing of gait. PLoS ONE 2014, 9, e102493. [Google Scholar] [CrossRef] [Green Version]
- Winter, D.A.; Prince, F.; Frank, J.S.; Powell, C.; Zabjek, K.F. Unified theory regarding A/P and M/L balance in quiet stance. J. Neurophysiol. 1996, 75, 2334–2343. [Google Scholar] [CrossRef]
- Winter, D.A. Human balance and posture control during standing and walking. Gait Posture 1995, 3, 193–214. [Google Scholar] [CrossRef]
- Pickerill, M.L.; Harter, R.A. Validity and reliability of limits-of-stability testing: A comparison of 2 postural stability evaluation devices. J. Athl. Train. 2011, 46, 600–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragnarsdóttir, M. The Concept of Balance. Physiotherapy 1996, 82, 368–375. [Google Scholar] [CrossRef]
- Nantel, J.; McDonald, J.C.; Bronte-Stewart, H. Effect of medication and STN-DBS on postural control in subjects with Parkinson’s disease. Park. Relat. Disord. 2012, 18, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Nilsson, M.H.; Rehncrona, S.; Tjernström, F.; Magnusson, M.; Johansson, R.; Fransson, P.-A. Effects of Deep Brain Stimulation on Postural Control in Parkinson’s Disease. Comput. Biol. Med. 2020, 122, 103828. [Google Scholar] [CrossRef] [PubMed]
- Benabid, A.-L.; Koudsié, A.; Benazzouz, A.; Fraix, V.; Ashraf, A.; Le Bas, J.F.; Chabardes, S.; Pollak, P. Subthalamic Stimulation for Parkinson’s Disease. Arch. Med. Res. 2000, 31, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthi, N.; Mulligan, S.; Mahant, P.; Samanta, J.; Abbas, J.J. Deep brain stimulation amplitude alters posture shift velocity in Parkinson’s disease. Cogn. Neurodyn. 2012, 6, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Kasahara, S.; Saito, H.; Anjiki, T.; Osanai, H. The effect of aging on vertical postural control during the forward and backward shift of the center of pressure. Gait Posture 2015, 42, 448–454. [Google Scholar] [CrossRef]
- Du Pasquier, R.A.; Blanc, Y.; Sinnreich, M.; Landis, T.; Burkhard, P.; Vingerhoets, F. The effect of aging on postural stability: A cross sectional and longitudinal study. Neurophysiol. Clin./Clin. Neurophysiol. 2003, 33, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Factor, S.A.; Steenland, N.K.; Higgins, D.S.; Molho, E.S.; Kay, D.M.; Montimurro, J.; Rosen, A.R.; Zabetian, C.P.; Payami, H. Postural instability/gait disturbance in Parkinson’s disease has distinct subtypes: An exploratory analysis. J. Neurol. Neurosurg. Psychiatry 2011, 82, 564–568. [Google Scholar] [CrossRef] [PubMed]



| PD-MED | PD-MED-DBS | HS | p-Values | ||
|---|---|---|---|---|---|
| demographic data: | n/gender | 38/ 29/ 29/ 9 9 | 31/ 22/ 22/ 9 9 | 30/ 19/ 19/ 11 11 | |
| age (years) | 68.2 ± 7.6 | 64.5 ± 7.5 # | 70.6 ± 5.7 # | # =0.007 | |
| clinical data: | self-rated balance confidence (0–100) (%) | 65.0 ± 16.8 * | 66.4 ± 17.3 # | 80.7 ± 9.4 *# | *# <0.001 |
| MMSE (0–30) | 28.6 ± 1.8 | 28.4 ± 1.8 | |||
| UPDRS III (0–108) | 15.9 ± 6.7 | 14.6 ± 5.9 | |||
| UPDRS total (0–199) | 26.1 ± 10.6 | 27.5 ± 9.5 | |||
| Hoehn and Yahr (0–5) | 2.0 ± 0.3 | 2.0 ± 0.3 | |||
| disease duration since diagnosis (months) | 78.6 ± 54.6 * | 184.4 ± 79.8 * | * <0.001 | ||
| disease-dominant body side | left: 20; right: 18 | left: 11; right: 20 | |||
| time between last neurological examination and perturbation test (months) | 5.2 ± 14.3 | 2.7 ± 2.8 | |||
| DBS duration since surgery (months) | 27.8 ± 10.3 | ||||
| self-rated satisfaction with DBS (%) | 77.9 ± 21.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heß, T.; Oehlwein, C.; Milani, T.L. Anticipatory Postural Adjustments and Compensatory Postural Responses to Multidirectional Perturbations—Effects of Medication and Subthalamic Nucleus Deep Brain Stimulation in Parkinson’s Disease. Brain Sci. 2023, 13, 454. https://doi.org/10.3390/brainsci13030454
Heß T, Oehlwein C, Milani TL. Anticipatory Postural Adjustments and Compensatory Postural Responses to Multidirectional Perturbations—Effects of Medication and Subthalamic Nucleus Deep Brain Stimulation in Parkinson’s Disease. Brain Sciences. 2023; 13(3):454. https://doi.org/10.3390/brainsci13030454
Chicago/Turabian StyleHeß, Tobias, Christian Oehlwein, and Thomas L. Milani. 2023. "Anticipatory Postural Adjustments and Compensatory Postural Responses to Multidirectional Perturbations—Effects of Medication and Subthalamic Nucleus Deep Brain Stimulation in Parkinson’s Disease" Brain Sciences 13, no. 3: 454. https://doi.org/10.3390/brainsci13030454
APA StyleHeß, T., Oehlwein, C., & Milani, T. L. (2023). Anticipatory Postural Adjustments and Compensatory Postural Responses to Multidirectional Perturbations—Effects of Medication and Subthalamic Nucleus Deep Brain Stimulation in Parkinson’s Disease. Brain Sciences, 13(3), 454. https://doi.org/10.3390/brainsci13030454







